Enhancing Photon Transfer Efficiency in Photocatalysis Using Suspended LED Lights for Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LED Light Bulbs
2.3. Experimental Setup
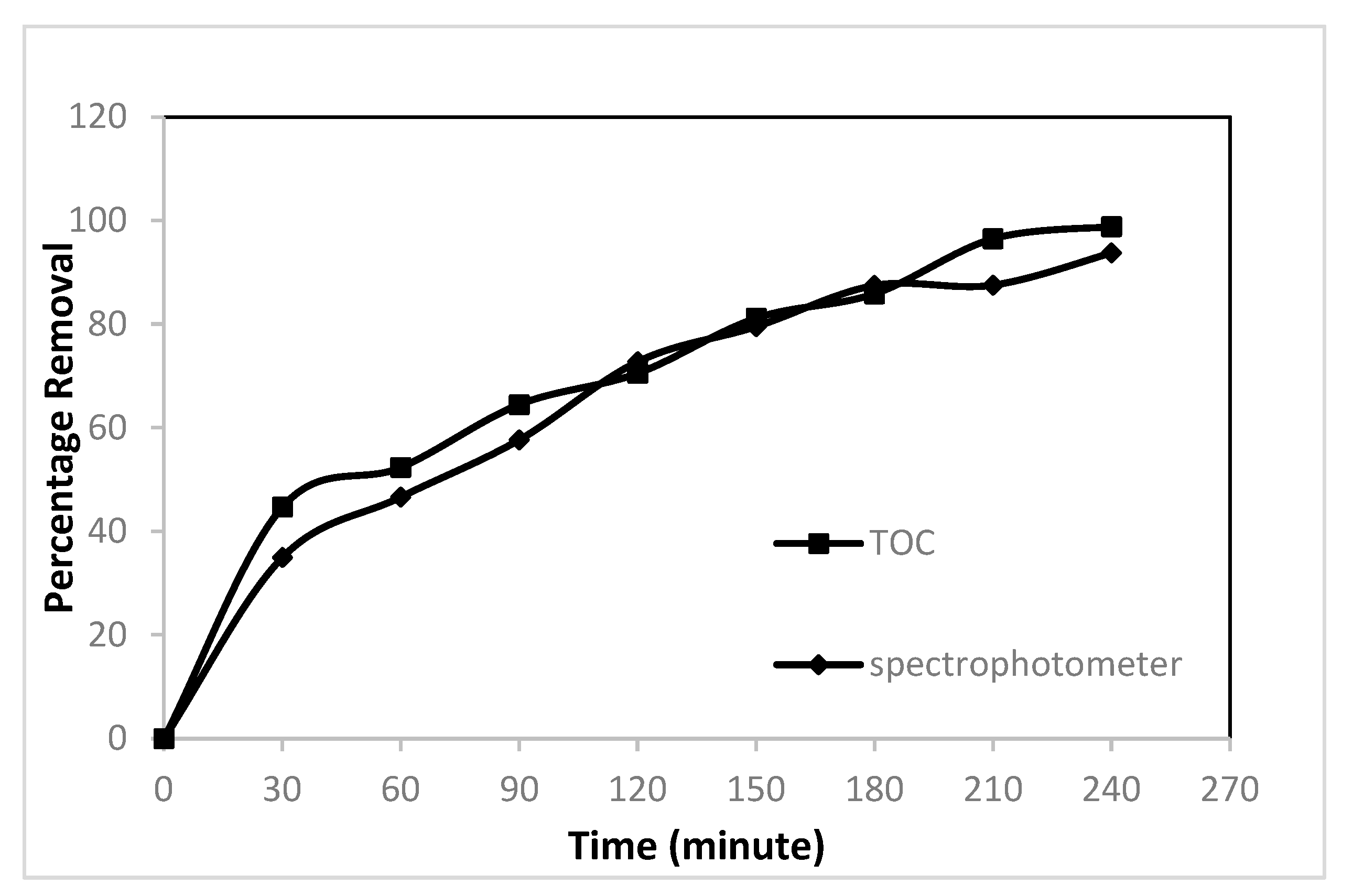
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mancuso, A.; Navarra, W.; Sacco, O.; Pragliola, S.; Vaiano, V.; Venditto, V. Photocatalytic Degradation of Thiacloprid Using Tri-Doped TiO2 Photocatalysts: A Preliminary Comparative Study. Catalysts 2021, 11, 927. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Jadaa, W.; Prakash, A.; Ray, A.K. Photocatalytic Degradation of Diazo Dye over Suspended and Immobilized TiO2 Catalyst in Swirl Flow Reactor: Kinetic Modeling. Processes 2021, 9, 1741. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Balasubramanian, G.; Suidan, M.T.; Khodadoust, A.P.; Baudin, I.; Laîné, J.M. Rotating disk photocatalytic reactor: Development, characterization, and evaluation for the destruction of organic pollutants in water. Water Res. 2000, 34, 2927–2940. [Google Scholar] [CrossRef]
- Lin, H.; Valsaraj, K.T. Development of an optical fiber monolith reactor for photocatalytic wastewater Treatment. J. Appl. Electrochem. 2005, 35, 699–708. [Google Scholar] [CrossRef]
- Aran, H.C.; Salamon, D.; Rijnaarts, T.; Mul, G.; Wessling, M.; Lammertink, R.G.H. Porous photocatalytic membrane microreactor (P2M2): A new reactor concept for photochemistry. J. Photochem. Photobiol. A Chem. 2011, 225, 36–41. [Google Scholar]
- Kuipers, J.; Bruning, H.; Yntema, D.; Rijnaarts, H. Wirelessly powered ultraviolet light emitting diodes for photocatalytic oxidation. J. Photochem. Photobiol. A Chem. 2015, 299, 25–30. [Google Scholar] [CrossRef]
- Kuhlmann, K.; Matthews, D.R. Current Assignee Meridian Design Inc. U.S. Patent No. US7550089B2, 23 June 2009. [Google Scholar]
- Yao, Z.; Wang, M.; Jia, R.; Zhao, Q.; Liu, L.; Sun, S. Comparison of UV-based advanced oxidation processes for the removal of different fractions of NOM from drinking water. J. Environ. Sci. 2023, 126, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [PubMed]
- Rojviroon, T.; Sirivithayapakorn, S. Properties of TiO2 thin films prepared using sol–gel process. Surf. Eng. 2013, 29, 77–80. [Google Scholar] [CrossRef]
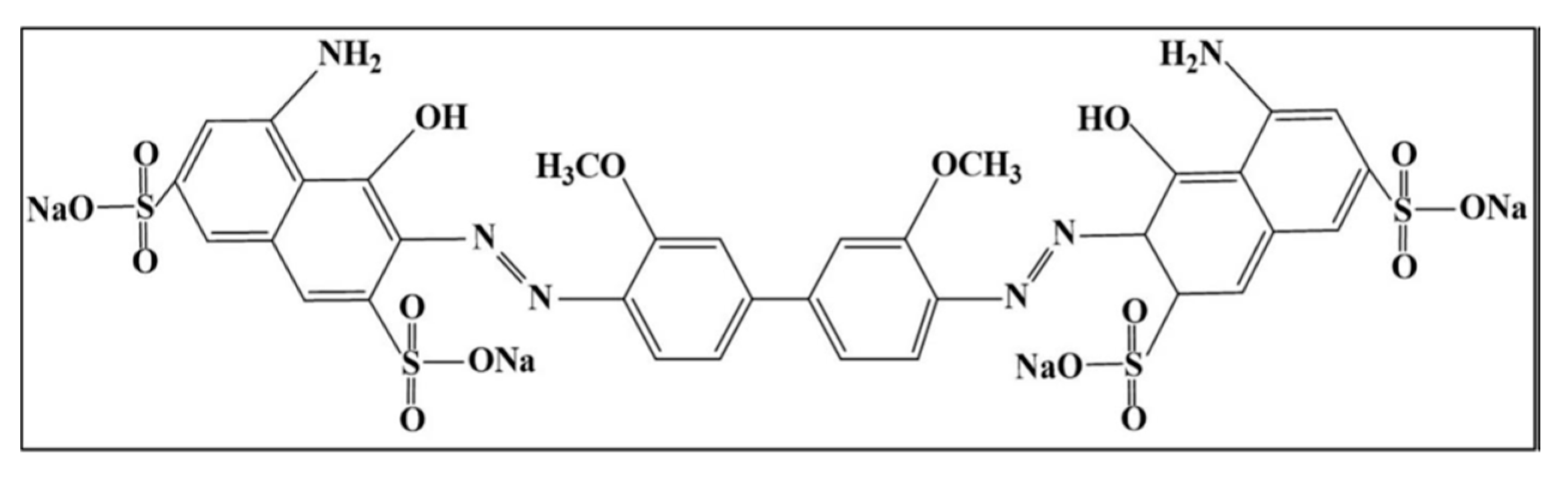

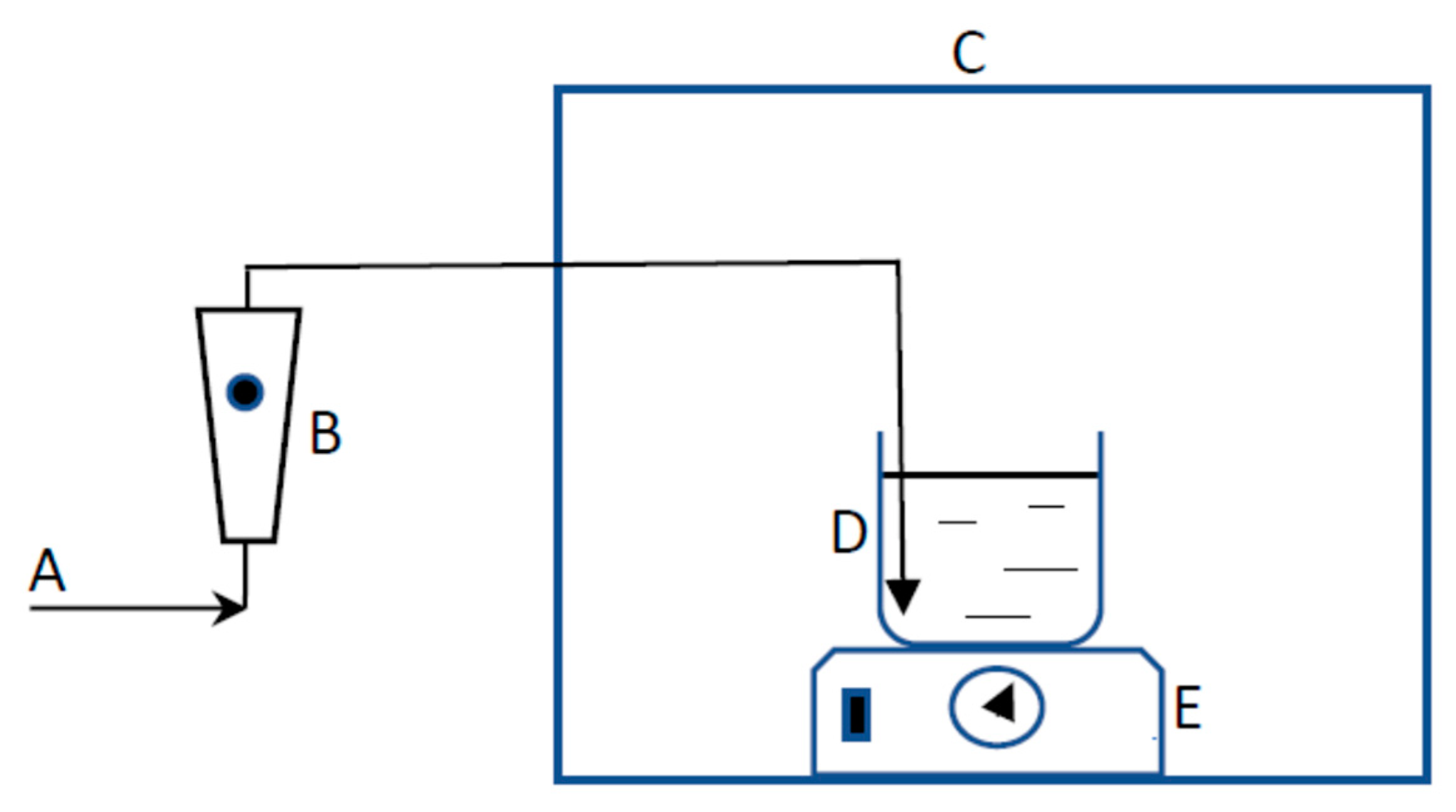
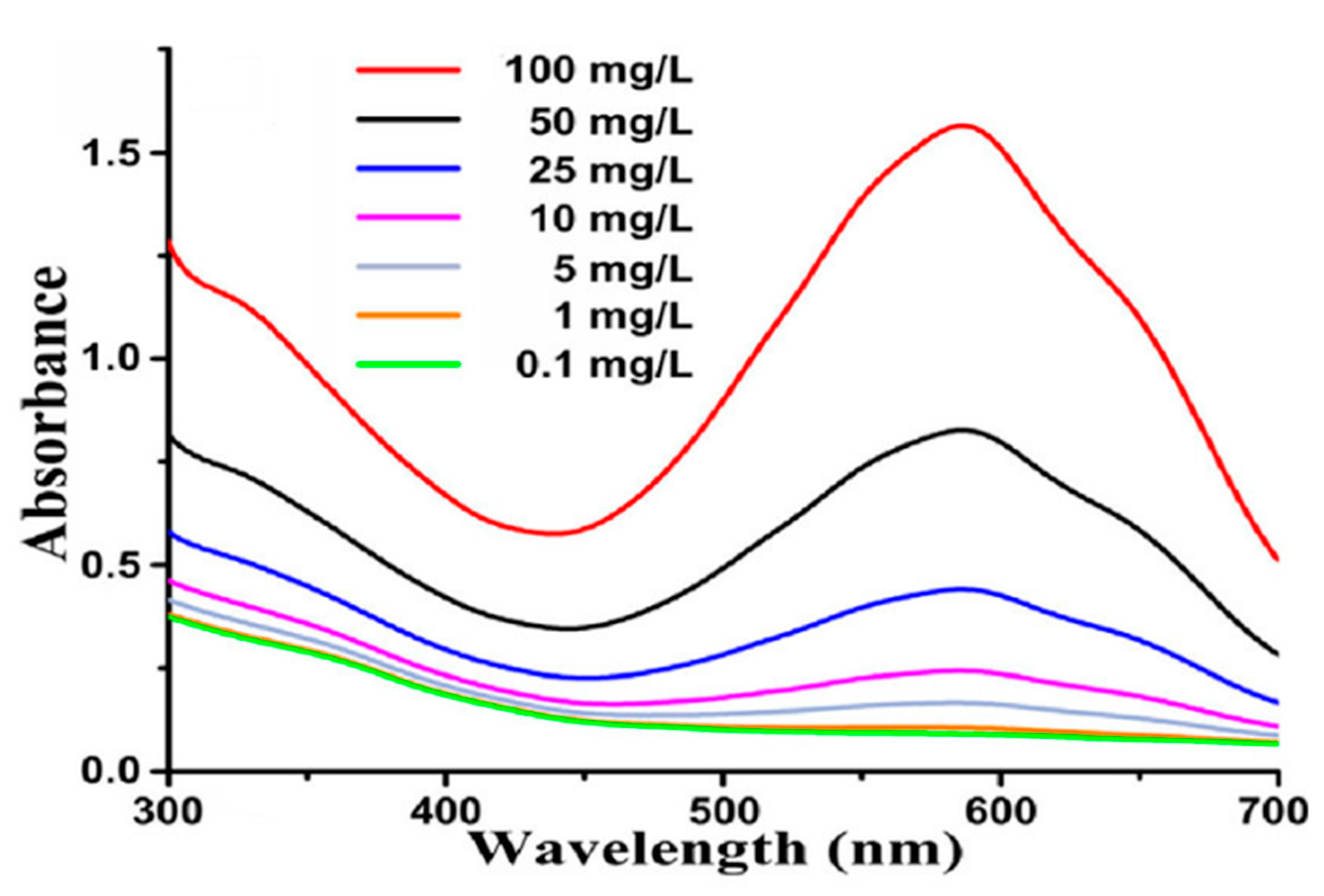
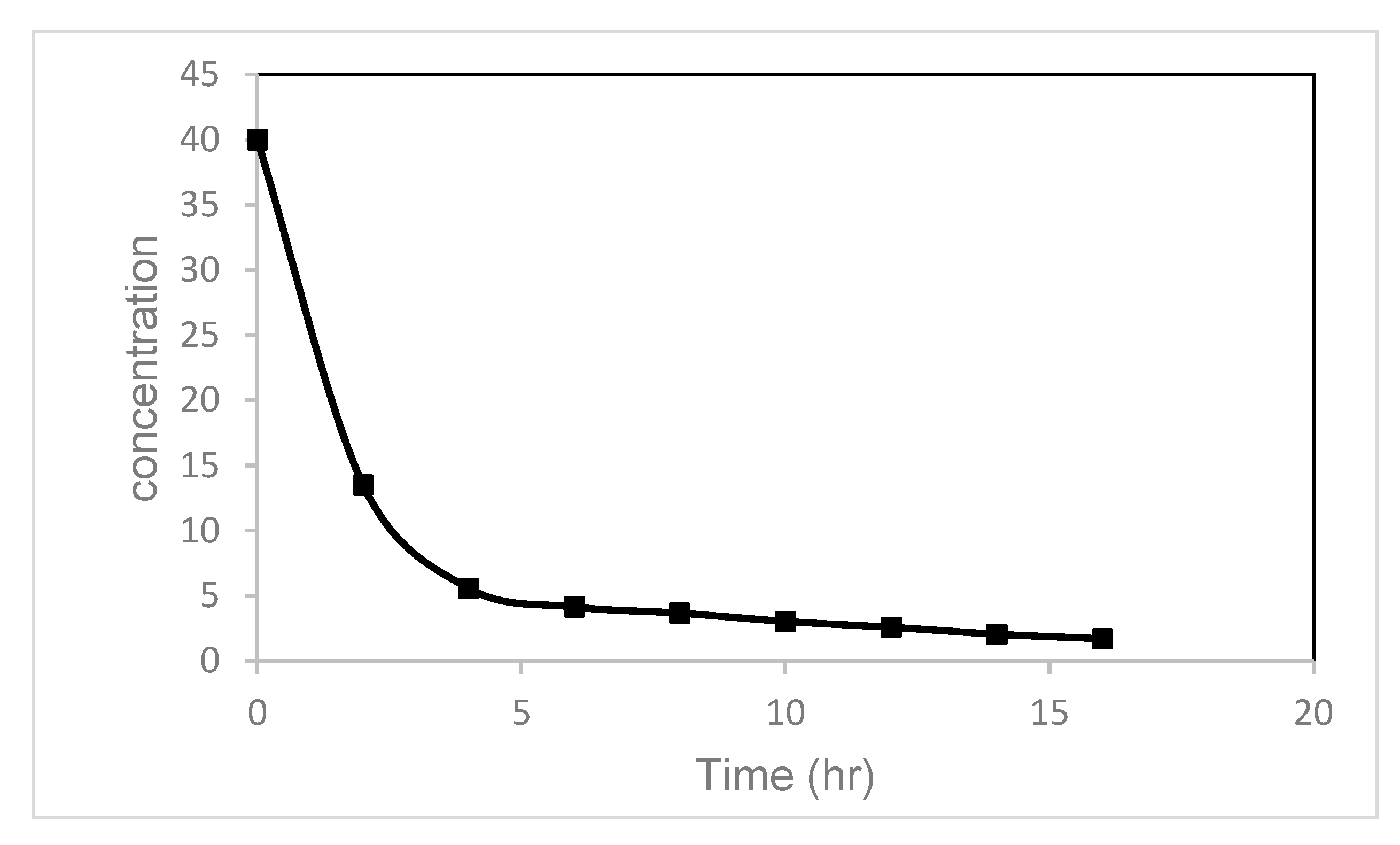
| Reactor Shape, Volume, mL | UV/Visible Light Source Wattage, Watt | Watt per Unit Volume W/mL | Reference |
|---|---|---|---|
| Cylindrical 100 mL | 200 W high pressure mercury lamp | 2 | Yao et al., 2023 [11] |
| Dish type 100 mL | 75 W low pressure mercury lamp | 0.75 | Yao et al., 2023 [11] |
| Cylindrical 25 mL | 18 W UV + 500 W Xe lamp | 20.75 | Yang Yu et al., 2017 [12] |
| Cylindrical 1100 mL | 500 W UV-LED | 0.45 | Thammasak Rojviroon et al., 2013 [13] |
| Cylindrical 500 mL | 1.7 W (total) * | 0.0035 | This study |
| Sample Initial Concentration mg/L | Degradation % | Time Requirements | |
|---|---|---|---|
| This Study | Waleed et al. [5] | ||
| 40 | 75 | 10 (h) | 14 (h) |
| 18 | 90 | 210 (min) | 270 (min) |
| 14 | 95 | 140 (min) | 150 (min) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rad, S.M.; Ray, A.K.; Barghi, S. Enhancing Photon Transfer Efficiency in Photocatalysis Using Suspended LED Lights for Water Treatment. Reactions 2023, 4, 246-253. https://doi.org/10.3390/reactions4020014
Rad SM, Ray AK, Barghi S. Enhancing Photon Transfer Efficiency in Photocatalysis Using Suspended LED Lights for Water Treatment. Reactions. 2023; 4(2):246-253. https://doi.org/10.3390/reactions4020014
Chicago/Turabian StyleRad, Samira Mosalaei, Ajay K. Ray, and Shahzad Barghi. 2023. "Enhancing Photon Transfer Efficiency in Photocatalysis Using Suspended LED Lights for Water Treatment" Reactions 4, no. 2: 246-253. https://doi.org/10.3390/reactions4020014
APA StyleRad, S. M., Ray, A. K., & Barghi, S. (2023). Enhancing Photon Transfer Efficiency in Photocatalysis Using Suspended LED Lights for Water Treatment. Reactions, 4(2), 246-253. https://doi.org/10.3390/reactions4020014









