Thiol-Ene Reaction of Heparin Allyl Ester, Heparin 4-Vinylbenzyl Ester and Enoxaparin
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
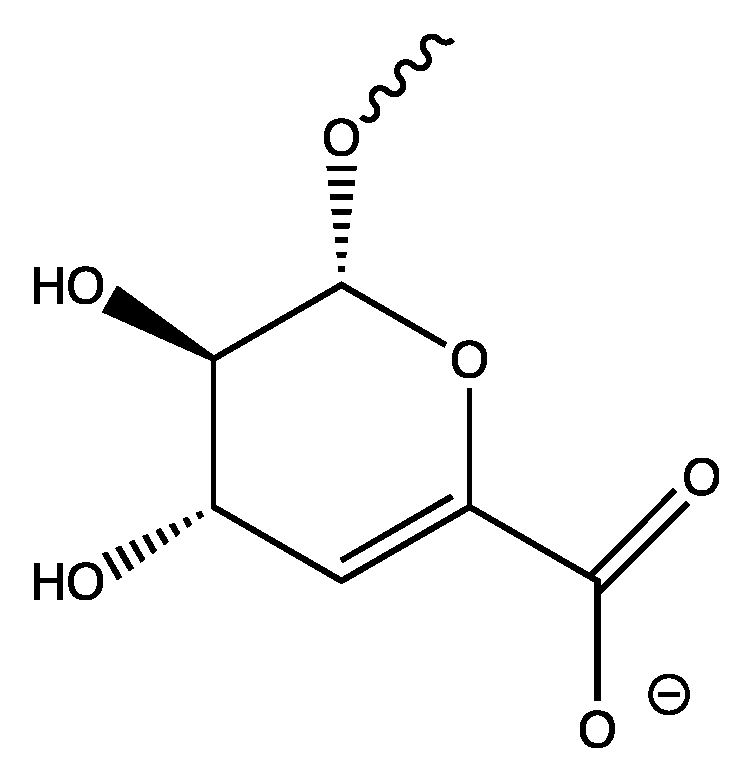
2.2. Preparation of Heparin Allyl Ester (1)
2.3. Preparation of Heparin 4-Vinylbenzyl Ester (2)
2.4. Thiol-Ene Reaction of Heparin Allyl Ester with Mercaptoacetic Acid (3)
2.4.1. Using Irradiation
2.4.2. Using Initiator
2.5. Thiol-Ene Reactions of Heparin 4-Vinylbenzyl Ester (2) General Procedure
2.5.1. Reaction with Cysteine (4)
2.5.2. Reaction with 1-Octanethiol (5)
2.5.3. Reaction with 4-Aminothiophenol (6)
2.6. Thiol-Ene Reactions of Enoxaparin
2.6.1. Reaction with Mercaptoacetic Acid Using Initiator (7)
2.6.2. Reaction with Mercaptoacetic Acid Using Irradiation
2.6.3. Reaction with Cysteine Using Irradiation (8)
3. Results
3.1. Preparation of Heparin Esters (Figure 3)
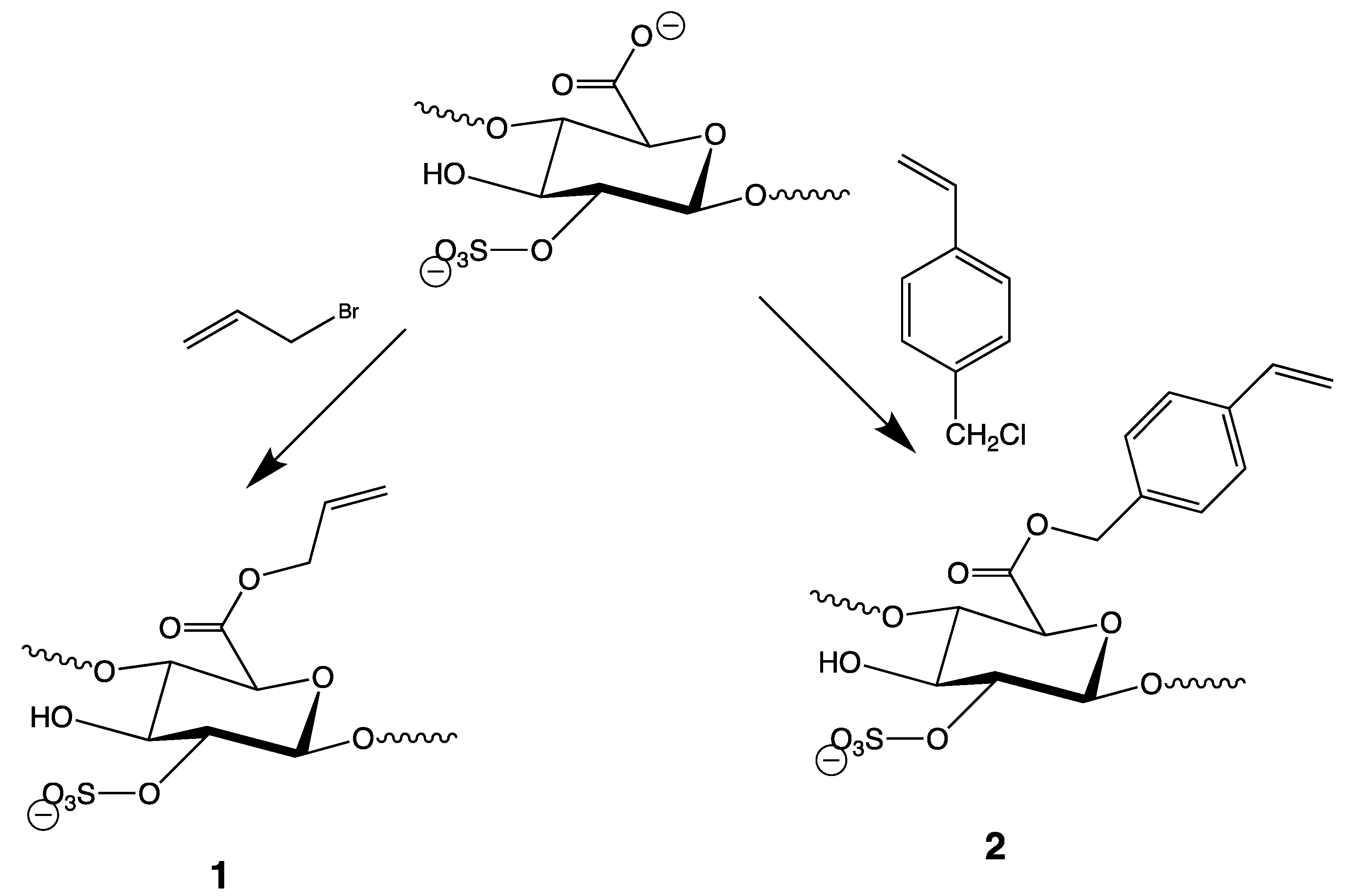
3.2. General Remarks on the Initiation of the Thiol-Ene Reactions
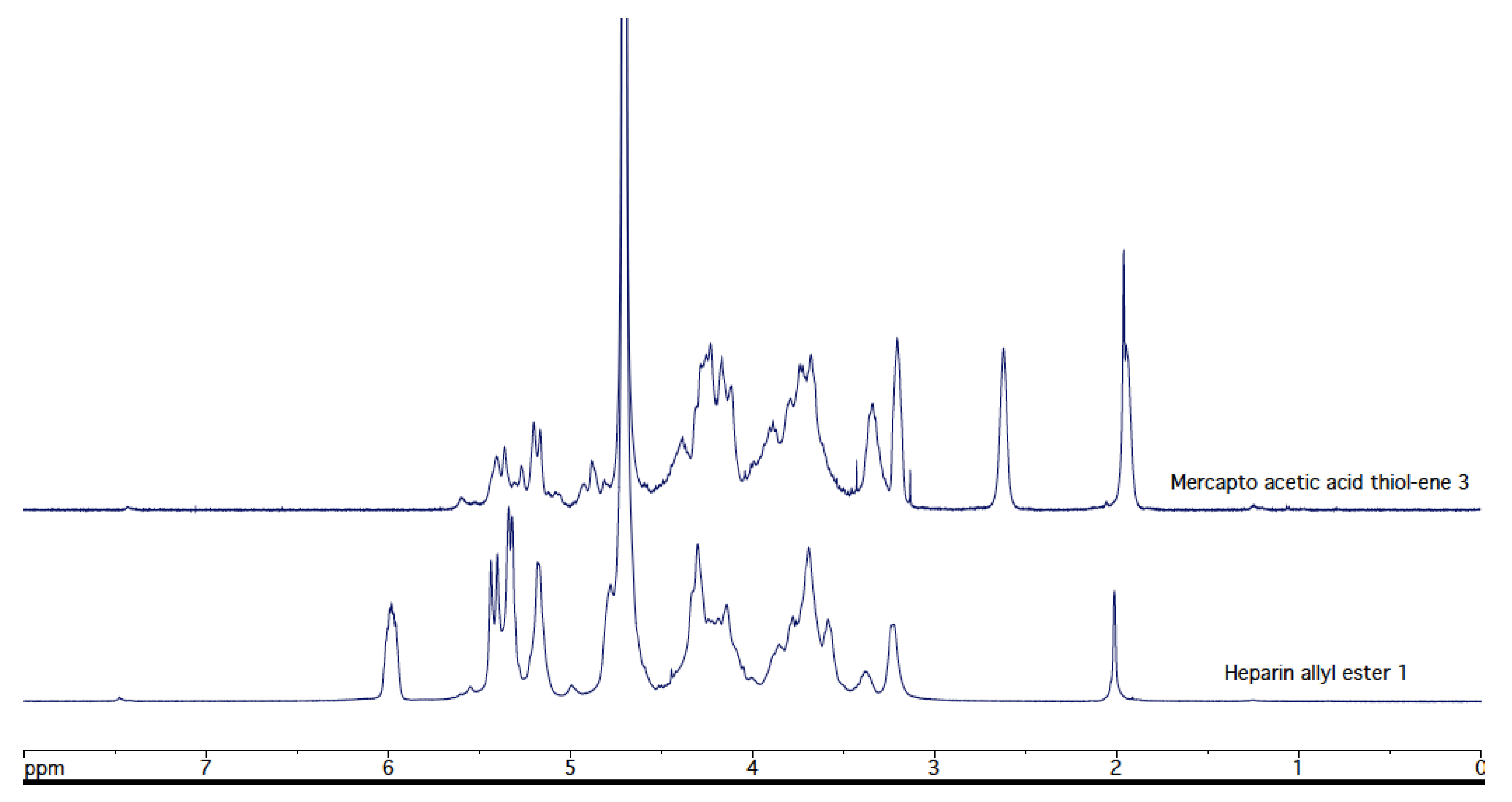
3.3. Thiol-Ene Reaction of Heparin Allyl Ester (1) with Mercaptoacetic Acid (Figure 4)
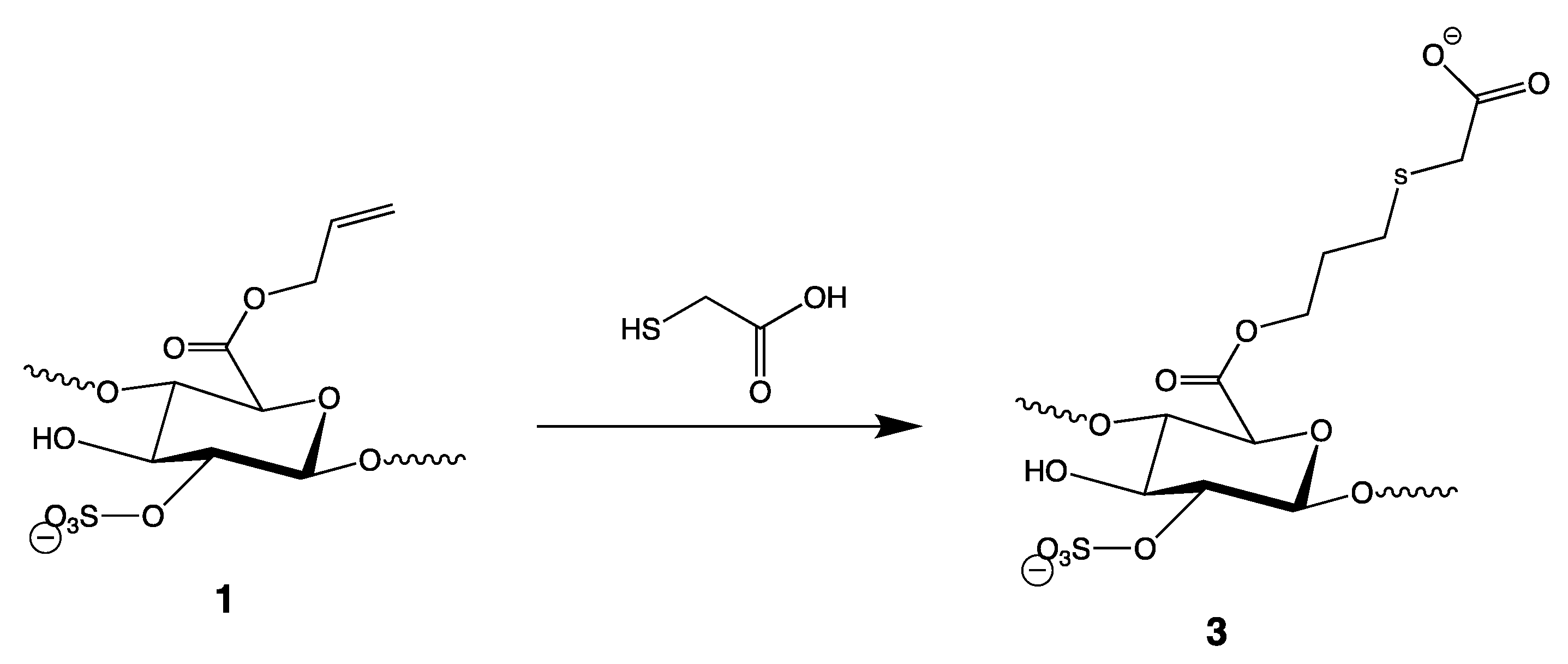
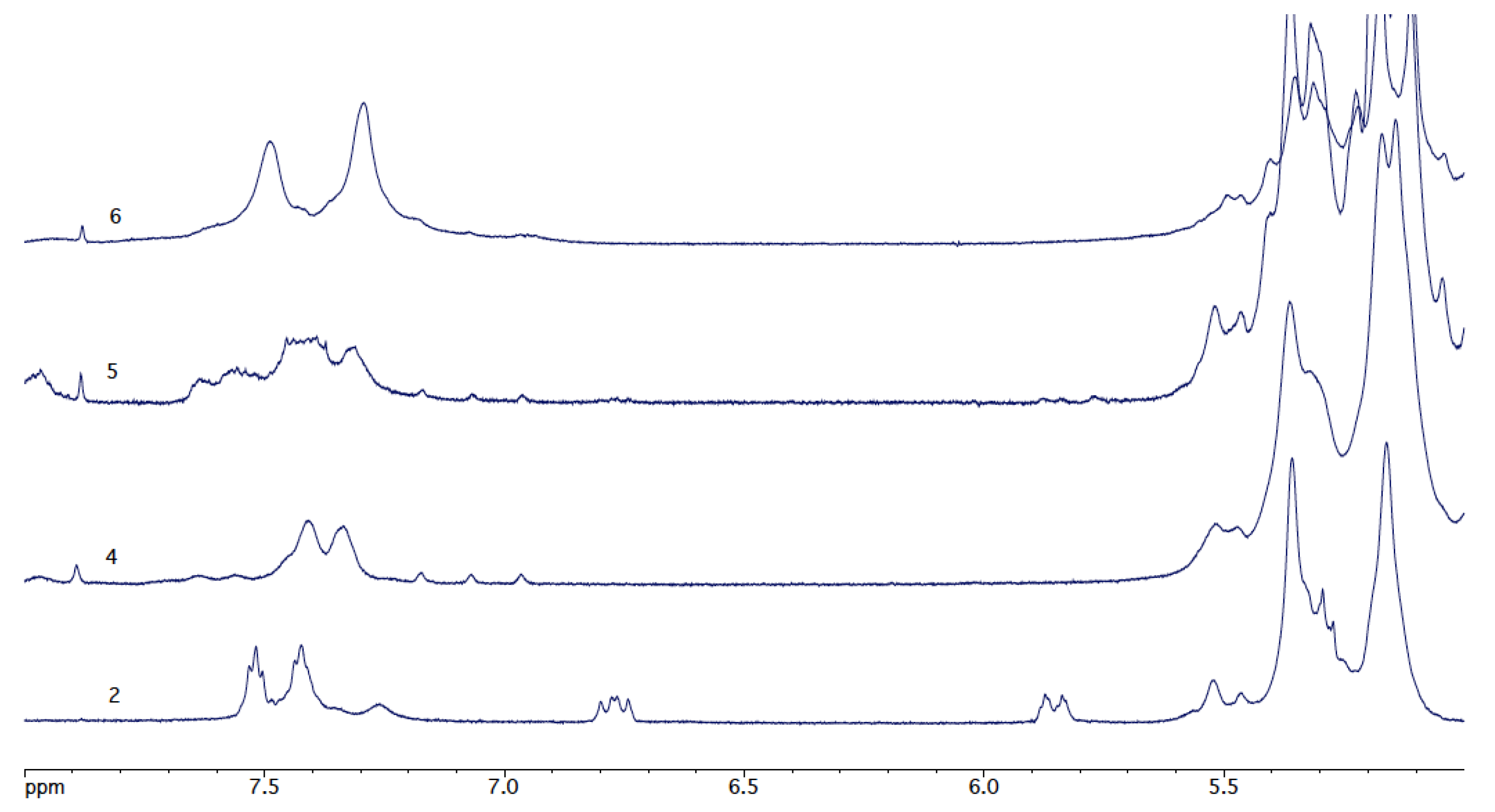
3.4. Thiol-Ene Reactions of Heparin 4-Vinylbenzyl Ester (Figure 6)

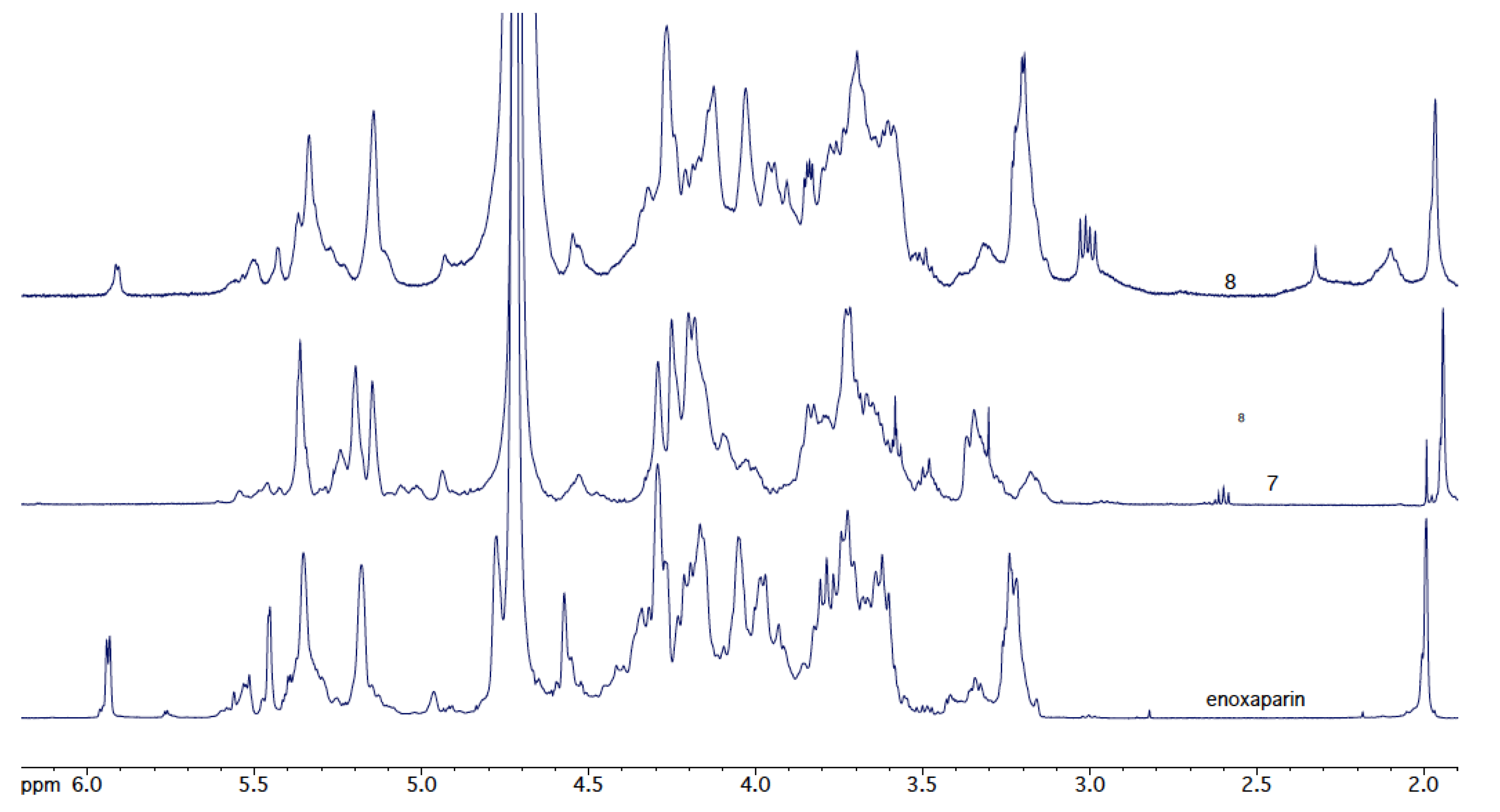
3.5. Thiol-Ene Reactions of Enoxaparin (Figure 8)
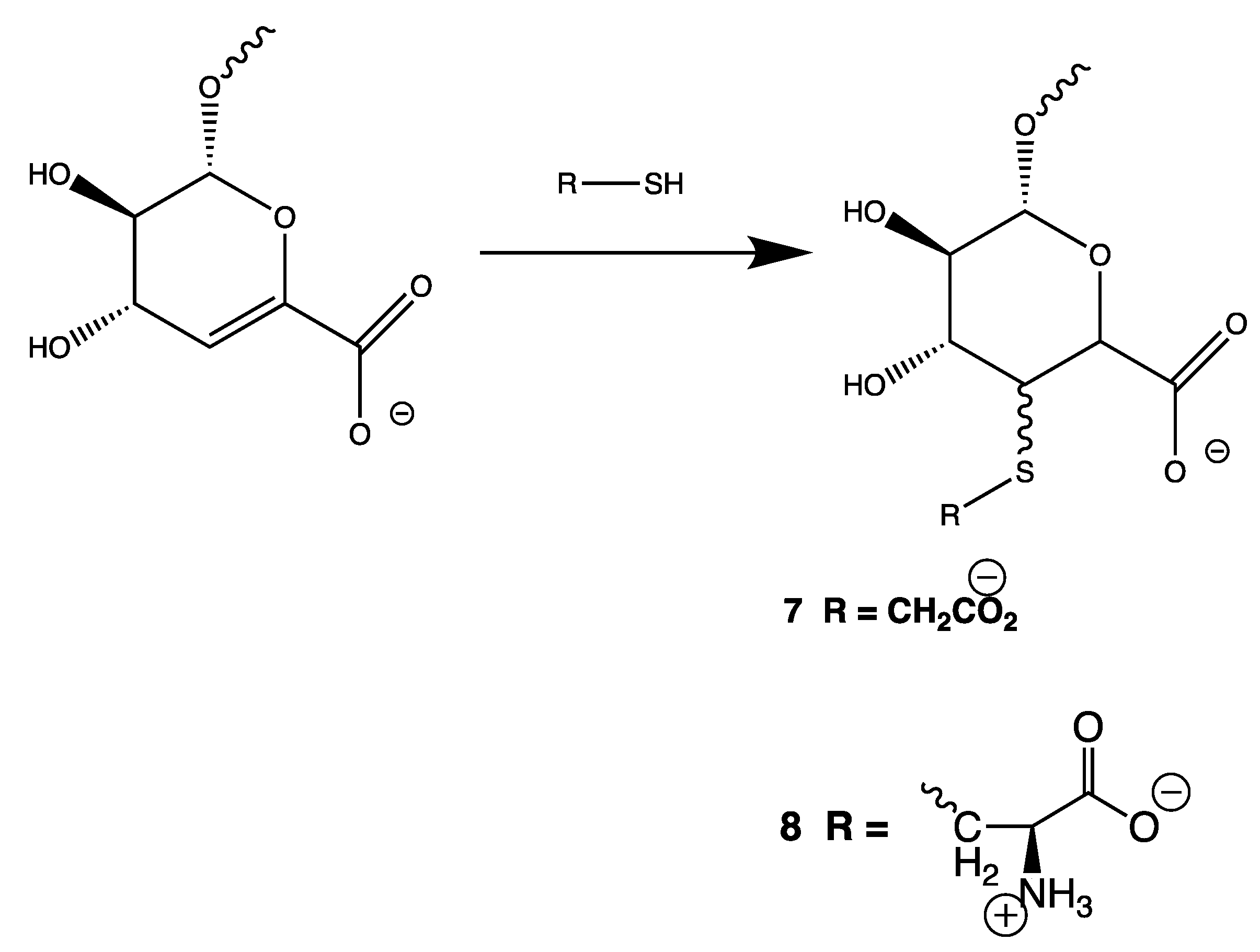
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stenzel, M.H. Glycopolymers for Drug Delivery: Opportunities and Challenges. Macromolecules 2022, 55, 4867–4890. [Google Scholar] [CrossRef]
- Drira, M.; Hentati, F.; Babich, O.; Sukhikh, S.; Larina, V.; Sharifian, S.; Homai, A.; Fendri, I.; Lemos, M.F.L.; Félix, C.; et al. Bioactive Carbohydrate Polymers—Between Myth and Reality. Molecules 2021, 26, 7068. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Poudel, D.P.; Taylor, R.T. Thiol-Ene Click-Inspired Late-Stage Modification of Long-Chain Polyurethane Dendrimers. Reactions 2022, 3, 12–29. [Google Scholar] [CrossRef]
- Hangyeol, C.; Myojeong, K.; Jaebong, J.; Sungwoo, H. Visible-Light-Induced Cysteine-Specific Bioconjugation: Biocompatible Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2020, 59, 22514–22522. [Google Scholar] [CrossRef]
- László, L.; Miklós, N.; Anikó, B.; Pál, H.; Miklós, Z.; Sándor, K. Conjugation of Bioactive Molecules to a Fluorescent Dithiomaleimide by Photoinduced and BEt3-Initiated Thio-Click Reactions. Eur. J. Org. Chem. 2015, 35, 7675–7681. [Google Scholar] [CrossRef]
- Neumann, K.; Conde-González, A.; Owens, M.; Venturato, A.; Zhang, Y.; Geng, J.; Bradley, M. An Approach to the High-Throughput Fabrication of Glycopolymer Microarrays through Thiol–Ene Chemistry. Macromolecules 2017, 50, 6026–6031. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, I.; Siskovic, R. Heparin derivatives prepared by modification of the uronic acid carboxyl groups. Thromb. Res. 1972, 1, 173–181. [Google Scholar] [CrossRef]
- Ingle, R.G.; Agarwal, A.S. A world of low molecular weight heparins (LMWHs) enoxaparin as a promising moiety—A review. Carbohydr. Polym. 2014, 106, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Hoppenstead, D.; Walenga, J.; Iqbal, O.; Ma, Q.; Jeske, W.; Sheikh, T. Pharmacodynamic and Pharmacokinetic Properties of Enoxaparin Implications for Clinical Practice. Clin. Pharmacokinet. 2003, 42, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. J. Virol. 2021, 95, e01987-20. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Elli, S.; Gaudesi, D.; Torri, G.; Casu, B.; Mourier, P.; Herman, F.; Boudier, C.; Lorenz, M.; Viskov, C. Effects on Molecular Conformation and Anticoagulant Activities of 1,6-Anhydrosugars at the Reducing Terminal of Antithrombin-Binding Octasaccharides Isolated from Low-Molecular-Weight Heparin Enoxaparin. J. Med. Chem. 2010, 53, 8030–8040. [Google Scholar] [CrossRef]
- Park, J.; Jeon, O.C.; Yun, J.; Nam, H.; Hwang, J.; Al-Hilal, T.A.; Kim, K.; Kim, K.; Byun, Y. End-Site-Specific Conjugation of Enoxaparin and Tetradeoxycholic Acid Using Nonenzymatic Glycosylation for Oral Delivery. J. Med. Chem. 2016, 59, 10520–10529. [Google Scholar] [CrossRef]
- Choubey, A.K.; Dora, C.P.; Bhatt, T.D.; Gill, M.S.; Suresh, S. Development and evaluation of PEGylated Enoxaparin: A novel approach for enhanced anti-Xa activity. Bioorg. Chem. 2014, 54, 1–6. [Google Scholar] [CrossRef]
- Masuko, S.; Higashi, K.; Wang, Z.; Bhaskar, U.; Hickey, A.M.; Zhang, F.; Toida, T.; Dordick, J.S.; Linhardt, R.J. Ozonolysis of the double bond of the unsaturated uronate residue in low-molecular-weight heparin and K5 heparosan. Carbohydr. Res. 2011, 346, 1962–1966. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C.; Wu, X.; Chen, Y. Efficient access to the non-reducing end of low molecular weight heparin for fluorescent labeling. Chem. Commun. 2014, 50, 7004–7006. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Postma, A.; Moad, G. Kinetics and mechanism for thermal and photochemical decomposition of 4,4′-azobis(4-cyanopentanoic acid) in aqueous media. Polym. Chem. 2019, 10, 3284–3287. [Google Scholar] [CrossRef]
- Cramer, N.B.; Scott, J.P.; Bowman, C.N. Photopolymerizations of Thiol-Ene Polymers without Photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.T.; Poudel, D.P. Thiol-Ene Reaction of Heparin Allyl Ester, Heparin 4-Vinylbenzyl Ester and Enoxaparin. Reactions 2022, 3, 442-450. https://doi.org/10.3390/reactions3030031
Taylor RT, Poudel DP. Thiol-Ene Reaction of Heparin Allyl Ester, Heparin 4-Vinylbenzyl Ester and Enoxaparin. Reactions. 2022; 3(3):442-450. https://doi.org/10.3390/reactions3030031
Chicago/Turabian StyleTaylor, Richard T., and Dhruba P. Poudel. 2022. "Thiol-Ene Reaction of Heparin Allyl Ester, Heparin 4-Vinylbenzyl Ester and Enoxaparin" Reactions 3, no. 3: 442-450. https://doi.org/10.3390/reactions3030031
APA StyleTaylor, R. T., & Poudel, D. P. (2022). Thiol-Ene Reaction of Heparin Allyl Ester, Heparin 4-Vinylbenzyl Ester and Enoxaparin. Reactions, 3(3), 442-450. https://doi.org/10.3390/reactions3030031





