Abstract
Herein, we report efficient visible light-induced photoredox reactions of C–H/N–H and C–X Bonds. These methods have provided access to varied portfolio of synthetically important γ-ketoesters, azaspirocyclic cyclohexadienones spirocyclohexadienones, multisubstituted benzimidazole derivatives, substituted N,2-diarylacetamide, 2-arylpyridines and 2-arylquinolines in good yields and under mild conditions. Moreover, we have successfully discussed the construction through visible light-induction by an intermolecular radical addition, dearomative cyclization, aryl migration and desulfonylation. Similarly, we also spotlight the visible light-catalyzed aerobic C–N bond activation from well-known building blocks through cyclization, elimination and aromatization. The potential use of a wide portfolio of simple ketones and available primary amines has made this transformation very attractive.
1. Introduction
As a new benign environment and effective catalytic strategy, visible light-induced photoredox catalysis has been well applied to modern organic synthesis. In this review, the recent advances in light-induced C-H and C-X bond activations and the accompanied reaction mechanisms are discussed in detail. Light energy is pollution-free, cheap, green and inexhaustible with potential industrial and medical applications (Figure 1). Therefore, an efficient and effective utilization of light has become one of the most active research topics. In fact, the connection between the use of light and environmental sustainability has been reported since the early 20th century, when the famous Italian organic photo-chemist Ciamician pointed out that the future of industrial chemical synthesis is low-maintenance, green and energy rate efficient [1,2,3]. The low-energy photochemical reactions will replace the traditional environment-friendly high-energy synthesis processes. Former scientists understood the mystery of photosynthesis to make better use of sunlight [4]. In the early 20th century, photochemists discovered that a light source can be involved in organic chemical reactions as an inexhaustible source of clean energy. Classic organic photochemical reactions usually use high-energy ultraviolet light to initiate the reactions. However, the content of ultraviolet light in sunlight is low. In addition, the UV generator generally uses high-pressure xenon lamps or mercury lamps, which are relatively expensive. Scaling up of the reaction is difficult due to the constraints with the light source limiting the industrial applicability of photo catalytic organic synthesis. Therefore, for the full use of visible light from sunlight (about 46%), efficient visible light catalyzed organic photochemical reactions widely used in industrial synthesis have been developed in recent years. This is the most effective way to solve the problem of industrial organic photosynthesis [2].
Figure 1.
Synthesis of biologically active compounds through C-H/N-H bond activation.
In recent years, the development of metal coordination chemistry has resulted in new advances in visible light-catalyzed chemical reactions. In this research field, the complexes of bipyridyl-based ruthenium and iridium displayed interesting characteristics, chemical stability and special photoredox properties (Scheme 1) [5,6,7,8,9,10].
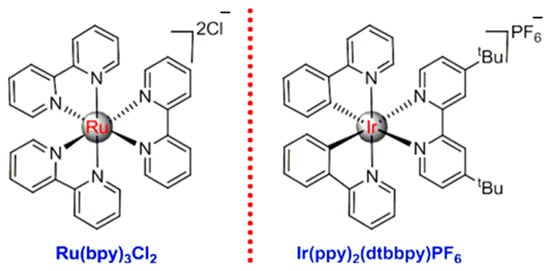
Scheme 1.
Typical bipyridyl complexes of ruthenium and iridium.
For example, a Ru(bpy)32+ complex showed a strong absorption in the visible wavelength range with an absorption maxima at 452 nm. In addition, the central metal ion in the ground state (S0) divalent ruthenium complex belongs to the d6 system (Scheme 2) [7]. When the metal center absorbs light, metal to ligand charge transfer (MLCT) reaches its excited singlet (S1), which usually excites singlet complexes, has a short lifetime (100–300 fs) and is not enough to promote an effective chemical conversion. The excited triplet state (T1) and the complex lifetime of the excited triplet state can be extended to 1100 ns. In the survival time, the complex in the excited state has high activity, which can, through an electron transfer, return to the ground state. As a result, the organic molecules are activated and trigger the chemical reactions. This innovation overcomes common problems such as the substrate limitations, as most organic molecules cannot be activated by conventional complexes under visible light. As a result, ruthenium bipyridyl complexes and their analogues are gradually being applied to organic synthesis.
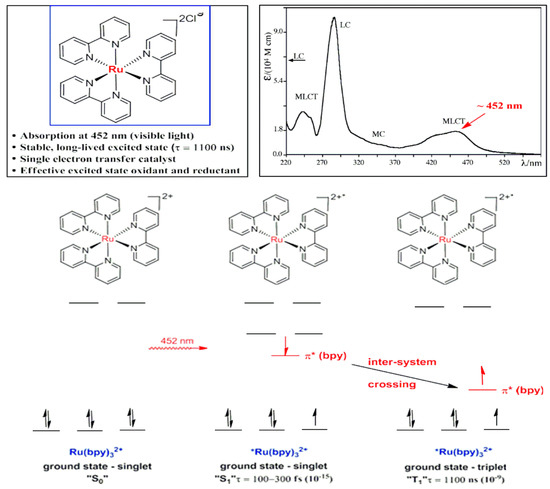
Scheme 2.
Strong absorption of a Ru(bpy)32+ complex in the visible wavelength range.
In 2008, in the early stages, Nicewicz and MacMillan et al. promoted the development of this field and used Ru(bpy)3Cl2 as a visible light catalyst for the asymmetric alkylation of aldehydes [11]. Ischey et al. [12] and Narayanam et al. [13] also reported the use of Ru(bpy)3Cl2 as a visible light catalyst and made progress in photo-catalysis and succeeded in a series of visible light catalyzed organic transformations.
Nowadays, visible light catalyzed organic reactions have become the focus of modern organic chemistry [14,15,16,17,18,19,20,21,22,23,24,25,26]. There are three methods of visible light photocatalytic transformations: oxidation quenching, reduction quenching and energy transfer. With a Ru(bpy)3Cl2 catalyst illuminated upon visible light, Ru(bpy)3 excitation occurs from the ground state transition of (2+) of a metal center to Ru (bpy)3 in the excited state 2+ * through a metal to ligand charge transfer (MLCT) (Scheme 3, path A). Ru (bpy)3 is reduced to a low energy state complex via a reductive quenching pathway (A). The metal complex in the excited state can interact with organic molecules. Similarly, the Ru (bpy)3 is oxidized to a high valence state by a single electron transfer to form a (3+) complex via an oxidative quenching path (B). Herein, organic molecules with electron gain or loss are called an oxidation quencher or reduction quencher. After gaining and losing electrons, organic molecules can undergo a series of transformations. At the same time, the excited state Ru(bpy)32+ * is also a good energy donor, which directly transfers energy to organic molecules through an energy transfer, leading to a chemical transformation (path C) (Scheme 3) [27].
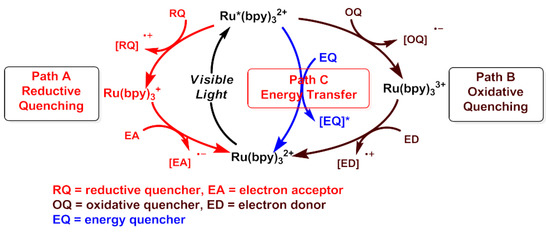
Scheme 3.
OQ = oxidative quencher, RQ =reductive quencher, ED = electron donor, EA = electron acceptor, EQ = energy quencher.
Following these findings, it was concluded that the occurrence of organic chemical reactions is mainly through electron transfer and energy transfer pathways. If there were an additional electron acceptor in the reaction system, then the electron transfer reaction is further divided into: (1) a net oxidation reaction, (2) a net reduction reaction, and (3) a redox neutral reaction. For visible light photocatalytic reactions, ruthenium and iridium bipyridine complexes are commonly used as a photocatalyst [28].
2. Visible Light Catalyzed Net Oxidation
A visible light catalyzed net oxidation usually refers to the addition of stoichiometric electron acceptors such as oxygen, high valence iodide, persulfide and other oxidizing substances to the reaction system with a photocatalyst. In the reaction cycle, the reaction substrate is activated, and the active intermediate produces participates in the subsequent chemical transformation.
2.1. Oxidative Production of Imine Ion Intermediates
Some amines are usually electron-rich and, therefore, they can be oxidized by a photocatalytic strategy. The study of imine ion intermediates is one of the mature fields in photocatalytic oxidation. The intermediate of an imine ion is produced by a chemical reaction wherein the organic amine molecule loses its electrons. Such reactions are initiated by the catalyst, and it is usually characterized by a reduction quenching process. For example, [Ru(bpy)32+] can promote the oxidation of electron rich organic molecules such as amines. During the course of the reaction, the photocatalyst can be reduced to a low valence complex (Ru(bpy)31+), resulting in the formation of a free radical cation intermediate 2. Similarly, Ru(bpy)32+ was generated by the action of oxygen [O].- species. Consequently, the iminium ion 4, formed from a radical intermediate 2, is further oxidated. The formed imine positive ions can react with various nucleophiles, introduce new amines at the α-position of amines and generate the corresponding target products [28,29]. The low valence complex needs the loss of electrons and to return to the initial valence state [Ru(bpy)32+], which requires the addition of stoichiometric electron acceptors (oxygen, high valence iodine, persulfides and other oxidizing substances) to perform the recycling and regeneration of photocatalysts (Scheme 4) [28,29].
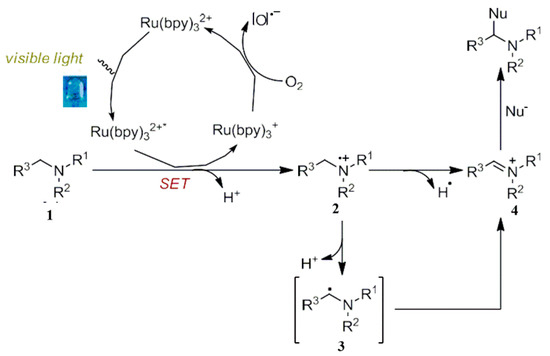
Scheme 4.
Visible light catalytic oxidation for the production of imine ion intermediates.
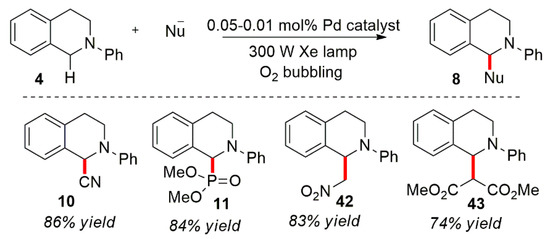
At the present stage, the research in this field is mainly focused on the following aspects: (1) exploration of a variety of nucleophiles, (2) expanding the application of the constructed products and looking for new chemical reactions and (3) developing new photocatalysts, chemical agents or catalytic systems. Firstly, from the perspective of nucleophiles, N-aryl-substituted tetrahydroisoquinoline was used as the model substrate to find various nucleophiles to directly construct new chemical bonds at the α-position of amines for the functionalization of its adjacent C-H bond. In 2010, Condie et al. described an ortho C-H bond functionalization in a visible light-catalyzed oxidation of organic amines (Scheme 5) [30] using Ru(bpy)3Cl2 or Ir(PPy)2(dtbbpy)PF6 as a photocatalyst and N-aryl-substituted tetrahydroisoquinoline 4 as a model substrate. The substrate was oxidized to the corresponding iminium ion intermediate under light. As a nucleophilic reagent, the C-H containing alkane attacks the intermediate of the iminium ion leading to the formation of a series of aza Henry products in high yields. In addition, the author conducted a series of control experiments, such as without light or a photocatalyst, and the reaction hardly occured. Oxygen is also necessary in the reaction system. Under the anaerobic conditions, the conversion rate of the reaction is significantly reduced. Fluorescence quenching experiments showed that the substrate can quench the fluorescence product by the excited photocatalyst.
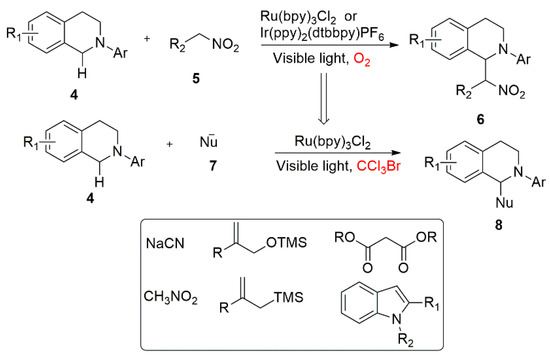
Scheme 5.
Visible light catalyzed aza Henry reactions.
The experimental results showed that this is a typical visible light catalytic reaction process, in order to avoid the amide and dimer by-products from α-amino radical and improve the slow catalyst turnover with O2 in the reaction system. Later, the team used trichlorobromomethane instead of oxygen as an oxidant. The reaction was attempted again, avoiding the production of amide by-products with good results [31].
In 2011, Rueping et al. proposed a series of synergistic catalytic modes combining a photocatalyst and a Lewis base catalyst to realize the photocatalytic Mannich reaction 9 (Scheme 6) [32]. Similar work was also reported by Zhao et al., wherein a comparative study of nucleophiles was performed. The formation of by-products was effectively avoided using enol silicone ethers as the nucleophilic reagents [33]. In the same year, 2011, Rueping et al. reported other typical nucleophiles facilitating the construction of C-C and C-P bonds [34,35]. They also reported the first case of photocatalysis and transition metal catalysis for the study of an alkynylation reaction (product 12) of N-substituted tetrahydroisoquinoline derivatives [36].
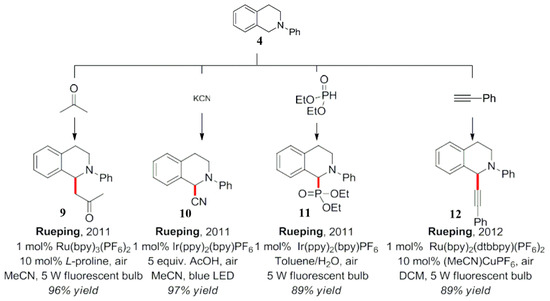
Scheme 6.
Study of an oxidative addition of different nucleophiles to an iminium ion.
Recently, the Zhu group reported the difluoromethylation of nitrophenyl substituted tetrahydroisoquinoline derivatives. The difluoro-substituted enol compounds formed in situ are used as nucleophilic reagents, and carbon tetrachloride was added as the reactive oxidant [37]. Firstly, the iminium ion intermediates were formed under photocatalytic conditions, and then organic compounds were added. The difluoromethylation of tetrahydroisoquinoline was accomplished by the addition of CH3CN/CCl4 and nucleophilic precursors (Scheme 7).
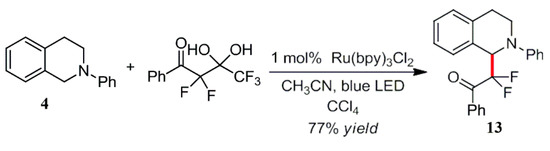
Scheme 7.
Study of a nucleophilic addition to tetrahydroisoquinoline derivatives.
In addition to the development of different nucleophiles for the addition reaction to iminium ions, a comparative study of the reaction substrates was also made. The Xiao team used the above strategy to realize an intramolecular nucleophilic reaction of nitrogen aryl substituted tetrahydroisoquinolines used for the synthesis of oxazine and pyrimidine derivatives (Scheme 8) [38].
Scheme 8.
Photocatalytic intramolecular cyclic reaction.
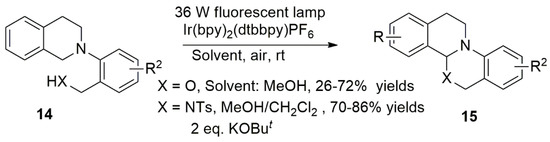
In 2011, Xiao et al. converted substituted nitrogen aryl tetrahydroisoquinoline substrates to nitrogen alkyl substituted tetrahydroquinoline and hydroisoquinoline derivatives to further expand the application of this reaction by adding electron poor olefin compounds through an [3 + 2] addition [39,40]. They reported cycloaddition and oxidative aromatization reactions for the synthesis of complex derivatives of pyrrolisoquinoline (Scheme 9). Importantly, there is a requirement of 1.1 equivalents of NBS to fulfill the oxidative aromatization reaction.
Scheme 9.
Synthesis of pyrrole isoquinoline derivatives.
Later, Rueping et al. published a report leading to the synthesis of nitrogen-containing quaternary ring skeleton compounds by employing photocatalytic oxidative conditions [41]. In addition to the above tetrahydroisoquinoline derivatives as reaction substrates, various types of tertiary amines participate in the photooxidation.
In 2011, Xuan et al. synthesized polysubstituted tetrahydroimidazole derivatives 25 by a visible light catalysis. In addition, the alkali plays a role in improving the diastereoselectivity of the reaction system (Scheme 10) [42].
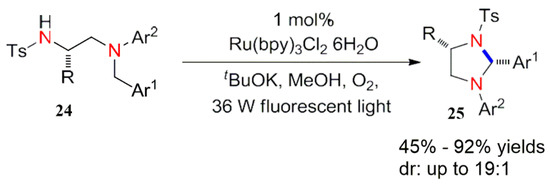
Scheme 10.
Synthesis of tetrahydroimidazole derivatives.
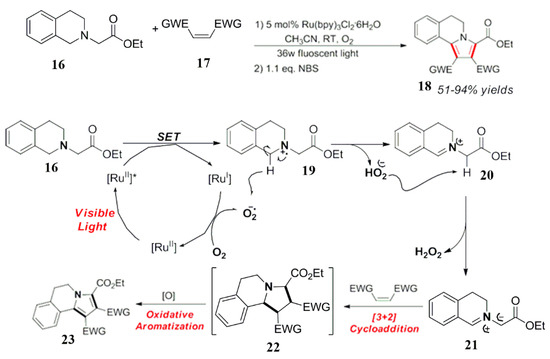
In 2012, Cai et al. used tetramethylethylenediamine as a raw material in the reaction (Scheme 11). It is a cationic intermediate of the nitrogen free radical which was produced by a photocatalytic oxidation under light conditions [43]. According to the structural characteristics, the intermediate further breaks the ortho C-C bond to form a free bond at the same time, but also a group intermediate and an iminium ion intermediate. The iminium ion intermediate is the main part of the reaction and is accepted in the system. The nucleophilic reagent nitroalkane attacks the iminium ion to generate the corresponding target product 28. Control experiments for the C-C cleavage of nitrogen radical cationic intermediates and photopolymerization reaction by amino radicals are also proved [43].
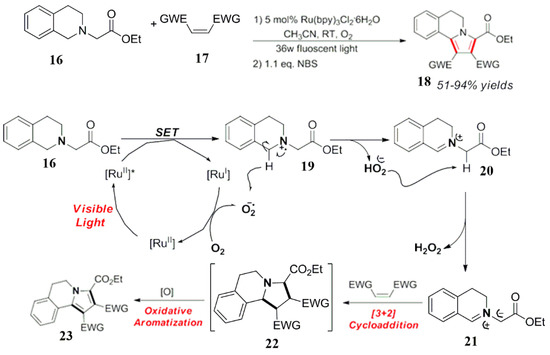
Scheme 11.
Synthesis of pyrrole isoquinoline derivatives.
In comparison to tertiary amines, there were relatively few reports on the photocatalytic transformation of secondary and primary amines. As their oxidation potential is high, they are not prone to oxidative reactions. Li et al. showed that the α-secondary amine substrate 29 can be oxidized to imines upon heating and light conditions, which is subsequently captured by indole derivatives to obtain carbonyl α-indole substituted amines [44]. Thereafter, Rueping et al. studied the combination of visible light and a PC photocatalyst for a α-secondary amine substrate 29. The addition of zinc acetate resulted in the activation of the imine intermediate formed in situ, which was conducted to the next nucleophilic addition (Scheme 12) [45].
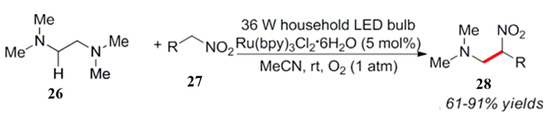
Scheme 12.
Visible light nitration of tetramethylethylenediamine.
In addition, in 2012, Rueping et al. reported the oxidation of a primary amine, benzylamine, under light [46]. Moreover, a self-condensation can occur to produce imine intermediates, and the further subsequent reaction is not complicated. However, this reaction makes possible the conversion of primary amines under photocatalytic conditions (Scheme 13) [46].
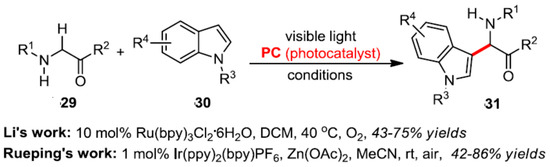
Scheme 13.
Visible light induced α-site arylation of carbonyl compounds.
In addition to the above organic dye photocatalysts, metal complexes have also been reported. The development of these complexes has further expanded the scope of metal photocatalysts and solved the problem of transmission, and some traditional metal photocatalysts are shown in Scheme 14.

Scheme 14.
Visible light induced self-condensation of primary amines.
Furthermore, as the core of the catalytic reaction, the development of new catalysts and catalytic systems is very important for the performance of reverse reactions. These efficient transformations play a vital role, and many groups have made in-depth research in relevant fields. Organic dye compounds have the advantages of a low price and easy access. They have similar physicochemical properties and light absorption properties, so organic dyes are widely used by organic chemists. Ravelli et al. used green and environmentally friendly common organic dye catalysts, as shown in Scheme 18, namely, Eosin Y, TBA Eosin Y and Rose Bengal [47,48,49,50].
Xue et al. successfully applied a gold pyridine complex as a photocatalyst for visible light oxidation (Scheme 15) [51]. Compared to previous works, various simple ketones and some simple organic amines can participate well in the reaction. The scope of substrates has been widely expanded with good to excellent yields (Scheme 15).
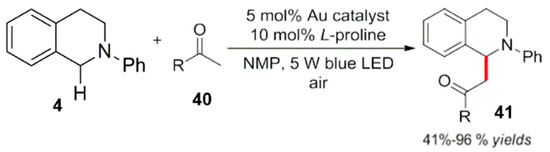
Scheme 15.
Au complex photocatalytic oxidative functionalization.
At the same time, To et al. used a Pd complex (PdF20TPP) formed by a highly active metal palladium and porphyrin as a catalyst upon the addition of the visible light catalyst [52]. Various types of nucleophiles and N-phenyl substituted tetrahydroisoquinoline derivatives were used. The iminium ion intermediates produced by the oxidation of 4 underwent an intermolecular reaction, and the corresponding products were generated smoothly.
In addition, Zhong et al. synthesized metal platinum pyridine complexes by analyzing their optical properties. It was found that a variety of nucleophiles (including electron rich indole) produced the corresponding products (Scheme 16). The functionalization of N-phenyltetrahydroisoquinoline at the α-position by indole, dimethyl malonate or nitromethane is shown in Scheme 17 [53]. It is worth noting that the addition of two equivalents of ferrous sulfate can effectively inhibit the reaction and generate by-products (amide compounds).
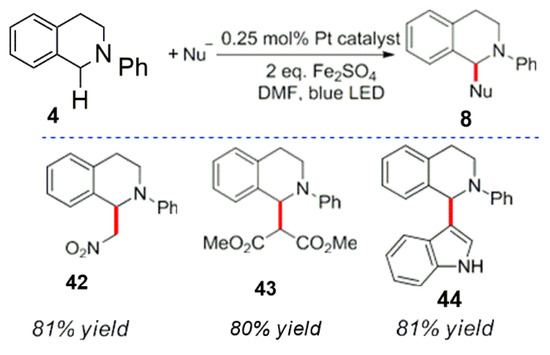
Scheme 16.
Photocatalytic reaction of hydroisoquinoline by a Pt complex.
Scheme 17.
Common photocatalysts dye used in organic reactions.
In 2022, Jiang et al. reported an oxidation of sulfides into sulfoxides using visible light under clean conditions [54]. In the same period, Zhang et al. synthesized hydrogen peroxide by advance oxidation processes (AOPs) through visible light photocatalyst [55].
In 2011, Hari and König et al. applied an organic dye (Eosin Y) to the catalytic reaction, using visible light for heteroaryl groups and oxidized various substrates with good results [56]. The metal palladium complex has the advantage of a high reaction efficiency and can be used with a very small amount of catalyst (Scheme 18) [57].
Scheme 18.
Photocatalytic reaction of a Pd metal complex.
Moreover, other similar organic dye compounds such as rose red and TBA Eosin Y [58] were successfully applied to the study of these photocatalytic reactions (Scheme 19).
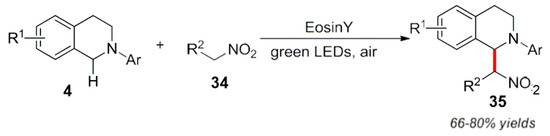
Scheme 19.
Oxidative functionalization catalyzed by organic dye catalysts.
At the same time, Li et al. used rose Bengal as a photocatalyst to oxidize tetramethylenediamine and introduced an aldehyde group at the indole 3-position. It should be pointed out that the reaction proceeds with a dual photocatalytic amine oxidation (Scheme 20) [59].
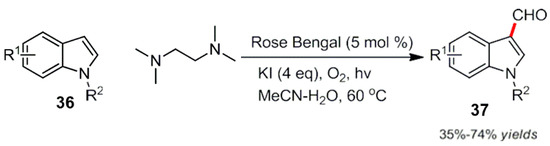
Scheme 20.
Oxidative functionalization catalyzed by organic dye catalysts.
Fu et al. used alkyne-containing nucleophiles for the photocatalytic trifluoromethylation and alkynylation with a dye (rose Bengal). This alkynylation reaction of aryl substituted tetrahydroisoquinoline substrates was achieved in good to excellent yields (Scheme 21) [60].
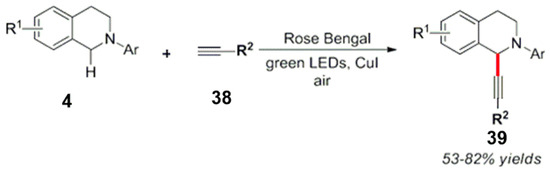
Scheme 21.
Organic dyes for the photocatalyzed alkynylation of tetrahydroisoquinolines.
2.2. Free Radical Intermediates Produced by Oxidation
As seen above, a visible light catalyzed oxidation of organic amines produces iminium ion intermediates, which are then subjected to be attacked by various nucleophiles for the synthesis of various products. Moreover, a series of chemical transformations has been widely studied. In fact, a photocatalytic strip can generate an amino radical cation 2 by SET (Single Electron Transfer). Acidity is also greatly enhanced, which can lead to the loss of a proton to obtain an α-amino radical intermediate 3. The radical can be stabilized by the adjacent nitrogen atom and then captured by other suitable organic molecules, resulting in the corresponding chemical conversion (Scheme 4).
Ju et al. reported a visible light-catalyzed [4 + 2] radical cycloaddition reaction from a tertiary amine 45 [61]. Starting from the maleimide 46, the polysubstituted tetrahydroisoquinoline derivative 47 was efficiently synthesized (Scheme 22). The mechanism was started by an oxidation under a visible light catalysis of an α-amino radical intermediate, followed by an addition to maleimide derivatives, and then the final product 47 was obtained through an intramolecular cyclization.
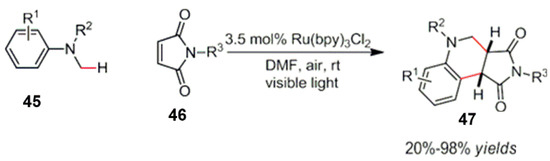
Scheme 22.
Visible light catalyzed radical cycloaddition reaction from tertiary amines.
Inspired by free radical cycloaddition reactions, Zhu et al. reported a reversible reaction in an oxygen atmosphere [62]. Photocatalytic reactions between various substituted tertiary aromatic amines and electron deficient olefins through an intermolecular free radical addition are shown in Scheme 23. The target compound 50 was obtained by an intramolecular cyclization via a series of reactions (Scheme 24) [62].
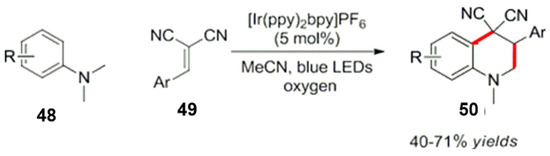
Scheme 23.
Reaction of amino ortho radical produced by a photocatalyst.
Scheme 24.
Free radical cycloaddition by a photocatalytic oxidation.
Douglas et al. used a photocatalytic oxidation for the C-H functionalization of amino ortho radicals with heterocycles (Scheme 24), a chemical reaction used for the synthesis of drug molecule [63]. Thus, photocatalytic strategies are widely used for the synthesis of medicines.
In addition, Bordwell et al. reported the addition reaction between mercaptan and olefin by a photocatalytic oxidation (Scheme 25) [64]. Upon oxidative light illumination, mercaptan generates mercaptan radical cations 57. After losing a proton, the mercaptan free radical 58 was generated, and the olefin in the reaction system captured the mercaptan free radical to form a free radical intermediate 59, then the free radical intermediate 59 and mercaptan were quenched by an electron transfer and, finally, sulfur-containing compounds were obtained.
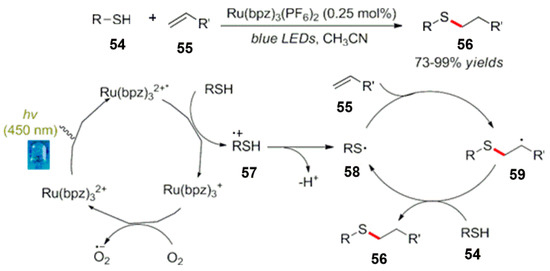
Scheme 25.
Reaction of sulfur radical produced by a photocatalytic oxidation.
2.3. Oxidation Reaction of Other Functional Groups
In addition to the oxidation of organic molecules with the aforementioned functional groups, the oxidation of other functional groups has also been improved and has developed rapidly. Yelo and Deronzier et al. studied this type of reaction in the early stages of development. The oxidation strategy was applied to the oxidation of benzyl alcohol derivatives and for the synthesis of aryl aldehydes under visible light catalysis (Scheme 26) [65]. The author did not need to add additional oxidants and oxygen as an oxidant. The photo-oxidation of benzyl alcohol occurred under mild light conditions to aryl aldehydes or ketones.
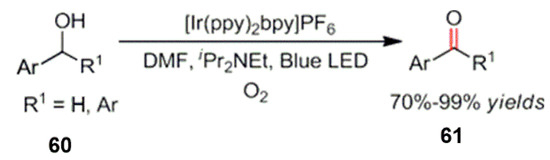
Scheme 26.
Photo-oxidation of benzyl alcohol to ketone derivatives.
Jiao et al. reported that a series of α-brominated esters or α-chlorinated esters was directly oxidized by a photocatalytic method to form α-ketoacid ester derivatives (Scheme 27) [66]. The authors used 4-methoxypyridine in a catalytic amount to activate the carbon bromine bond of the substrate. Oxygen was used as the oxidant in the system. Based on a series of control experiments, a reaction mechanism was proposed. The possible mechanism is: Firstly, 4-methoxypyridine reacts with α-bromoester to form a quaternary ammonium salt. The ammonium salt activates the carbon bromine bond of halogenated hydrocarbon, but also can be used as the initiator of the reaction to trigger the whole reaction. With the production of benzyl radicals, the free radical intermediate produced by benzyl radical reacts with the oxygen active species. Finally, the oxidative carbonylation reaction of bromo ester or chloro ester was realized to generate the corresponding oxidation product 63.
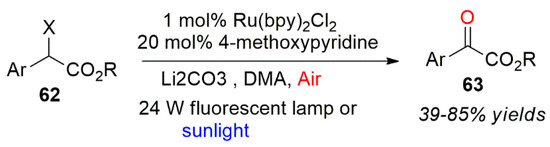
Scheme 27.
Photo-oxidation reaction for a C-X bond breakage.
In 2011, Zou et al. successfully synthesized arylboric acid derivatives using a photocatalytic oxidative strategy. Phenolic compounds were prepared by oxidative hydroxylation of the arylboric acid (Scheme 28) [67]. In addition, arylborate substrates are also suitable for this reaction, and the team determined the oxygen atom in the reaction product (phenol) through a series of controlled experiments. DFT (Density Functional Theory) calculations confirmed the possibility of the reaction process.
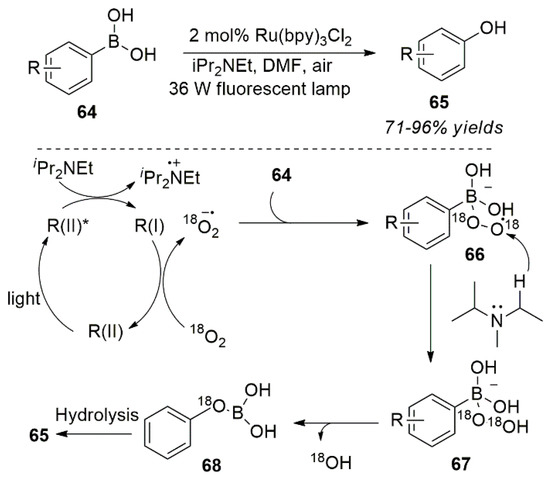
Scheme 28.
Photo-oxidation reaction for a C-B bond cleavage.
Cheng et al. performed the activation of a C-S bond of thiobenzoamide derivatives by means of a photo-oxidative quenching [68]. It was used in the synthesis of benzothiazole derivatives. In the reaction process, oxygen was used as an oxidative quencher, and alkali (DBU) was used in the reaction process. It is necessary to remove protons from the substrate 69 in the first step to form a more stable negative ion intermediate 71. After one electron oxidation, a free radical species 72 was formed, and then an intramolecular cyclization reaction occured by losing one electron. The targeted compound 70 was finally generated from hydrogen radical species. As a result of the oxidation reaction, the substrate 69 is completely converted. Removal of two electrons generated the corresponding target compound (70) (Scheme 29) [68]. Recently, Yong et al. efficiently synthesized unsaturated N-heteroarenes using a photo catalyst with excellent tolerance of functional groups [69].
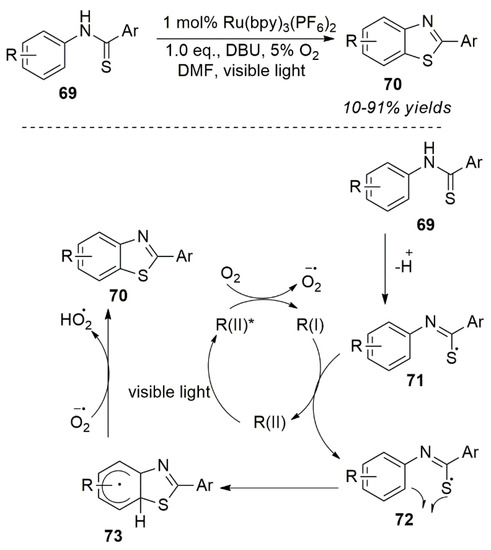
Scheme 29.
Photo-oxidation of C ̶ S bond of thiobenzoamide derivatives.
2.4. Other Types of Oxidation Reactions
In addition to the aforementioned reactions, another important research direction is the discovery of new methods of photo-oxidative catalysis. Recently, Bergonzini et al. made a perfect combination of visible light catalyst and a chiral thiourea catalyst OC. They synergistically activated the substrate molecules and small molecular catalysts in the reaction process. The interaction of hydrogen bonds forms chiral ion pairs, which then induces chirality in the process of a nucleophilic addition. The authors used an asymmetric Mannich reaction of nitroaryl substituted tetrahydroisoquinoline substrates with carbon tetrachloride as an oxidant (Scheme 30) [70].
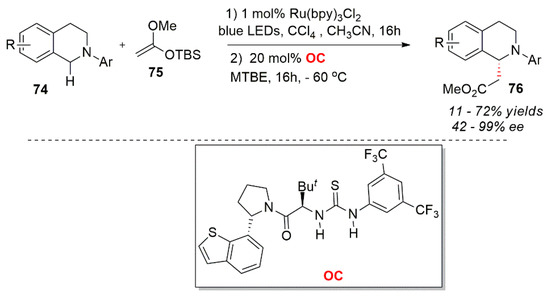
Scheme 30.
Photocatalytic asymmetric reaction of tetrahydroisoquinolines.
DiRocco and Rovis et al. studied the visible light oxidation with nitrogen heterocyclic carbine (NHC) synergistic catalysis amine for an asymmetry α-acylation (Scheme 31) [71]. In this process, dinitrobenzene was used as an oxidant through this oxidation process. The corresponding iminium ion intermediate was formed as an electrophilic reagent. At the same time, the nitrogen heterocyclic carbene (NHC) catalyst induced a polarity reversal of aldehyde to form an acyl anion or enol intermediates with strong nucleophilic properties and led to an intermolecular nucleophilic reaction. Feng et al. and Perephichka et al. have contributed to the growth of this field [72,73].
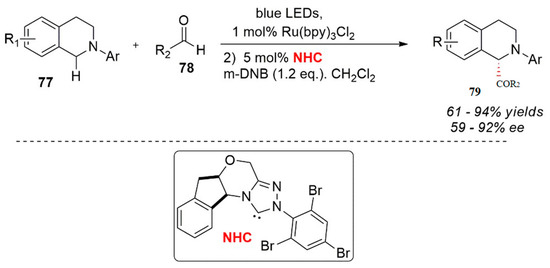
Scheme 31.
Photocatalytic asymmetric reaction.
In addition, many other types of visible light-catalyzed net oxidation reactions have been reported in the literature. Tucker et al. used a photo-oxidation reaction to solve the de-oxidation of p-methoxybenzyl protective groups under the photocatalytic conditions [74]. An, Ding et al. accomplished the oxidative amination reaction and an oxidative hemipinacol rearrangement reaction of indole derivatives [75,76]. Moreover, the Yoon group elaborated on a photocatalytic cycloaddition reaction involving oxygen [77,78]. The Lei group reported the decarboxylation/amination of photocatalytic oxidation of β-keto acids [79]. A photocatalytic net oxidation reaction includes the oxidative ring opening reaction of azacyclopropane [80], oxidative diaryl coupling reaction [81] and ether α-arylation [82].
3. Visible Light Catalyzed Net Reduction Reactions
Visible light-catalyzed net reduction reactions usually refer to the addition of a stoichiometric electron donor into the reaction system. The photocatalyst is a solid in the catalytic cycle. The active intermediates produced by activating the reaction substrate participate in the subsequent chemical transformation.
3.1. Reduction in Electron Deficient Olefins
As early as 1981, Pac et al. reported the visible light-catalyzed reduction in electron deficient olefins [83]. The authors used Ru(bpy)3Cl2 as a photocatalyst and 1-benzyl-1,4-dihydronicotinamide (BNAH) as a substrate. Dimethyl maleate was reduced to dimethyl succinate, and the reduction system was applied to electron deficient aromatics. Good results were obtained with olefin series (Scheme 32). Moreover, Miller et al. reported a strategy for the reductive hydrodifluoroalkylation of alkenes using triethylamine as the terminal reductant [84]. Recently, Willis et al. studied sulfonamides to convert sulfonyl radical intermediates. This method exploits a photocatalytic approach to access the radical, which is harnessed by relating pharmaceutically sulfonamides with a range of olefine fragments [85].
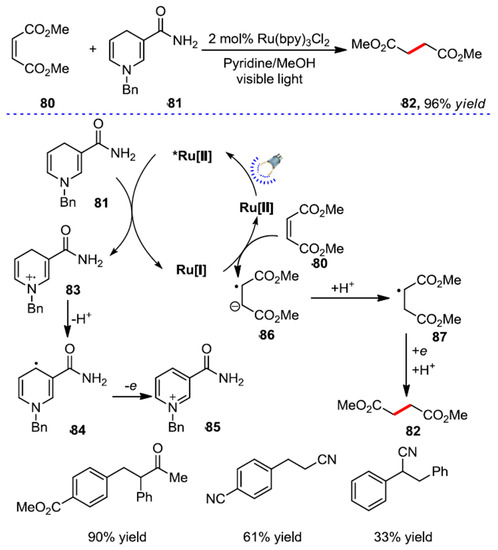
Scheme 32.
Photocatalytic reduction in electron deficient olefins.
3.2. Reductive Dehalogenation Reactions
The dehalogenation reaction widely exists in organic chemical transformations, wherein a free radical dehalogenation is an effective strategy and method. This kind of reaction system usually needs an addition of organotin reagents or it is initiated by a free radical initiator, with disadvantages of harsh reaction conditions and an unfriendly environment [86]. As early as the 1980s and 1990s, new methods, such as a photocatalytic reductions and dehalogenation, were reported [87,88]. However, for various reasons, they have not been further studied. Recently, visible light catalysis has attracted the attention of scientists with a need for mild and efficient catalysis [89]. The reductive dehalogenation method has become a research hotspot for scientists. Kellogg and Willner, et al. used Ru(bpy)3Cl2 as a photocatalyst and organic base diisopropyl ethyl carbamate as a reducing quencher. The carbon bromine or carbon chlorine bonds of carbonyl and benzyl sites were systematically studied, except for the inactive aryl groups. Besides alkenyl halides, other organic compounds were also dehalogenated in high yields and with good selectivities (Scheme 33) [90,91,92]. Regarding the mechanism, firstly, under the light excitation, the excited divalent ruthenium catalyst Ru [II]* was reduced with iPr2NEt to form a low valence state Ru(I), and then the substrate was activated by a low valence state catalyst. The free radical intermediate 92 was formed by an electron transfer from Ru (I), and then the intermediate grabbed a hydrogen from 93. The dehalogenation product was generated from the base. The labeling experiments proved that both iPr2NEt and HCOOH are the hydrogen sources. Again, Neumann et al. used the organic dye Eosin Y as the photoreduction catalyst and Hantzsch ester as the reduction quencher. The reaction was also studied further, with promising results, in combination with a continuous flow chemistry technology for the conversion. The reaction time is greatly shortened, and an efficient conversion of the reaction was realized [93].
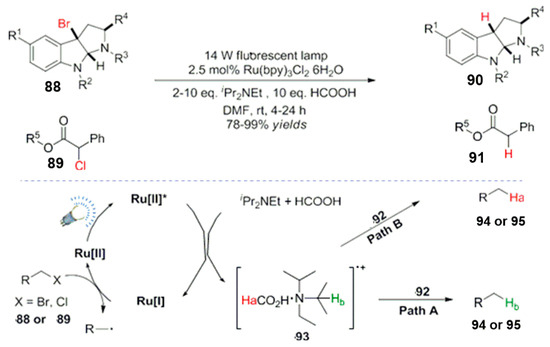
Scheme 33.
Ru(bpy)3Cl2 6H2O catalyzed dehalogenation.
In addition, Goren and Willner reported earlier that 1,2-dibromo biphenyl ethane was separated into two phases under photocatalytic conditions with the successful synthesis of stilbene [94]. Some research groups have also explored this aspect in depth. Their research mainly focuses on a diversity of substrates as well as on improving the efficiency of the reaction conditions. In 2011, Maji et al. successfully used a visible light catalytic reduction method for the dehalogenation of 1,2-dibromoaromatic compounds. A series of unsaturated ketones, esters and amides was successfully synthesized using 1,5-dimethoxynaphthalene as a reducing quencher [95]. In 2014, Mc Tieman et al. used organic small molecule catalysts to transfer electrons under light conditions. This strategy enabled the synthesis of dehalogenated compounds (Scheme 34) [96].
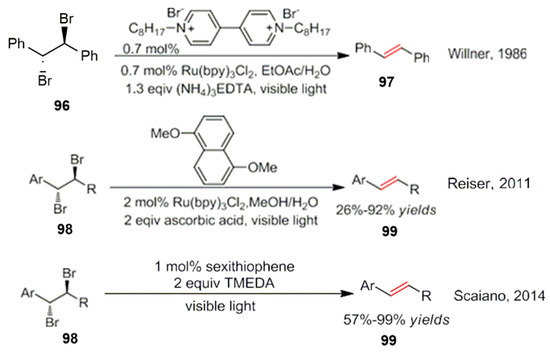
Scheme 34.
Photocatalyzed dehalogenation reactions.
To expand the applicability of a dehalogenation reaction, Nguyen et al. used fac-Ir(PPy)3 as the photocatalyst, tributylamine as the reducing and quenching agent and Hantzsch ester or formic acid as an additive, and used them for the activation of iodinated compounds. Reduced and deiodinated products were obtained in a high yield under the optimized conditions (Scheme 35) [97]. In addition, when the molecule contains unsaturated chemical bonds, such as carbon–carbon double bonds, alkyl or alkenyl groups, the reductive dehalogenation of iodine compounds produces free radical intermediates, and then the intramolecular free radical cyclization reaction occurred, leading to the corresponding cyclized products.
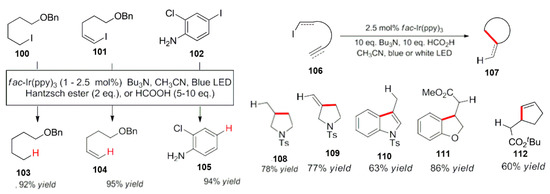
Scheme 35.
Photocatalytic reductive dehalogenation.
In addition, the Okada team reported a photocatalytic deprotection of phthalimide protected ester compounds [98,99,100]. The ester compound 127 produced a free radical anion 129 under photocatalytic conditions. The free radical anion was unstable, was converted and released CO2 to form a free radical intermediate 128 accompanied by a Phthaloyl production of amine 126. The free radical intermediate 128 was captured by coupling reagents to form the corresponding compound (Scheme 36).
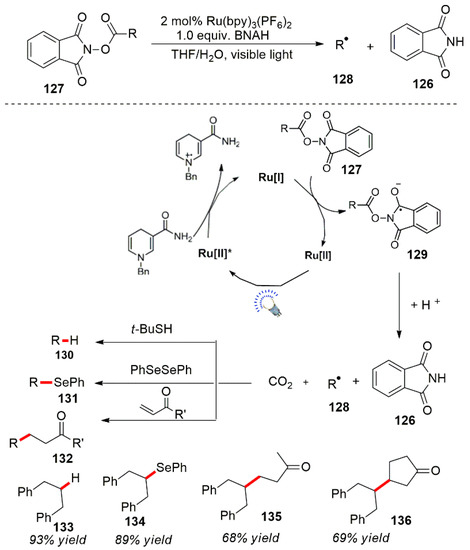
Scheme 36.
Azacyclopropane compounds’ ring opening photocatalysis.
Many other research groups have also worked on a reductive deiodination reaction. Kim and Lee completed the de-iodination and de-iodination cyclization of similar compounds using iridium catalysts with the reaction products in a good yield [101]. Wang et al. applied this method for the reduction and de-oxidation of some iodized sugars [102]. Recently, Pillegrin et al. also demonstrated photocatalyzed dehalogenation by homoleptic Cu(I) complexes under ambient conditions [103].
3.3. Deductive Desulfonation of Benzene Sulfonyl and Other Sulfur Salts
Hantzsch esters are often used as reducing agents in organic reactions to provide a hydrogen source for the synthesis of C-S bonds. In the early stages, Nakamura et al. studied C-S bonds in compounds containing sulfonyl groups at the ortho position of carbonyl groups. The visible light photocatalytic cleavage process was studied, and various carbonyl compounds were obtained in a high yield [104]. In the experiment, bipyridine ruthenium was used as a photocatalyst, and Hantzsch ester was used as a reducing quencher [104]. Similarly, Yang et al. have successfully reduced these compounds using cheap and easily available organic photo dye catalysts (Scheme 37) [105].
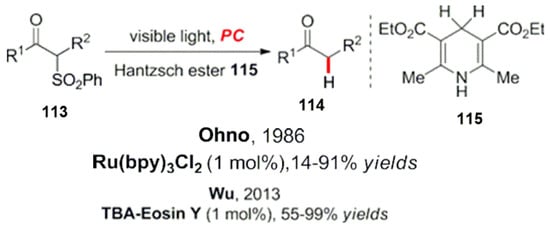
Scheme 37.
Photocatalytic reduction desulfone reaction.
Recently, Xuan et al. used Hantzsch ester as a hydrogen source to realize the production of hydrogen through visible light-catalyzed reactions in the deprotection process of tosyl-protected amide compounds (Scheme 38). This process occurs under mild reaction conditions and for a wide range of substrates. It has good functional group compatibility and provides a new method for the removal of benzene sulfonyl protective groups [106].
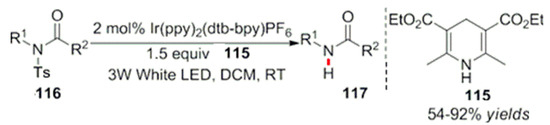
Scheme 38.
Photocatalytic reduction in benzene sulfonyl groups.
However, the basic concept for the reaction of visible light-catalyzed desulfurization was also reported by the Kellogg group in 1978 [107]. The reduction and fracture process of C-S bonds of benzoyl sulfonium salts under visible light catalysis was studied (Scheme 39) [108]. In this work, the author proposed that the reducing agent Hantzsch ester salt may directly absorb light to produce a radical intermediate, which was confirmed by controlled experiments. The free radical intermediate 122 is involved in the reduction process of the catalyst and then reacts with the free radical 120. The possible photocatalytic reaction mechanism was proposed. In 2019, the Wenger group reported a visible light photocatalyst for dehalogenation by producing a reductive electron source for the decomposition of quaternary ammonium that converts trifluoromethyl to difluoromethyl groups. In this method, detoxification can be achieved for chlorinated compounds with 200 turnover numbers [109].
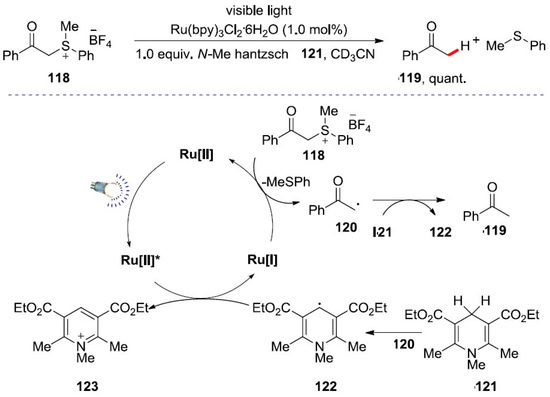
Scheme 39.
Visible light catalyzed desulfurization.
3.4. Reductive Deprotection
In 2011, Zlotorzynska and Sammis reported a visible light catalyzed reduction in benzyl alcohols protected by phthalimide [110]. In this work, the bipyridine ruthenium complex was used as a photocatalyst, and diisopropyl ethylamine was used as a reduction quencher. The deprotection of these compounds is thus realized, and aldehydes and phthalimide are obtained. Controlled experiments showed that the reaction is a visible light-catalyzed process (Scheme 40) [110].
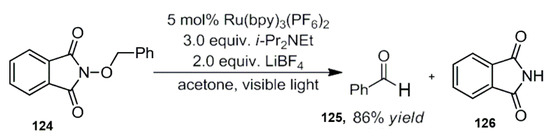
Scheme 40.
Photocatalytic reduction deprotection reaction.
3.5. Reductive Ring Opening of Azacyclopropane
In 2011, the Ollivier group realized the photocatalytic ring opening reaction of oxo or aza cyclopropane compounds adjacent to a carbonyl group (Scheme 41) [111]. In this reaction, Hantzsch ester was used as both a reducing quencher and a hydrogen donor. As a hydrogen donor, it is used for the reduction in a free radical intermediate 142 produced by a photocatalytic reaction, and the formed intermediate vanished. It can grab a hydrogen to produce the reductive product 138. In addition, it can also interact with suitable free radical receptors, such as the allyl sulfonate 143.
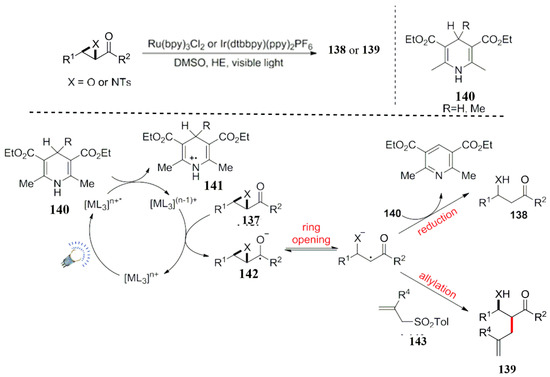
Scheme 41.
Visible light-catalyzed atom transfer radical addition reaction.
In addition to the types of aforementioned reductive reactions, other types of visible light-catalyzed net reduction reactions have also been extensively studied. For example, Hironaka et al. performed the reduction of benzyl bromide by a photocatalytic reductive reaction [112]. Ru(bpy)3Cl2 was used as a photocatalyst, and 1-benzyl-1,4-dihydronicotinamide (BNAH) was used as a reducing agent. The Kern and the Sauvage teams developed a new copper complex photocatalyst for the reduction in benzyl bromide compounds, and similar results were obtained [113]. In addition, a visible light photocatalytic reduction was also applied to nitro and azide compounds. The reduction in hydrazine produced the corresponding amine compounds. The reaction conditions were mild, efficient and have a wide range of potential applications [114,115,116,117]. In 2022, the Yamaguchi group made progress in this field and performed catalytic reductive ring opening of epoxides by photocatalysis [118]. In addition, the Oda et al. reported a photocatalytic deprotection of phthalimide-protected ester compounds [119]. The ester compound 127 produced a free radical anion 129 under photocatalytic conditions. The free radical anion was unstable, was converted and released CO2 to form a free radical intermediate 128 accompanied by a Phthaloyl production of amine 126. The free radical intermediate 128 was captured by coupling reagents to form the corresponding compound (Scheme 42).
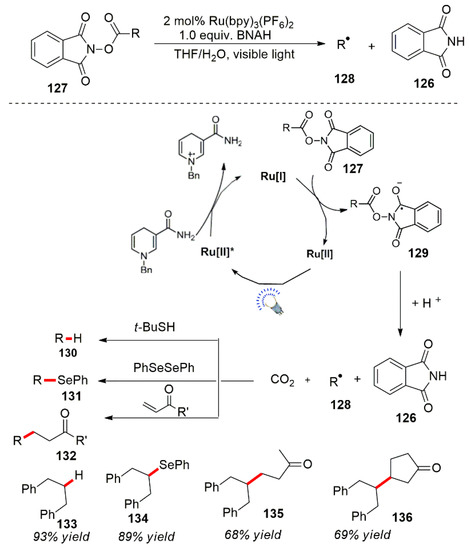
Scheme 42.
Azacyclopropane compounds’ ring opening photocatalysis.
4. Visible Light-Catalyzed Redox Neutral Reaction
Visible light-catalyzed redox neutral reaction usually refers to the reaction system without the addition of additional stoichiometric electrons. The acceptor or donor uses the reaction substrate itself and the intermediate obtained by the reaction as the electron acceptor or donor for the cycle of the whole photocatalytic reaction.
4.1. Atom Transfer Radical Addition Reaction
As a kind of redox neutral reaction, the visible light-catalyzed atom transfer radical addition reaction can be explained by the following Scheme 41.
In Scheme 43, the substrate 144 receives foreign electrons from a metal catalyst to form free radicals 145 and negative ions X. The unsaturated nature of the species helped to react with the compound 146 and to form more stable free radical species 147, which are then transformed with the loss of an electron. At the same time, the free radical species themselves formed positive ions and interacted with the system X (usually X is halogen) to form a new compound 148. For the occurrence of the reaction, there is no need to add any electric field. The substrate acceptor or donor can realize the catalytic cycle, and the substrate itself has a free radical addition reaction (Scheme 43).
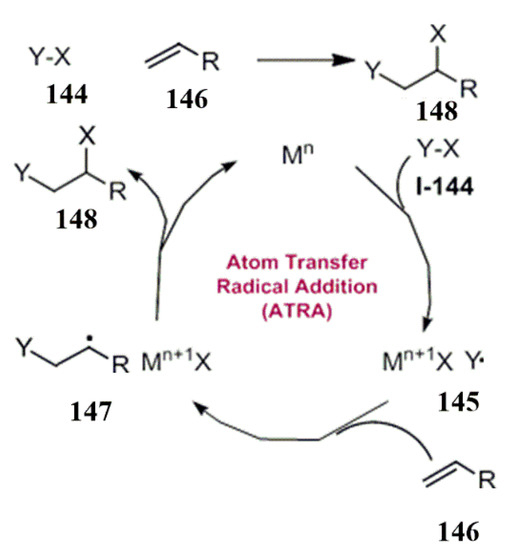
Scheme 43.
Photo-catalyzed atom transfer radical addition reaction.
In 1994, Barton et al. was the first to report a visible light catalytic strip. The free radical addition reaction of the atom transfer between p-toluenesulfonic acid selenium compounds and alkenyl ethers was carried out to obtain the target compound in high yields (Scheme 44) [120].
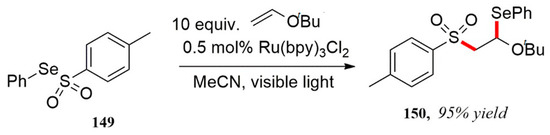
Scheme 44.
Reaction of p-toluenesulfonic acid selenium and alkenyl ethers.
In 2011, Nguyen et al. reported an example of visible light-catalyzed atomic oxidation based on a transfer radical addition reaction (Scheme 45) [121]. They expanded the use of substrates to a wider range, such as active halogenates: α-carbonyl halide, trifluoromethane iodide, p-methylbenzene sulfonyl chloride and carbon tetrachloride. The mechanistic scheme is described as the following: upon illumination, the catalyst absorbs visible light to form an excited state. The electron transfer between Ir(III)* oxidized to a high valence state Ir(IV), accompanied by the free radicals’ formation 152. Then, the free radical intermediate facilitates an intermolecular reaction on the unsaturated substrates to produce a new free radical intermediate 154 and then participates in the reduction of a metal catalyst to complete the photocatalytic cycle. The intermediate 154 lost electrons to form a new carbocationic intermediate 155. A subsequent attack of a halogen anion yielded the olefin addition product 156 (Scheme 45) [121]. The Reiser et al. used metal copper complexes as a photocatalyst, and the reaction was studied under light conditions [122].
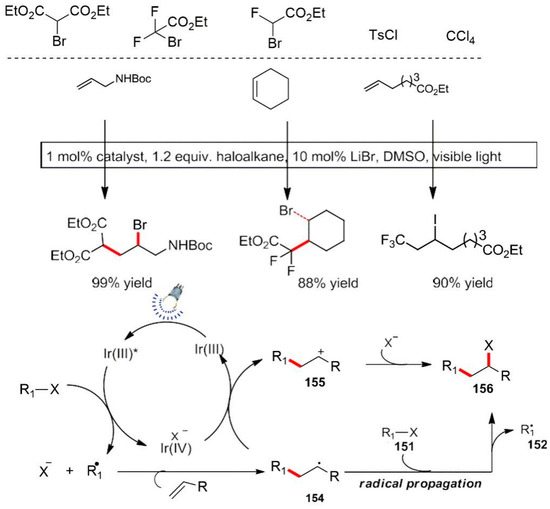
Scheme 45.
Radical transfer addition reaction.
4.2. Combination of Visible Light Catalysis and Asymmetric Catalysis
Yao et al. used a 2-iodine visible light-catalyzed redox reaction with methylcyclopropane as the substrate, owing to the instability of a ternary ring structure [123]. After the ring opening rearrangement reaction, the free radical species produced and reacted with unsaturated olefins or alkynes. Intermolecular free radical [3 + 2] cycloaddition reaction and the subsequent transformation produce the target compound (Scheme 46). The addition of stoichiometric amounts of zinc greatly promoted the reaction. In addition, the authors adopted the same method for the visible light-catalyzed intramolecular cyclization reaction [124].
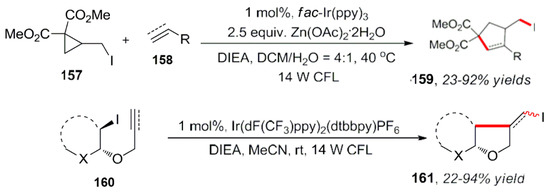
Scheme 46.
Intermolecular free radical [3 + 2] cycloaddition reaction.
In 2010, Macmillan et al. successfully combined visible light catalysis with molecule catalysis to achieve α-Site asymmetric alkylation reactions with aldehydes (Scheme 47) [125]. In the reaction process, two catalysts cooperated during the reaction process. First, the α-bromocarbonyl compound 163 was converted into a free radical intermediate 165 under light conditions with a Ru(bpy)3 photocatalyst. Then, the free radical 165 reacted with the enamine intermediate 166 in an intermolecular free radical addition reaction.
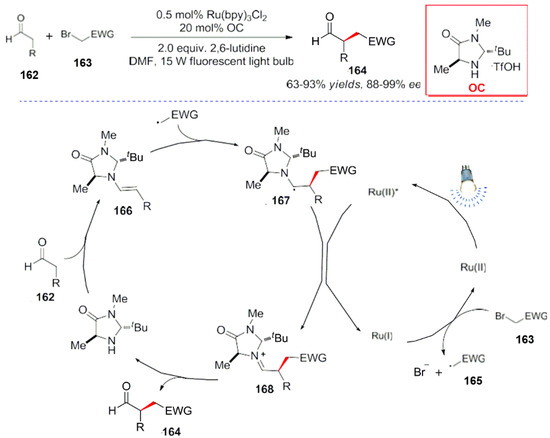
Scheme 47.
Aldehydes’ α-site asymmetric alkylation reaction.
The α-amino radical intermediate 167 was created in the process of a radical addition, and then the free radical intermediate 167 transferred one electron to the catalyst for a self oxidation. Similarly, the iminium ion intermediate 168 was hydrolyzed to produce the product with a catalytic regeneration.
Later, they expanded the application and realized an α-reverse benzylation, trifluoromethylation reactions [126] and ammoniation reactions [127]. At the same time, many research groups were focused on carbonyl compounds, and a series of studies has been carried out on the functionalization at the α-position [128,129,130]. Recently, the combination of chiral primary amine catalysts and photocatalysis developed by the Luo group has been successfully employed for the reaction of 1,3-di asymmetric alkylations of carbonyl compounds [131]. The reaction conditions are mild, with high yields and high enantioselectivities. The construction of a quaternary carbon center was realized in the product (Scheme 48). Aldehydes or ketones were synthesized with the above α-alkylation reactions.
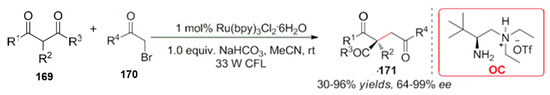
Scheme 48.
Ru-catalyzed asymmetric alkylation of carbonyl compounds.
Recently, Ooi et al. combined visible light and a chiral Brønsted acid catalyst for a free radical addition reaction between arylmethylamine and methylsulfonyl substituted imines (Scheme 49) [132].
Scheme 49.
Visible light and chiral Brønsted Acid Salt catalysis.
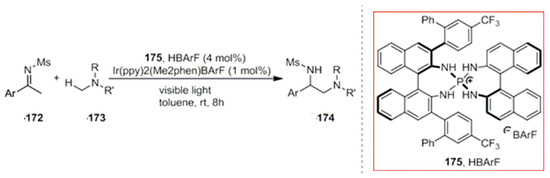
In addition, Huo et al. developed a new chiral metal iridium complex as a photocatalyst. The asymmetric complex combines with the photocatalyst, resulting in the formation of a new type of asymmetric reactions. For example, they applied it to carbonyl compounds containing imidazole moieties for an asymmetric alkylation. The new complex formed by the coordination with the catalyst acts as a photocatalyst to activate the halogenated hydrocarbon 177 and produces a free radical intermediate 179. Finally, the active intermediate 181 can form an alkylated product in high yields with excellent enantioselectivities (Scheme 50) [133]. This emerging catalyst leads to a new process for the development of new reactions. In 2021, Gong et al. highlighted visible light-promoted asymmetric catalysis by chiral complexes [134]; a combination of transition metal catalysts with chiral ligands [135,136,137,138,139,140,141,142] was reported. Moreover, various methods are reported in literature to induce the chirality with chiral transition metals such as Cr, Co, Ni and Cu complexes [143,144,145,146].
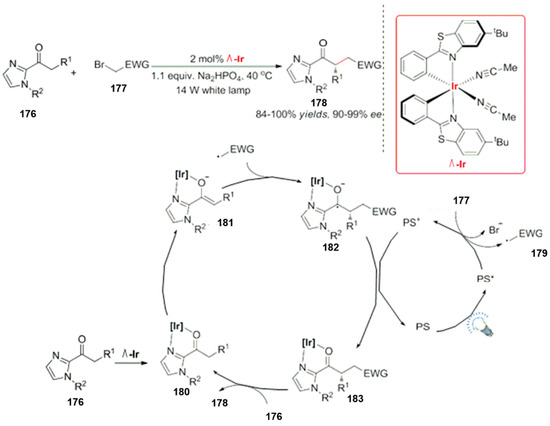
Scheme 50.
Photocatalyst and asymmetric catalyst Λ-Ir.
4.3. Combination of Visible Light Catalysis and Transition Metal Catalysis
In addition to the combination of photocatalysis and asymmetric catalysis, visible light catalysis and transition metal catalysis were combined, and their cooperative study has also been reported. A series of transition metal catalysts, such as Pd, Cu, Au and Ni, was applied to expand the scope of the reactions. In 2011, Kalyani et al. reported the combination of visible light and a palladium catalyst for the first time at room temperature, and good results were obtained for a carbon–hydrogen bond activation of aromatic compounds (Scheme 51) [147]. The corresponding reaction mechanism is also mentioned in Scheme 52.[148] Firstly, the palladium catalyst was used with the guiding group in the pyridine fragment of the substrate 184 and produced an intermediate 187. At the same time, the diazonium salt 185 and an aryl radical intermediate were formed under the action of a photocatalyst. Moreover, the radical is added to the intermediate 187 to form a new palladium intermediate 188. Then, the palladium intermediate transfers electrons to the high valence Ru[III] catalyst. After completing the photocatalytic cycle, it was oxidized to tetravalent species to form an intermediate 189 by a reduction. The arylation product 186 was obtained by the elimination step with the release of the palladium catalyst.
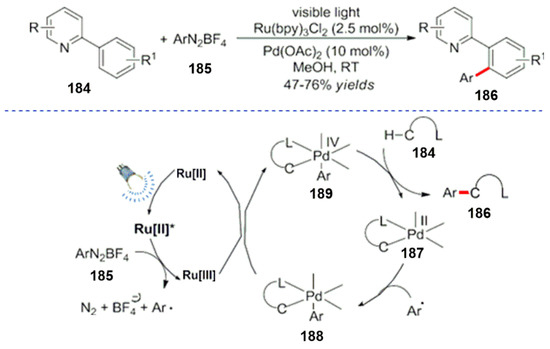
Scheme 51.
Photocatalysis and palladium catalyzed arylation of C-H bonds.
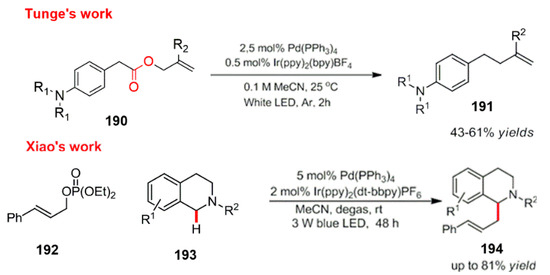
Scheme 52.
Allylation catalyzed by photocatalysis and transition metal (Pd) catalysts.
The report on the organic catalytic transformation via the combination of a transition metal catalyst and visible light catalysis provides the possibility of, and important perspectives for, the formation of diverse sustainable products. Simultaneously, Tunge et al. and Xiao et al. reported an organic catalytic reaction by the combination of a transition metal and visible light catalysis. They studied a palladium-catalyzed allylation under visible light [149,150]. Moreover, they solved the problem with the traditional palladium catalyzed allylation, which proceeds through harsh reaction conditions. However, their reactions can proceed smoothly under mild conditions (Scheme 52).
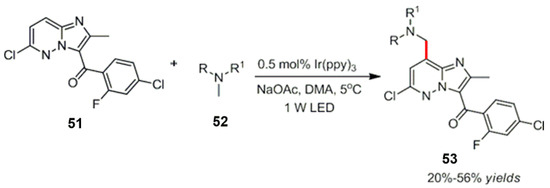
Based on preliminary work, Ye et al. combined a photocatalyst with a cheap copper catalyst. An arylboric acid derivative reacted with trifluoromethylation in good yields (Scheme 53) [151].
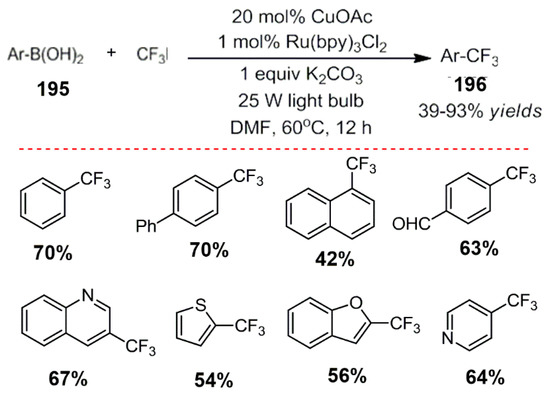
Scheme 53.
Photocatalysis and palladium catalyzed arylation of C-H bonds.
As a metal catalyst, Au is usually used for the activation of unsaturated double bonds- or triple bonds-containing compounds and their subsequent chemical transformations. In 2013, Glorius et al. reported the reaction of alkenyl alcohols and aryl compounds by a combination of visible light and an Au catalysis. Based on the study of an intermolecular addition ring-closing reaction of nitrogen salts, substituted oxygen-containing heterocyclic compounds were successfully constructed (Scheme 54) [152].
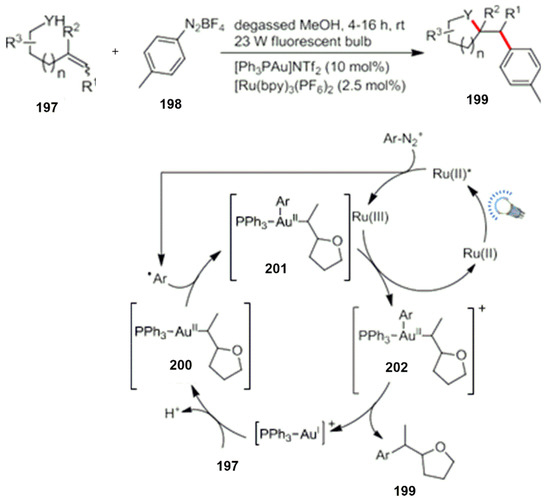
Scheme 54.
Photocatalysis and metal Au catalyzed cyclization.
The combination of visible light catalysis and a transition metal catalyst such as Ni is one of the most rapidly developing research directions in this field. Macmillan et al. used the double catalytic reaction of a photocatalyst and a nickel catalyst for the functionalization of ortho sp3 carbons of amino acids, and the benzylic C-H bond of dimethylaniline also works well (Scheme 55) [153]. However, the aryl halide substrate is limited to aryl iodide and bromide. At the same time, Tellis et al. carried out a pioneering work in the research of photocatalytic coupling reactions involving an organic boron reagent and an aryl halide [154]. Since then, the field of photocatalysis has led to a broad range of photo and metal catalysts.
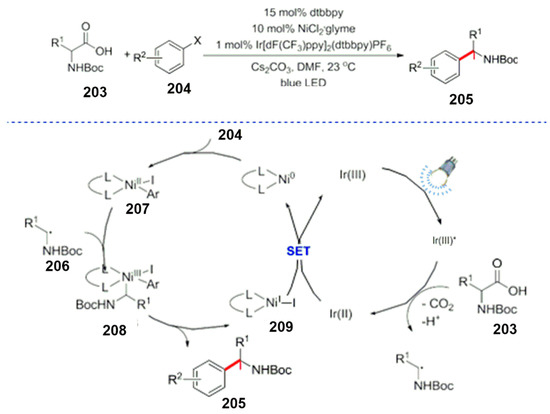
Scheme 55.
Photocatalysis and metal Ni-catalyzed cyclization.
4.4. Free Radical Addition Reaction to π-System Species
Aromatic or heteroaromatic compounds as one of the species of the π-system have been widely used in the field of photocatalysis. For the research in this direction, we summarized the cycle mechanism, as shown in Scheme 56. Firstly, the substrate 210 underwent a radical intermediate 211 with the combined action of a photocatalyst and visible light. Then, the aromatic ring compound 212 was molecularly synthesized by free radical intermediates. The meta addition reaction forms an aryl radical intermediate 213, and the newly generated radical intermediate continues to participate in the photo-polymerization. The catalytic cycle forms a free radical cation 214 and completes the regeneration of the metal catalyst. Lastly, the free cation 214 generated the corresponding aromatization product 215 by losing a proton.
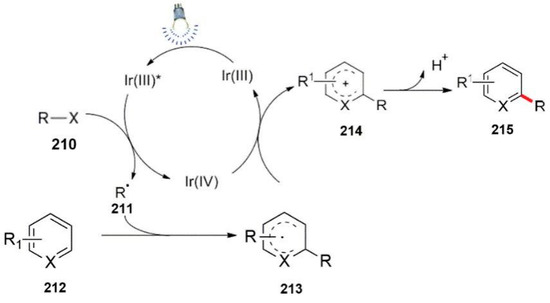
Scheme 56.
General process of a photocatalytic aryl radical addition reaction.
Moreover, Yelo and Deronzier widely used diazonium salts in photocatalytic reactions as aryl radical precursors [155]. The study was on C-H activation using visible light-catalyzed electron rich aromatization with an organic dye, Eosin Y, as a photocatalyst under mild conditions (Scheme 57) [156]. Later, Xue et al. used Ru(bpy)3Cl2 as a photocatalyst and water as the reaction solvent, until completion of the reaction, with a scope including a wide range of substrates and applications, including electron deficient heterocyclic compounds [157]. In addition, Wang et al. have also applied diaryl iodine compounds to the study of this kind of reaction, and good results have been obtained [158]. The Cheng group turned their research direction towards aryl halogenation, which is cheaper and easier. The reaction can proceed smoothly under heating conditions, but the reaction conditions are relatively harsh [159].
Scheme 57.
Photocatalytic arylation of aromatic rings.
Fluorinated compounds are a hot spot and a new research direction in the field of organic chemistry. Many scientists have targeted the synthesis of fluorinated compounds catalyzed by visible light. In 2011, Macmillan et al. used trifluoromethyl sulfonyl chloride as the source of trifluoromethyl radicals to complete the reverse trifluoromethylation of aromatic compounds (Scheme 58) [160]. It was also applied to the synthesis of drug molecules. Later, Choi et al. reported the use of trifluoromethyliodomethane as a trifluoromethyl source which was successfully attached to the aromatic compounds through a photochemical reaction [161]. Then, the Zhu group used the commercial Togni reagent as the precursor of trifluoromethyl radicals, and the corresponding fragment units were successfully introduced into aromatic amines [162].
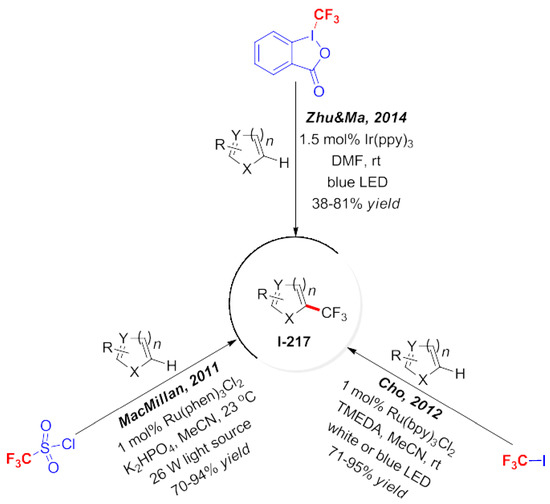
Scheme 58.
Photocatalytic trifluoromethylation of aromatic rings.
In addition, compounds containing difluoromethyl radicals have also been employed for further aromatic ring functionalizations through a photocatalytic reaction (Scheme 59). For example, the Liu group used difluorobromomethyl phosphate [163] and difluorobromomethylamide for the difluoromethylation of aromatic compounds [164]. The Wang group reported the use of iododifluoromethylsulfonic acid with good results [165]. The Cho group used ethyl difluorobromoacetate as a fluorine-containing agent as a radical source and completed a series of difluoroalkylation reactions of aromatic compounds [166].
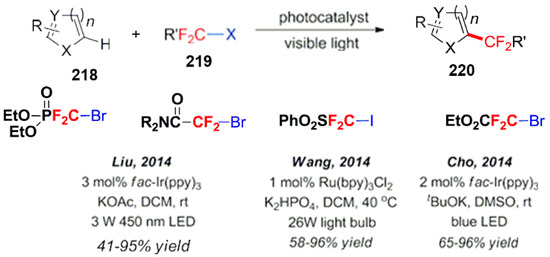
Scheme 59.
Difluoromethyl radicals applied to photocatalytic aromatic functionalizations.
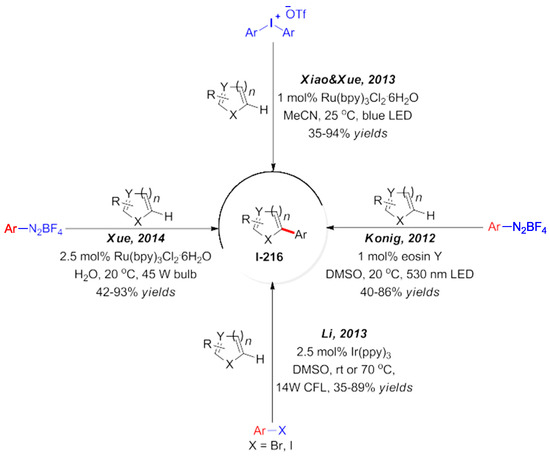
In addition, there are relevant literature reports on the amination of heterocyclic compounds catalyzed by visible light. Nitrogen-containing compounds are used as nitrogen-containing radical sources, such as phthalimide compounds and their derivatives [167,168]. In addition to the above aromatic compounds, other π-system compounds, such as carbon–carbon double and triple bond compounds, are also widely used in the study of photoredox reactions. In this research field, Yasu et al. [169,170,171,172,173] and Iqbal et al. [174,175] and other research groups have made systematic progress in this field. They used different trifluoromethylation reagents to treat unsaturated carbon–carbon double bonds or carbon–carbon triple bonds. Grandjean and Nicewiz successively reported C-H chlorination and C-H amination reactions under visible light catalysis, as shown in Scheme 60 [176,177,178,179,180]. This organic dye with a strong oxidation ability is used as an organic photocatalyst. Olefin compounds were oxidized into free radical cationic intermediates and then attacked by different nucleophiles [181,182,183,184,185]. A lot of research work has been conducted on radical addition reactions, and very systematic research results have been obtained. Ischay and Miyake et al. completed an intramolecular [2 + 2] cycloaddition reaction of unsaturated ketones induced by visible light [12,186]. A series of polysubstituted cyclobutanes was synthesized. The addition of lithium tetrafluoroborate greatly promoted the reaction. The main reason is that lithium salt can coordinate with carbonyls to activate substrates but also can stabilize the reverse reaction. The authors have successively completed a variety of unsaturated ketones for the study of an intramolecular cycloaddition reaction [187]. In addition, other research groups have also made relevant reports on this work, such as the production of α-amino self-oxidation by an amine oxidation. The addition reaction of a sulfur radical and olefin is produced by the addition reaction of mercaptan [188,189] and the oxidation of mercaptan [190,191].
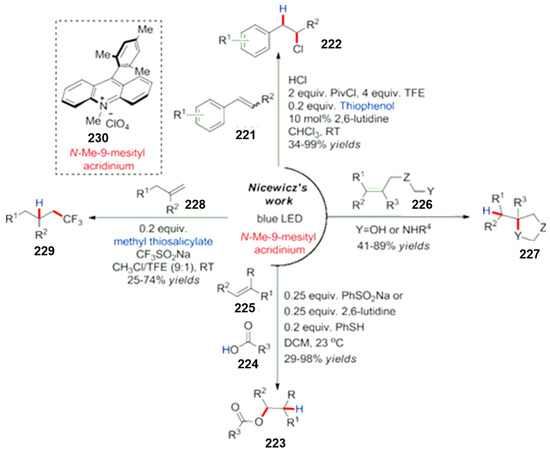
Scheme 60.
Nicewicz’s work on N-Me-9-mesityl acridinium.
5. Visible Light Catalyzed Energy Transfer Reaction
The Xiao group reported azide styrene derivatives under light conditions to synthesize indole derivatives without substituents on nitrogen through an energy transfer reaction (Scheme 61) [192,193].
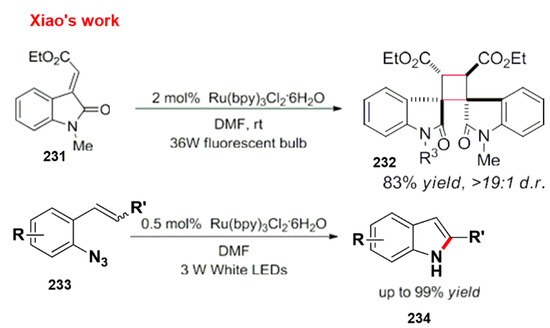
Scheme 61.
Synthesis of azide styrene derivatives by a fluorescent bulb.
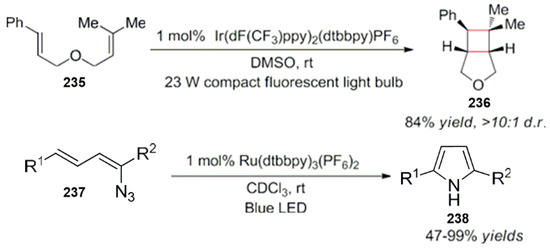
Lu and Yoon made relevant studies on this work. They have successively carried out photocatalytic energy transfer reactions. Moreover, the [2 + 2] cycloaddition reaction of olefins and the intramolecular cyclization of alkenylazide were used for the synthesis of pyrrole compounds (Scheme 62) [194,195].
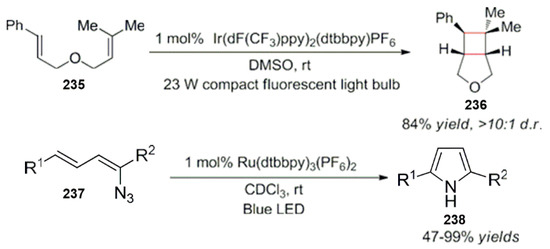
Scheme 62.
Cycloaddition of olefins and intramolecular cyclization of alkenylazide derivatives.
In addition to initiate chemical reactions between the photocatalyst and organic molecules through electron transfer, the photocatalyst in an excited state (Ru (bpy)32 + *, e = 2.12 eV) is also a good energy donor, which can transfer energy directly to organic molecules, thereby initiating subsequent chemical conversion in the reaction process (Scheme 63). The description is as follows: First, the catalyst absorbs visible light and is excited to the excited singlet state (S1). The instability of the excited triplet state jumps to a more stable excited triplet state (T1) through the inter-system crossing.
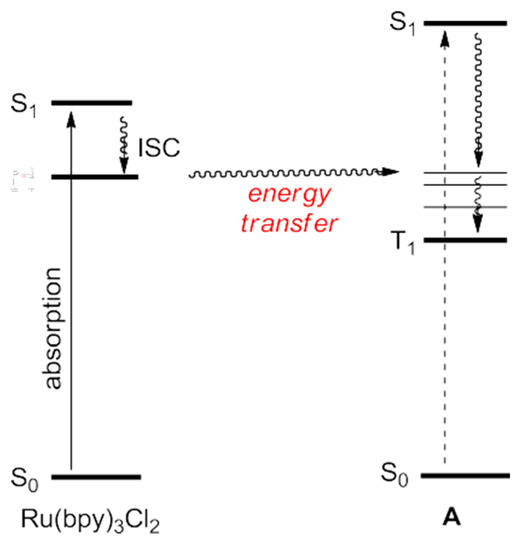
Scheme 63.
Chemical reactions between a Ru-based photocatalyst and organic molecules.
(T1) transfers energy to the substrate receptor, and this activated substrate has an active nature involved in the subsequent chemical reaction. Compared with the previous reports, there are relatively few literature reports on this work. For example, Xiao et al. reported a cycloaddition reaction of indole [2 + 2] by a 3-alkenyl oxidation via a photocatalytic process with good yields and high selectivities (Scheme 64).
Scheme 64.
Cycloaddition of olefins and intramolecular cyclization of alkenylazide derivatives.
Fan and Li reported a synthesis of enaminones through an energy transfer between a catalyst and oxygen and a photocatalytic acetyl migration reaction, and they proposed a possible reaction mechanism through controlled experiments [196]. Recently, various reports have been presented in the literature for photo-catalyzed reactions and C-H and N-H bond activations [197,198,199,200,201,202,203,204,205,206,207].
These advances highlight various kinds of reactions such as cycloaddition, oxidative aromatization and migration reactions. Moreover, these methodologies can be used for the construction of synthetically important drugs and industrial products and may fulfill the research requirements.
6. Conclusions
In this review, we have discussed novel access to various synthetically important γ-ketoesters, azaspirocyclic cyclohexadienones spirocyclohexadienones, multisubstituted benzimidazole derivatives, substituted N,2-diarylacetamide, 2-arylpyridines and 2-arylquinolines with mechanistic studies in good yields and under mild conditions. These efficient visible light-induced photoredox reactions of C-H/N-H and C-X bonds made this transformation very attractive. These reactions proceed through visible light-induced cover intermolecular radical additions, de-aromative cyclizations, aryl migrations and desulfonylations. Similarly, we also mentioned a visible light-catalyzed aerobic C–N bond activation through cyclization, elimination and aromatization.
Author Contributions
M.S.A. have share main idea and wrote the manuscript and K.M. also share same idea about review and polished the manuscript. Q.Z. and B.Z. also contribute in participate in correct the references. P.-H.L. and Q.W. polished the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No research data is available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morton, O. Solar energy: A new day dawning?: Silicon Valley sunrise. Nature 2006, 443, 19–23. [Google Scholar]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef]
- Nocera, D.G. On the future of global energy. Daedalus 2006, 135, 112–115. [Google Scholar]
- Ciamician, G. The photochemistry of the future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Corrd. Chem. Rev. 1982, 46, 159–244. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Von Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar]
- Campagna, S.; Puntoriero, F.; Nastasi, F.; Bergamini, G.; Balzani, V. Photochemistry and photophysics of coordination compounds: Ruthenium. Top. Curr. Chem. 2007, 280, 117–214. [Google Scholar]
- Wang, X.-Y.; Guerzo, A.D.; Tunuguntla, H.; Schehl, R.H. Advanced Ceramics for Energy and Environmental Applications. Res. Intermed. 2007, 33, 63. [Google Scholar] [CrossRef]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and photophysics of coordination compounds: Iridium. Top. Curr. Chem. 2007, 281, 143–203. [Google Scholar]
- Balzani, V.; Credi, A.; Venturi, M. Photochemical conversion of solar energy. ChemSusChem 2008, 1, 26–58. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Tucker, J.W.; Stephenson, C.R.J. Electron-transfer photoredox catalysis: Development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A.; Moore, A.L. Molecular mimicry of photosynthetic energy and electron transfer. Acc. Chem. Res. 1993, 26, 198–205. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fangnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Acc. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar]
- Zeitler, K. Photoredox catalysis with visible light. Angew. Chem. Int. Ed. 2009, 48, 9785–9789. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 40, 120. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R. Shining light on photoredox catalysis: Theory and synthetic applications. Chem. Soc. Rev. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Teply, F. Photoredox catalysis by [Ru(bpy)3]2+ to trigger transformations of organic molecules. Organic synthesis using visible-light photocatalysis and its 20th century roots. Collect. Czech. Chem. Commun. 2011, 76, 859–917. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Zheng, N. A photo touch on amines: New synthetic adventures of nitrogen radical cations. Synlett 2012, 23, 1851–1856. [Google Scholar]
- Shi, L.; Xia, W. New Cy5 photosensitizers for cancer phototherapy: A low singlet–triplet gap provides high quantum yield of singlet oxygen. Chem. Soc. Rev. 2012, 41, 7061. [Google Scholar]
- Hoffmann, N. Homogeneous Photocatalytic Reactions with Organometallic and Coordination Compounds—Perspectives for Sustainable Chemistry. ChemSusChem 2012, 5, 352–371. [Google Scholar] [CrossRef]
- Xi, Y.; Yi, H.; Lei, A. Synthetic applications of photoredox catalysis with visible light. Org. Biomol. Chem. 2013, 11, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Hari, D.P.; Kong, B. The photocatalyzed meerwein arylation: Classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed. 2013, 52, 4734–4743. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xu, X.; Li, X. Tandem trifluoromethylthiolation/aryl migration of aryl alkynoates to trifluoromethylthiolated alkenes. Chin. J. Chem. 2013, 33, 2046. [Google Scholar] [CrossRef]
- Xuan, J.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Visible-light-induced formal [3 + 2] cycloaddition for pyrrole synthesis under metal-free conditions. Eur. J. Org. Chem. 2013, 2013, 6755. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Groger, H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem. Rev. 2003, 103, 2795–2828. [Google Scholar] [CrossRef]
- Cordova, A. The direct catalytic asymmetric Mannich reaction. Acc. Chem. Res. 2004, 37, 102–112. [Google Scholar] [CrossRef]
- Condie, A.G.; Gonzalelez-Gomez, J.C.; Stephenson, C.R. Visible-light photoredox catalysis: Aza-Henry reactions via C−H functionalization. J. Am. Chem. Soc. 2010, 132, 1464–1465. [Google Scholar] [CrossRef]
- Freeman, D.B.; Furst, L.; Condie, A.G.; Stephenson, C.R. Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light. J. Org. Lett. 2012, 14, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Vila, C.; Koenings, R.M.; Poscharny, K.; Fabry, D.C. Dual catalysis: Combining photoredox and Lewis base catalysis for direct Mannich reactions. Chem. Commun. 2011, 47, 2360–2362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yang, C.; Guo, L.; Sun, H.; Chen, C.; Xia, W. Visible light-induced oxidative coupling reaction: Easy access to Mannich-type products. Chem. Commun. 2012, 48, 2337–2339. [Google Scholar]
- Rueping, M.; Zhu, S.; Koenigs, R.M. Visible-light photoredox catalyzed oxidative Strecker reaction. Chem. Commun. 2011, 47, 12709–12711. [Google Scholar] [CrossRef]
- Rueping, M.; Zhu, S.; Koenings, R.M. Photoredox catalyzed C–P bond forming reactions—Visible light mediated oxidative phosphonylations of amines. Chem. Commun. 2011, 47, 8679–8681. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Koenigs, R.M.; Poscharny, K.; Fabry, D.C.; Leonori, D.; Vila, C. Dual Catalysis: Combination of Photocatalytic Aerobic Oxidation and Metal Catalyzed Alkynylation Reactions—C-C Bond Formation Using Visible Light. Chem. Eur. J. 2012, 18, 5170–5174. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, X.; Mao, H.; Tang, Z.; Cheng, Y.; Zhu, C. Visible-light-induced direct C(sp3)–H difluromethylation of tetrahydroisoquinolines with the in situ generated difluoroenolates. Chem. Commun. 2014, 50, 7521–7523. [Google Scholar]
- Xuan, J.; Feng, Z.-J.; Duan, S.-W.; Xiao, W.-J. Room temperature synthesis of isoquino [2,1-a][3,1] oxazine and isoquino[2,1-a]pyrimidine derivatives via visible light photoredox catalysis. RSC Adv. 2012, 2, 4065–4068. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Lu, L.Q.; Fu, L.; Chang, N.J.; Rong, J.; Chen, J.R.; Xiao, W.J. Visible-light-induced oxidation/[3 + 2] cycloaddition/oxidative aromatization sequence: A photocatalytic strategy to construct pyrrolo [2,1-a] isoquinolines. Angew. Chem. Int. Ed. 2011, 50, 7171–7175. [Google Scholar] [CrossRef]
- Rueping, M.; Leonori, D.; Poisson, T. Visible light mediated azomethine ylide formation—Photoredox catalyzed [3 + 2] cycloadditions. Chem. Commun. 2011, 47, 9615–9617. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Cheng, Y.; An, J.; Lu, L.-Q.; Zhang, X.-X.; Xiao, W.-J. Visible light-induced intramolecular cyclization reactions of diamines: A new strategy to construct tetrahydroimidazoles. Chem. Commun. 2011, 47, 8337–8339. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhao, X.; Wang, X.; Liu, Q.; Li, Z.; Wang, D.Z. Visible-Light-Promoted C-C Bond Cleavage: Photocatalytic Generation of Iminium Ions and Amino Radicals. Angew. Chem. Int. Ed. 2012, 51, 8050–8053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Hu, M.; Huang, X.-C.; Gong, L.-B.; Xie, Y.-X.; Li, J.-H. Direct α-arylation of α-amino carbonyl compounds with indoles using visible light photoredox catalysis. J. Org. Chem. 2012, 77, 8705–8711. [Google Scholar] [CrossRef]
- Zhu, S.; Rueping, M. Merging visible-light photoredox and Lewis acid catalysis for the functionalization and arylation of glycine derivatives and peptides. Chem. Commun. 2012, 48, 11960–11962. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Vila, C.; Szadkowska, A.; Koenigs, R.M.; Fronert, J. Photoredox Catalysis as an Efficient Tool for the Aerobic Oxidation of Amines and Alcohols: Bioinspired Demethylations and Condensations. ACS Catal. 2012, 2, 2810–2815. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Albini, A. Photoorganocatalysis. What for? Chem. Soc. Rev. 2013, 42, 97–113. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; Hamilton, D.S. Organic photoredox catalysis as a general strategy for anti-Markovnikov alkene hydrofunctionalization. Synlett 2014, 25, 1191–1196. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Xue, Q.; Xie, J.; Jin, H.; Cheng, Y.; Zhu, C. Highly efficient visible-light-induced aerobic oxidative C–C, C–P coupling from C–H bonds catalyzed by a gold(III)-complex. Org. Biomol. Chem. 2013, 11, 1606–1609. [Google Scholar] [CrossRef]
- To, W.P.; Liu, Y.; Lau, T.C.; Che, C.M. A Robust Palladium(II)–Porphyrin Complex as Catalyst for Visible Light Induced Oxidative C—H Functionalization. Chem. Eur. J. 2013, 19, 5654–5664. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Wang, G.-X.; Liu, Q.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. A Highly Efficient and Selective Aerobic Cross-Dehydrogenative-Coupling Reaction Photocatalyzed by a Platinum(II) Terpyridyl Complex. Chem. Eur. J. 2013, 19, 6443–6450. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Liu, Q.; Ren, Y.; Jiang, H. Visible light-driven efficient palladium catalyst turnover in oxidative transformations within confined frameworks. Nat. Commun. 2022, 13, 928. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.L.; Gu, Q.S.; Liu, X.Y. Catalytic enantioselective C(sp3)–H functionalization involving radical intermediates. Nat. Commun. 2021, 12, 475. [Google Scholar] [CrossRef]
- Hari, D.P.; Konig, B. Eosin Y catalyzed visible light oxidative C–C and C–P bond formation. Org. Lett. 2011, 13, 3852–3855. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kee, C.W.; Chen, L.; Tan, C.-H. Dehydrogenative coupling reactions catalysed by Rose Bengal using visible light irradiation. Green Chem. 2011, 13, 2682–2685. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Reactivity and Mechanistic Insight into Visible-Light-Induced Aerobic Cross-Dehydrogenative Coupling Reaction by Organophotocatalysts. Chem. Eur. J. 2012, 18, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, X.Y.; Li, Y.J.; Li, P.X. Aerobic transition-metal-free visible-light photoredox indole C-3 formylation reaction. ACS Catal. 2014, 4, 1897–1900. [Google Scholar] [CrossRef]
- Fu, W.; Guo, W.; Zou, G.; Xu, C. Selective trifluoromethylation and alkynylation of tetrahydroisoquinolines using visible light irradiation by Rose Bengal. J. Fluor. Chem. 2012, 140, 88–94. [Google Scholar] [CrossRef]
- Ju, X.; Li, D.; Li, W.; Yu, W.; Bian, F. The reaction of tertiary anilines with maleimides under visible light redox. Adv. Synth. Catal. 2012, 354, 3561–3567. [Google Scholar] [CrossRef]
- Zhu, S.; Das, A.; Bui, L.; Zhou, H.; Curran, D.P.; Rueping, M. Oxygen switch in visible-light photoredox catalysis: Radical additions and cyclizations and unexpected C–C-bond cleavage reactions. J. Am. Chem. Soc. 2013, 135, 1823–1829. [Google Scholar] [CrossRef]
- Douglas, J.J.; Cole, K.P.; Stephenson, C.R.J. Photoredox catalysis in a complex pharmaceutical setting: Toward the preparation of JAK2 inhibitor LY2784544. J. Org. Chem. 2014, 79, 11631–11643. [Google Scholar] [CrossRef] [PubMed]
- Bordwell, F.G.; Zhang, X.-M.; Satish, A.V.; Cheng, J.-P. Assessment of the importance of changes in ground-state energies on the bond dissociation enthalpies of the OH bonds in phenols and the SH bonds in thiophenols. J. Am. Chem. Soc. 1994, 116, 6605–6610. [Google Scholar] [CrossRef]
- Cano-Yelo, H.; Deronzier, A. Photo-oxidation of some carbinols by the Ru(II) polypyridyl complex-aryl diazonium salt system. Tetrahedron Lett. 1984, 25, 5517–5520. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Jiao, N. Utilization of natural sunlight and air in the aerobic oxidation of benzyl halides. Org. Lett. 2011, 13, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-Q.; Chen, J.-R.; Liu, X.-P.; Lu, L.-Q.; Davis, R.L.; Jorgensen, K.A.; Xiao, W.-J. Highly efficient aerobic oxidative hydroxylation of arylboronic acids: Photoredox catalysis using visible light. Angew. Chem. Int. Ed. 2012, 51, 784. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, J.; Qu, Y.; Li, P. Aerobic visible-light photoredox radical C–H functionalization: Catalytic synthesis of 2-substituted benzothiazoles. Org. Lett. 2012, 14, 98–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Zhou, X.; Yang, Y. Oxygen-vacancy-boosted visible light driven photocatalytic oxidative dehydrogenation of saturated N-heterocycles over Nb2O5 nanorods. Appl. Catal. B Environ. 2022, 316, 121622. [Google Scholar] [CrossRef]
- Bergonzini, G.; Schindler, C.S.; Wallentin, C.-J.; Jacobsen, E.N.; Stephenson, C.R.J. Photoredox activation and anion binding catalysis in the dual catalytic enantioselective synthesis of β-amino esters. Chem. Sci. 2014, 5, 112–116. [Google Scholar] [CrossRef]
- DiRocco, D.A.; Rovis, T. Catalytic Asymmetric α-Acylation of Tertiary Amines Mediated by a Dual Catalysis Mode: N-Heterocyclic Carbene and Photoredox Catalysis. J. Am. Chem. Soc. 2012, 134, 8094–8097. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Xuan, J.; Xia, X.-D.; Ding, W.; Guo, W.; Chen, J.-R.; Zou, Y.-Q.; Lu, L.-Q.; Xiao, W.-J. Direct sp3 C–H acroleination of N-aryl-tetrahydroisoquinolines by merging photoredox catalysis with nucleophilic catalysis. Org. Biomol. Chem. 2014, 12, 2037–2040. [Google Scholar] [CrossRef]
- Perepichka, I.; Kundu, S.; Hearne, Z.; Li, C.-J. Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenative-coupling of alkynes and tetrahydroisoquinolines. Org. Biomol. Chem. 2015, 13, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Narayanam, J.M.R.; Shah, P.S.; Stephenson, C.R.J. Oxidative photoredox catalysis: Mild and selective deprotection of PMB ethers mediated by visible light. Chem. Commun. 2011, 47, 5040–5042. [Google Scholar] [CrossRef]
- An, J.; Zou, Y.-Q.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Visible Light-Induced Aerobic Oxyamidation of Indoles: A Photocatalytic Strategy for the Preparation of Tetrahydro-5H-indolo [2,3-b] quinolinols. Adv. Synth. Cat. 2013, 355, 1483–1489. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, Q.-Q.; Xuan, J.; Li, T.-R.; Lu, L.-Q.; Xiao, W.-J. Photocatalytic aerobic oxidation/semipinacol rearrangement sequence: A concise route to the core of pseudoindoxyl alkaloids. Tetrahedron Lett. 2014, 55, 4648–4652. [Google Scholar] [CrossRef]
- Parrish, J.D.; Ischay, M.A.; Lu, Z.; Guo, S.; Peters, N.R.; Yoon, T.P. Endoperoxide synthesis by photocatalytic aerobic [2 + 2 + 2] cycloadditions. Org. Lett. 2012, 14, 1640–1643. [Google Scholar] [CrossRef]
- Lu, Z.; Parrish, J.D.; Yoon, T.P. [3 + 2] Photooxygenation of aryl cyclopropanes via visible light photocatalysis. Tetrahedron 2014, 70, 4270–4278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Yi, H.; Qin, C.; Bai, R.; Qi, X.; Lan, Y.; Lei, A. Visible-Light-Mediated Decarboxylation/Oxidative Amidation of α-Keto Acids with Amines under Mild Reaction Conditions Using O2. Angew. Chem. Int. Ed. 2014, 53, 502–506. [Google Scholar] [CrossRef]
- Sun, H.; Yang, C.; Lin, R.; Xia, W. Regioselective Ring-Opening Nucleophilic Addition of Aziridines through Photoredox Catalyst. Adv. Synth. Cat. 2014, 356, 2775–2780. [Google Scholar] [CrossRef]
- Hamada, T.; Ishida, H.; Usui, S.; Watanabe, Y.; Tsumura, K.; Ohkubo, K. A novel photocatalytic asymmetric synthesis of (R)-(+)-1,1′-bi-2-naphthol derivatives by oxidative coupling of 3-substituted-2-naphthol with Δ-[Ru(menbpy)3]2+[menbpy = 4,4′-di (1R,2S,5R)-(–)-menthoxycarbonyl-2,2′-bipyridine], which posseses molecular helicity. Chem. Commun. 1993, 11, 909–911. [Google Scholar]
- Jin, J.; MacMillan, D.W.C. Direct α-Arylation of Ethers through the Combination of Photoredox-Mediated C-H Functionalization and the Minisci Reaction. Angew. Chem. Int. Ed. 2014, 54, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pac, C.; Ihama, M.; Yasuda, M.; Miyauchi, Y.; Sakurai, H. Tris (2,2′-bipyridine) ruthenium(2+)-mediated photoreduction of olefins with 1-benzyl-1,4-dihydronicotinamide: A mechanistic probe for electron-transfer reactions of NAD(P)H-model compounds. J. Am. Chem. Soc. 1981, 103, 6495–6497. [Google Scholar] [CrossRef]
- Ellefsen, J.D.; Miller, S.J. Photocatalytic Reductive Olefin Hydrodifluoroalkylation Enabled by Tertiary Amine Reductants Compatible with Complex Systems. J. Org. Chem. 2022, 87, 10250–10255. [Google Scholar] [CrossRef] [PubMed]
- Tilby, M.J.; Dewez, D.F.; Pantaine, L.R.; Hall, A.; Martínez-Lamenca, C.; Willis, M.C. Photocatalytic late-stage functionalization of sulfonamides via sulfonyl radical intermediates. ACS Catal. 2022, 12, 6060–6067. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002, 102, 4009–4092. [Google Scholar] [CrossRef]
- Goren, Z.; Willner, I. Photochemical and chemical reduction of vicinal dibromides via phase transfer of 4,4′-bipyridinium radical: The role of radical disproportionation. J. Am. Chem. Soc. 1983, 105, 7764–7765. [Google Scholar] [CrossRef]
- Hironaka, K.; Fukuzumi, S.; Tanaka, T. Tris(bipyridyl)ruthenium(II)-photosensitized reaction of 1-benzyl-1,4-dihydronicotinamide with benzyl bromide. J. Chem. Soc. Perkin Trans. 2 1984, 10, 1705–1709. [Google Scholar] [CrossRef]
- Maidan, R.; Goren, Z.; Becker, J.Y.; Willner, I. Application of multielectron charge relays in chemical and photochemical debromination processes. The role of induced disproportionation of N,N’-dioctyl-4,4′-bipyridinium radical cation in two-phase systems. J. Am. Chem. Soc. 1984, 106, 6217–6222. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Kellogg, R.M. 3-Methyl-2,3-dihydrobenzothiazoles as reducing agent. Dye enhanced photoreactions. Tetrahedron Lett. 1985, 26, 1453–1456. [Google Scholar] [CrossRef]
- Maidan, R.; Willner, I. Photochemical and chemical enzyme catalyzed debromination of meso-1,2-dibromostilbene in multiphase systems. J. Am. Chem. Soc. 1986, 108, 1080–1082. [Google Scholar] [CrossRef]
- Willner, I.; Tsfania, T.; Eichen, Y. Photocatalyzed and electrocatalyzed reduction of vicinal dibromides and activated ketones using ruthenium(I) tris(bipyridine) as electron-transfer mediator. J. Org. Chem. 1990, 55, 2656–2662. [Google Scholar] [CrossRef]
- Neumann, M.; Fiildner, S.; Konig, B.; Zeitler, K. Metal-free, cooperative asymmetric organophotoredox catalysis with visible light. Angew. Chem. Int. Ed. 2011, 50, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Goren, Z.; Mandler, D.; Maidan, R.; Degani, Y.; Oegani, J. Transformation of single-electron transfer photoproducts into multielectron charge relays: The functions of water—Oil two-phase systems and enzyme catalysis. J. Photochem. 1985, 28, 215–228. [Google Scholar] [CrossRef]
- Maji, T.; Karmakar, A.; Reiser, O. Comprehensive Organic Synthesis. J. Org. Chem. 2011, 76, 736. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Pitre, S.P.; Scaiano, J.C. Photocatalytic dehalogenation of vicinal dibromo compounds utilizing sexithiophene and visible-light irradiation. ACS Catal. 2014, 4, 4034. [Google Scholar] [CrossRef]
- Nguyen, J.O.; O’Amato, E.M.; Narayanam, J.M.; Stephenson, C.R. Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions. Nat. Chem. 2012, 4, 854. [Google Scholar] [CrossRef]
- Okada, K.; Okamoto, K.; Morita, N.; Okubo, K.; Oda, M. Photosensitized decarboxylative Michael addition through N-(acyloxy) phthalimides via an electron-transfer mechanism. J. Am. Chem. Soc. 1991, 113, 9401–9402. [Google Scholar] [CrossRef]
- Okada, K.; Okubo, K.; Morita, N.; Oda, M. Redox-mediated decarboxylative photo-phenylselenenylation of N-acyloxyphthalimides. Chem. Lett. 1993, 22, 2021–2024. [Google Scholar] [CrossRef]
- Okada, K.; Okubo, K.; Morita, N.; Oda, M. Reductive decarboxylation of N-(acyloxy) phthalimides via redox-initiated radical chain mechanism. Tetrahedron Lett. 1992, 33, 7377–7380. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C. Visible-light-induced photocatalytic reductive transformations of organohalides. Angew. Chem. Int. Ed. 2012, 51, 12303–12306. [Google Scholar] [CrossRef]
- Wang, H.; Tao, J.; Cai, X.; Chen, W.; Zhao, Y.; Xu, Y.; Yao, W.; Zeng, J.; Wan, Q. Stereoselective Synthesis of α-Linked 2-Deoxy Glycosides Enabled by Visible-Light-Mediated Reductive Deiodination. Chem. Eur. J. 2014, 20, 17319–17323. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, L.; Queffélec, C.; Haidaraly, K.M.; Blart, E.; Pellegrin, Y. Dehalogenation reaction photocatalyzed by homoleptic copper(i) complexes associated with strongly reductive sacrificial donors. Catal. Sci. Technol. 2021, 11, 6041–6047. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujii, M.; Mekata, H.; Oka, S.; Ohno, A. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 1986, 897–898. [Google Scholar]
- Yang, D.-T.; Meng, Q.-Y.; Zhong, J.-J.; Xiang, M.; Liu, Q.; Wu, L.-Z. Metal-free desulfonylation reaction through visible-light photoredox catalysis. Eur. J. Org. Chem. 2013, 2013, 7528–7532. [Google Scholar] [CrossRef]
- Xuan, J.; Li, B.-J.; Feng, Z.-J.; Sun, G.-D.; Ma, H.-H.; Yuan, Z.-W.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Desulfonylation of Tosyl Amides through Catalytic Photoredox Cleavage of N-S Bond Under Visible-Light Irradiation. Chem. Asian. J. 2013, 8, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Hedstrand, D.M.; Kruizinga, W.M.; Kellogg, R.M. Light induced and dye accelerated reductions of phenacyl onium salts by 1,4-dihydropyridines. Tetrahedron Lett. 1978, 19, 1255–1258. [Google Scholar] [CrossRef]
- Van Bergen, T.J.; Hedstrand, D.M.; Kruizinga, W.H.; Kellogg, R.M. Reduction of aldehydes and ketones by sodium dithionite. J. Org. Chem. 1979, 44, 4953. [Google Scholar] [CrossRef]
- Kerzig, C.; Guo, X.; Wenger, O.S. Unexpected hydrated electron source for preparative visible-light driven photoredox catalysis. J. Am. Chem. Soc. 2019, 141, 2122–2127. [Google Scholar] [CrossRef]
- Zlotorzynska, M.; Sammis, G.M. Photoinduced electron-transfer-promoted redox fragmentation of N-alkoxyphthalimides. Org. Lett. 2011, 13, 6264–6267. [Google Scholar] [CrossRef]
- Larraufie, M.H.; Pellet, R.; Fensterbank, L.; Goddard, J.P.; Lacote, E.; Malacria, M.; Ollivier, C. Visible-light-induced photoreductive generation of radicals from epoxides and aziridines. Angew. Chem. Int. Ed. 2011, 50, 4463. [Google Scholar] [CrossRef]
- Hiraonaka, T.; Shiori, J.; Okahata, N. Ruthenium–bipyridine complex-catalyzed photo-induced reduction of nitrobenzenes with hydrazine. Bull. Chem. Soc. Jpn. 2004, 77, 1763–1764. [Google Scholar]
- Kern, J.-M.; Sauvage, J.-P. Photochemical deposition of electrically conducting polypyrrole. Chem. Commun. 1987, 10, 657–658. [Google Scholar] [CrossRef]
- Zhang, W.; Carpenter, K.L.; Lin, S. Electrochemistry broadens the scope of flavin photocatalysis: Photoelectrocatalytic oxidation of unactivated alcohols. Angew. Chem. 2020, 132, 417–425. [Google Scholar] [CrossRef]
- Gazi, S.; Ananthakrishnan, R. Metal-free-photocatalytic reduction of 4-nitrophenol by resin-supported dye under the visible irradiation. Appl. Catal. B Environ. 2011, 105, 317–325. [Google Scholar] [CrossRef]
- Chen, Y.; Kamlet, A.S.; Steinman, J.B.; Liu, D.R. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 2011, 3, 146–153. [Google Scholar] [CrossRef]
- Willner, I.; Ford, W.E.; Otvos, J.W.; Calvin, M. Photoinduced electron transfer across a water-oil boundary: A model for redox reaction separation. Nature 1979, 280, 823–824. [Google Scholar] [CrossRef]
- Aida, K.; Hirao, M.; Funabashi, A.; Sugimura, N.; Ota, E.; Yamaguchi, J. Reductive Ring Opening of Epoxides under Dual Zirconocene and Photoredox Catalysis. J. Chem. 2022, 8, 1762. [Google Scholar]
- Aida, K.; Hirao, M.; Funabashi, A.; Sugimura, N.; Ota, E.; Yamaguchi, J. Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis. Chem 2022, 8, 1762–1774. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Csiba, M.A.; Jaszberenyi, J.C. Ru(bpy)32+-mediated addition of Se-phenyl p-tolueneselenosulfonate to electron rich olefins. Tetrahedron Lett. 1994, 35, 2869–2872. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Tucker, J.W.; Konieczynska, M.D.; Stephenson, C.R.J. Intermolecular atom transfer radical addition to olefins mediated by oxidative quenching of photoredox catalysts. J. Am. Chem. Soc. 2011, 133, 4160–4163. [Google Scholar] [CrossRef] [PubMed]
- Pirtsch, M.; Paria, S.; Matsuno, T.; Isobe, H.; Reiser, O. [Cu(dap)2Cl] As an Efficient Visible-Light-Driven Photoredox Catalyst in Carbon–Carbon Bond-Forming Reactions. Chem. Eur. J. 2012, 18, 7336–7340. [Google Scholar] [CrossRef]
- Gu, X.; Li, X.; Qu, Y.; Yang, Q.; Li, P.; Yao, Y. Intermolecular Visible-Light Photoredox Atom-Transfer Radical [3 + 2]-Cyclization of 2-(Iodomethyl) cyclopropane-1,1-dicarboxylate with Alkenes and Alkynes. Chem. Eur. J. 2013, 19, 11878–11882. [Google Scholar] [CrossRef]
- Gu, X.; Lu, P.; Fan, W.; Li, P.; Yao, Y. Visible light photoredox atom transfer Ueno–Stork reaction. Org. Biomol. Chem. 2013, 11, 7088–7091. [Google Scholar] [CrossRef]
- Shih, H.W.; Vander Wal, M.N.; Grange, R.L.; MacMillan, D.W. Enantioselective α-benzylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2010, 132, 13600–13603. [Google Scholar] [CrossRef] [PubMed]
- Nagib, D.A.; Scott, M.E.; MacMillan, D.W. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Nagib, D.A.; MacMillan, D.W. Photoredox Catalysis: A Mild, Operationally Simple Approach to the Synthesis of α-Trifluoromethyl Carbonyl Compounds. Angew. Chem. Int. Ed. 2011, 50, 6119. [Google Scholar] [CrossRef]
- Cecere, G.; Konig, C.M.; Alleva, J.L.; MacMillan, D.W.C. Enantioselective direct α-amination of aldehydes via a photoredox mechanism: A strategy for asymmetric amine fragment coupling. J. Am. Chem. Soc. 2013, 135, 11521–11524. [Google Scholar] [CrossRef]
- Cherevatskaya, M.; Neumann, M.; Ftildner, S.; Harlander, C.; Klimmel, S.; Dankesreiter, S.; Pfitzner, A.; Zeitler, K.; Konig, B. Visible-light-promoted stereoselective alkylation by combining heterogeneous photocatalysis with organocatalysis. Angew. Chem. Int. Ed. 2012, 51, 4062–4066. [Google Scholar] [CrossRef]
- Arceo, E.; Jurberg, I.D.; Alvarez-Fernandez, A.; Melchiorre, P. Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 2013, 5, 750–756. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Luo, S. Asymmetric α-photoalkylation of β-ketocarbonyls by primary amine catalysis: Facile access to acyclic all-carbon quaternary stereocenters. J. Am. Chem. Soc. 2014, 136, 14642–14654. [Google Scholar] [CrossRef]
- Uraguchi, D.; Kinoshita, N.; Kizu, T.; Ooi, T. Synergistic catalysis of ionic Brønsted acid and photosensitizer for a redox neutral asymmetric α-coupling of N-arylaminomethanes with aldimines. J. Am. Chem. Soc. 2015, 137, 13768–13771. [Google Scholar] [CrossRef]
- Huo, H.; Shen, X.; Wang, C.; Zhang, L.; Rose, P.; Chen, L.-A.; Harms, K.; Marsch, M.; Hilt, G.; Meggers, E. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 2014, 515, 100. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Cai, J.; Gong, L. Visible-light-promoted asymmetric catalysis by chiral complexes of first-row transition metals. Synthesis 2021, 53, 1570. [Google Scholar]
- Zhou, K.; Yu, Y.; Lin, Y.M.; Li, Y.; Gong, L. Copper-catalyzed aerobic asymmetric cross-dehydrogenative coupling of C (sp3)–H bonds driven by visible light. Green Chem. 2020, 22, 4597–4603. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Chen, B.; Xu, M.; Li, C.; Zhang, D.; Zhang, G. Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 2020, 22, 1490. [Google Scholar] [CrossRef]
- Xia, H.-D.; Li, Z.-L.; Gu, Q.-S.; Dong, X.-Y.; Fang, J.-H.; Du, X.-Y.; Wang, L.-L.; Liu, X.-Y. Photoinduced Copper-Catalyzed Asymmetric Decarboxylative Alkynylation with Terminal Alkynes. Angew. Chem. Int. Ed. 2020, 59, 16926. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, W.; Yu, S. Enantioselective remote C (sp3)–H cyanation via dual photoredox and copper catalysis. Org. Lett. 2020, 22, 5910. [Google Scholar] [CrossRef]
- Saha, D. Catalytic enantioselective radical transformations enabled by visible light. Chem. Asian J. 2020, 15, 2129–2152. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.-C. Enantioselective synthesis enabled by visible light photocatalysis. Org. Biomol. Chem. 2020, 18, 4298–4353. [Google Scholar] [CrossRef] [PubMed]
- Prentice, C.; Morrisson, J.; Smith, A.D.; Zysman-Colman, E. Beilstein Recent developments in enantioselective photocatalysis. J. Org. Chem. 2020, 16, 2363. [Google Scholar]
- Zhang, H.-H.; Chen, H.; Zhu, C.; Yu, S. A review of enantioselective dual transition metal/photoredox catalysis. Sci. China Chem. 2020, 63, 637–647. [Google Scholar] [CrossRef]
- Huang, X.; Meggers, E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes. Acc. Chem. Res. 2019, 52, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Vega-Peñaloza, A.; Paria, S.; Bonchio, M.; Dell’Amico, L.; Companyó, X. Profiling the privileges of pyrrolidine-based catalysts in asymmetric synthesis: From polar to light-driven radical chemistry. ACS Catal. 2019, 9, 6058. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, W.; Zheng, W.-H.; Lu, H. Advances in asymmetric visible-light photocatalysis. Org. Biomol. Chem. 2019, 17, 8673–8689. [Google Scholar] [CrossRef]
- Lipp, A.; Badir, S.O.; Molander, G.A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed. 2021, 60, 1714–1726. [Google Scholar] [CrossRef]
- Kalyani, D.; McMurtrey, K.B.; Neufeldt, S.R.; Sanford, M.S. Room-temperature C–H arylation: Merger of Pd-catalyzed C–H functionalization and visible-light photocatalysis. J. Am. Chem. Soc. 2011, 133, 18566–18569. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, S.R.; Sanford, M.S. The Catalytic Potential of Coptis japonica NCS2 Revealed–Development and Utilisation of a Fluorescamine-Based Assay. Adv. Synth. Cata. 2012, 354, 2997–3008. [Google Scholar]
- Lang, S.B.; O’Nele, K.M.; Tunge, J.A. Decarboxylative allylation of amino alkanoic acids and esters via dual catalysis. J. Am. Chem. Soc. 2014, 136, 13606–13609. [Google Scholar] [CrossRef]
- Xuan, J.; Zeng, T.-T.; Feng, Z.-J.; Deng, Q.-H.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J.; Alper, H. Redox-Neutral α-Allylation of Amines by Combining Palladium Catalysis and Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 1625–1628. [Google Scholar] [CrossRef]
- Ye, Y.; Sanford, M.S. Merging visible-light photocatalysis and transition-metal catalysis in the copper-catalyzed trifluoromethylation of boronic acids with CF3I. J. Am. Chem. Soc. 2012, 134, 9034. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Hopkinson, M.N.; Glorius, F. Combining gold and photoredox catalysis: Visible light-mediated oxy-and aminoarylation of alkenes. J. Am. Chem. Soc. 2013, 135, 5505. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Ahneman, D.T.; Chu, L.; Terrett, J.A.; Doyle, A.G.; MacMillan, D.W.C. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Tellis, J.C.; Primer, D.N.; Molander, G.A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 2014, 345, 433. [Google Scholar] [CrossRef] [PubMed]
- Cano-Yelo, H.; Deronzier, A. Photocatalysis of the Pschorr reaction by tris-(2,2′-bipyridyl) ruthenium(II) in the phenanthrene series. J. Chem. Soc. Perkin Trans. 2 1984, 6, 1093–1098. [Google Scholar] [CrossRef]
- Hari, D.P.; Schroll, P.; Konig, B. Metal-free, visible-light-mediated direct C–H arylation of heteroarenes with aryl diazonium salts. J. Am. Chem. Soc. 2012, 134, 2958. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Jia, Z.H.; Zhao, C.J.; Zhang, Y.Y.; Wang, C.; Xiao, J. Direct Arylation of N-Heteroarenes with Aryldiazonium Salts by Photoredox Catalysis in Water. Chem. Eur. J. 2014, 20, 2960–2965. [Google Scholar] [CrossRef]
- Xiao, J.; Xue, D.; Liu, Y.-X.; Wang, J.-D.; Zhao, C.-J.; Zou, Q.-Z.; Wang, C. Room-temperature arylation of arenes and heteroarenes with diaryliodonium salts by photoredox catalysis. Synlett 2013, 24, 507. [Google Scholar]
- Cheng, Y.; Gu, X.; Li, P. Visible-light photoredox in homolytic aromatic substitution: Direct arylation of arenes with aryl halides. Org. Lett. 2013, 15, 2664–2667. [Google Scholar] [CrossRef]
- Nagib, D.A.; MacMillan, D.W.C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 2011, 480, 224–228. [Google Scholar] [CrossRef]
- Iqbal, N.; Choi, S.; Ko, E.; Cho, E.J. Trifluoromethylation of heterocycles via visible light photoredox catalysis. Tetrahedron Lett. 2012, 53, 2005. [Google Scholar] [CrossRef]
- Xie, J.; Yuan, X.; Abdukader, A.; Zhu, C.; Ma, J. Visible-light-promoted radical C–H trifluoromethylation of free anilines. Org. Lett. 2014, 16, 1768–1771. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X.J.; Lei, W.L.; Chen, H.; Wu, L.Z.; Liu, Q. Direct C–H difluoromethylenephosphonation of arenes and heteroarenes with bromodifluoromethyl phosphonate via visible-light photocatalysis. Chem. Commun. 2014, 50, 15916. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, X.J.; Jia, W.L.; Zhong, J.J.; Wu, L.Z.; Liu, Q. Visible-Light-Driven Difluoroacetamidation of Unactive Arenes and Heteroarenes by Direct C–H Functionalization at Room Temperature. Org. Lett. 2014, 16, 5842–5845. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-M.; Hou, Y.; Yin, F.; Xu, Y.-M.; Li, Y.; Zheng, X.; Wang, X.-S. Visible light-mediated C–H difluoromethylation of electron-rich heteroarenes. Org. Lett. 2014, 16, 2958. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, E.; You, Y.; Cho, E.J. Visible Light-Induced Aromatic Difluoroalkylation. Adv. Synth. Cat. 2014, 356, 2741–2748. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Lee, D.G.; Roh, S.W.; Lee, C. Nitrogen-centered radical-mediated C–H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 2014, 50, 9273–9276. [Google Scholar] [CrossRef]
- Allen, L.J.; Cabrera, P.J.; Lee, M.; Sanford, M.S. N-Acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 2014, 136, 5607–5610. [Google Scholar] [CrossRef]
- Qin, Q.; Yu, S. Visible-light-promoted redox neutral C–H amidation of heteroarenes with hydroxylamine derivatives. Org. Lett. 2014, 16, 3504–3507. [Google Scholar] [CrossRef]
- Yasu, Y.; Koike, T.; Akita, M. Three-component Oxytrifluoromethylation of Alkenes: Highly Efficient and Regioselective Difunctionalization of C—C Bonds Mediated by Photoredox Catalysts. Angew. Chem. Int. Ed. 2012, 51, 9567–9571. [Google Scholar] [CrossRef]
- Yasu, Y.; Koike, T.; Akita, M. Intermolecular aminotrifluoromethylation of alkenes by visible-light-driven photoredox catalysis. Org. Lett. 2013, 15, 2136–2139. [Google Scholar] [CrossRef]
- Yasu, Y.; Arai, Y.; Tomita, R.; Koike, T.; Akita, M. Highly regio-and diastereoselective synthesis of CF3-substituted lactones via photoredox-catalyzed carbolactonization of alkenoic acids. Org. Lett. 2014, 16, 780–783. [Google Scholar] [CrossRef]
- Tomita, R.; Yasu, Y.; Koike, T.; Akita, M. Combining photoredox-catalyzed trifluoromethylation and oxidation with DMSO: Facile synthesis of α-trifluoromethylated ketones from aromatic alkenes. Angew. Chem. Int. Ed. 2014, 53, 7144–7148. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Choi, S.; Kim, E.; Cho, E.J. Trifluoromethylation of alkenes by visible light photoredox catalysis. J. Org. Chem. 2012, 77, 11383–11387. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Jung, J.; Park, S.; Cho, E.J. Controlled trifluoromethylation reactions of alkynes through visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2014, 53, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, J.M.; Nicewicz, D.A. Synthesis of Highly Substituted Tetrahydrofurans by Catalytic Polar-Radical-Crossover Cycloadditions of Alkenes and Alkenols. Angew. Chem. Int. Ed. 2013, 52, 3967–3971. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Nicewicz, D.A. Anti-Markovnikov hydroamination of alkenes catalyzed by an organic photoredox system. J. Am. Chem. Soc. 2013, 135, 9588–9591. [Google Scholar] [CrossRef]
- Perkowski, A.J.; Nicewicz, D.A. Direct catalytic anti-Markovnikov addition of carboxylic acids to alkenes. J. Am. Chem. Soc. 2013, 135, 10334–10337. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Manohar, N.; Nicewicz, D.A. anti-Markovnikov Hydroamination of Alkenes Catalyzed by a Two-Component Organic Photoredox System. Angew. Chem. Int. Ed. 2014, 53, 6198–6201. [Google Scholar] [CrossRef]
- Wilger, D.J.; Grandjean, J.M.; Lammert, T.R.; Nicewicz, D.A. The direct anti-Markovnikov addition of mineral acids to styrenes. Nat. Chem. 2014, 6, 720–726. [Google Scholar] [CrossRef]
- Du, J.; Yoon, T.P. Crossed intermolecular [2 + 2] cycloadditions of acyclic enones via visible light photocatalysis. J. Am. Chem. Soc. 2009, 131, 14604–14605. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Shen, M.; Yoon, T.P. [3 + 2] Cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis. J. Am. Chem. Soc. 2011, 133, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Skubi, K.L.; Schultz, D.M.; Yoon, T.P. A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light. Science 2014, 344, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Kohls, P.; Jadhav, D.; Pandey, G.; Reiser, O. Visible light photoredox catalysis: Generation and addition of N-aryltetrahydroisoquinoline-derived α-amino radicals to michael acceptors. Org. Lett. 2012, 14, 672–675. [Google Scholar] [CrossRef]
- Miyake, Y.; Nakajima, K.; Nishibayashi, Y. Visible-light-mediated utilization of α-aminoalkyl radicals: Addition to electron-deficient alkenes using photoredox catalysts. J. Am. Chem. Soc. 2012, 134, 3338–3341. [Google Scholar] [CrossRef]
- Miyake, Y.; Ashida, Y.; Nakajima, K.; Nishibayashi, Y. Visible-light-mediated addition of α-aminoalkyl radicals generated from α-silylamines to α,β-unsaturated carbonyl compounds. Chem. Commun. 2012, 48, 6966–6968. [Google Scholar] [CrossRef]
- Yoon, U.C.; Mariano, P.S. Mechanistic and synthetic aspects of amine-enone single electron transfer photochemistry. Acc. Chem. Res. 1992, 25, 233–240. [Google Scholar] [CrossRef]
- Xu, W.; Jeon, Y.T.; Hasegawa, E.; Yoon, U.C.; Mariano, P.S. Novel electron-transfer photocyclization reactions of. alpha.-silyl amine. alpha., beta.-unsaturated ketone and ester systems. J. Am. Chem. Soc. 1989, 111, 406–408. [Google Scholar] [CrossRef]
- Jeon, Y.T.; Lee, C.-P.; Mariano, P.S. Radical cyclization reactions of. alpha.-silyl amine. alpha., beta.-unsaturated ketone and ester systems promoted by single electron transfer photosensitization. J. Am. Chem. Soc. 1991, 113, 8847–8863. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.-M.; Mariano, P.S. Single electron transfer promoted photocyclization reactions of (aminoalkyl) cyclohexenones. Mechanistic and synthetic features of processes involving the generation and reactions of amine cation and. alpha.-amino radicals. J. Am. Chem. Soc. 1991, 113, 8863–8878. [Google Scholar] [CrossRef]
- Hamilton, D.S.; Nicewicz, D.A. Direct catalytic anti-Markovnikov hydroetherification of alkenols. J. Am. Chem. Soc. 2012, 134, 18577–18580. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-Q.; Duan, S.-W.; Meng, X.-G.; Hu, X.-Q.; Gao, S.; Chen, J.-R.; Xiao, W.-J. Visible light induced intermolecular [2 + 2]-cycloaddition reactions of 3-ylideneoxindoles through energy transfer pathway. Tetrahedron 2012, 68, 6914–6919. [Google Scholar] [CrossRef]
- Xia, X.-D.; Xuan, J.; Wang, Q.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Synthesis of 2-Substituted Indoles through Visible Light-Induced Photocatalytic Cyclizations of Styryl Azides. Adv. Synth. Cat. 2014, 356, 2807–2812. [Google Scholar] [CrossRef]
- Lu, Z.; Yoon, T.P. Visible light photocatalysis of [2 + 2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 2012, 51, 10329. [Google Scholar] [CrossRef] [PubMed]
- Farney, E.P.; Yoon, T.P. Visible-Light Sensitization of Vinyl Azides by Transition-Metal Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 793–797. [Google Scholar] [CrossRef]
- Fan, W.G.; Li, P.X. Visible-Light-Mediated 1,2-Acyl Migration: The Reaction of Secondary Enamino Ketones with Singlet Oxygen. Angew. Chem. Int. Ed. 2014, 53, 12201. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Dong, W.; Xie, X.; Wan, J.; Zhang, Z. Visible light-induced aerobic C–N bond activation: A photocatalytic strategy for the preparation of 2-arylpyridines and 2-arylquinolines. RSC Adv. 2016, 54, 48315–48318. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, B.; Ding, G.; Ren, K.; Xie, X.; Zhang, Z. Ru-catalyzed asymmetric hydrogenation of δ-keto Weinreb amides: Enantioselective synthesis of (+)-Centrolobine. Org. Biomol. Chem. 2016, 14, 2723–2730. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Dong, W.; Ren, K.; Xie, X.; Wan, J.; Zhang, Z. Visible light-induced intramolecular dearomative cyclization of α-bromo-N-benzyl-alkylamides: Efficient construction of 2-azaspiro[4.5]decanes. Chem. Commun. 2016, 52, 3709–3712. [Google Scholar] [CrossRef]
- Gao, X.; Dong, W.; Hu, B.; Gao, H.; Yuan, Y.; Xie, X.; Zhang, Z. Visible-light induced tandem radical cyanomethylation and cyclization of N-aryl acrylamides: Access to cyanomethylated oxindoles. RSC Adv. 2017, 78, 49299–49302. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, Y.; Xie, X.; Zhang, Z. Visible-light-induced cascade dearomatization cyclization between alkynes and indole-derived bromides: A facile strategy to synthesize spiroindolenines. Chem. Commun. 2020, 56, 14047–14050. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; Yuan, Y.; Xie, X.; Zhang, Z. Visible-light-induced intramolecular radical cascade of α-bromo-N-benzyl-alkylamides: A new strategy to synthesize tetracyclic N-fused indolo [2,1-a] isoquinolin-6 (5 H)-ones. Org. Biomol. Chem. 2020, 18, 263. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yang, L.; Zhang, Z.; Xie, X. Visible-light-promoted aerobic oxidation of sulfides and sulfoxides in ketone solvents. Tetrahedron 2022, 110, 132708. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, S.Y.; Dong, W.H.; Wu, F.; Xie, X.M.; Zhang, Z.G. Visible-Light-Induced Radical Cascade Cyclization of o-Diisocyanoarenes: Synthesis of Diethyl Benzo[a]phenazine-6,6(5H)-Dicarboxylate. Adv. Synth. Catal. 2021, 363, 4216–4221. [Google Scholar] [CrossRef]
- Dong, W.; Yuan, Y.; Liang, C.; Wu, F.; Zhang, S.; Xie, X.; Zhang, Z. Photocatalytic radical ortho-dearomative cyclization: Access to spiro[4.5]deca-1,7,9-trien-6-ones. J. Org. Chem. 2021, 86, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; MacMillan, D.W. Photoredox α-vinylation of α-amino acids and N-aryl amines. J. Am. Chem. Soc. 2014, 136, 11602–11605. [Google Scholar] [CrossRef]
- Affron, D.P.; Davis, O.A.; Bull, J.A. Regio-and stereospecific synthesis of C-3 functionalized proline derivatives by palladium catalyzed directed C(sp3)–H arylation. Org. Lett. 2014, 16, 4956–4959. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).