Thermal Input/Concentration Output Systems Processed by Chemical Reactions of Helicene Oligomers
Abstract
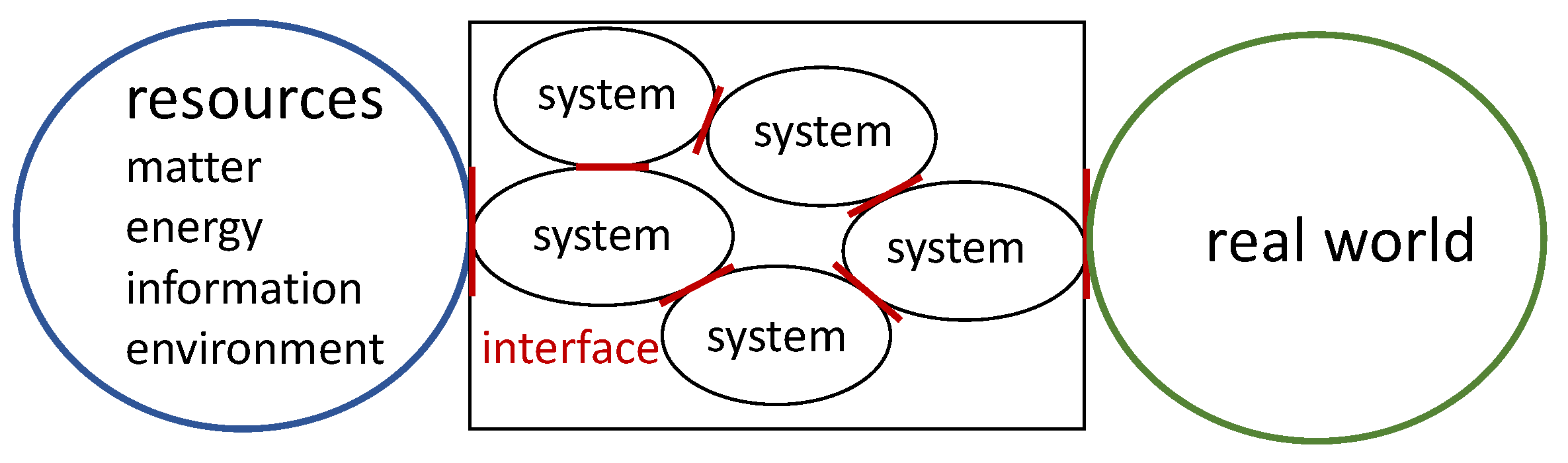
1. Input/Output Systems
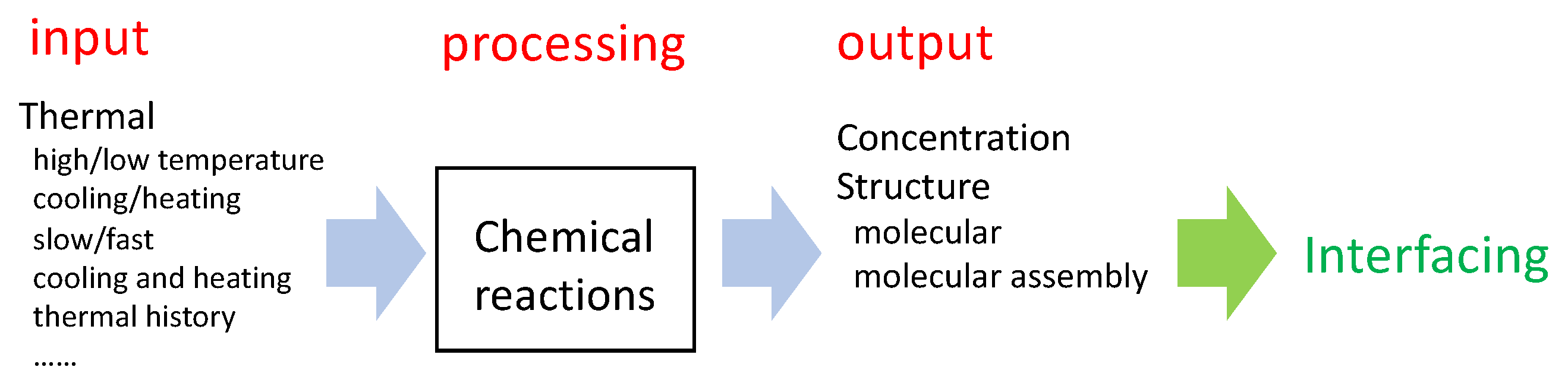
2. Thermal Input/Concentration Output Systems Processed by Chemical Reactions
2.1. Processing by Chemical Reactions
2.2. Temperature Dependency of Chemical Reactions
2.2.1. Chemical Equilibrium
2.2.2. Irreversible Chemical Reactions out of Chemical Equilibrium
3. Properties of Chemical Reactions for Thermal Input/Concentration Output Systems
3.1. Reversible Chemical Reactions out of Chemical Equilibrium
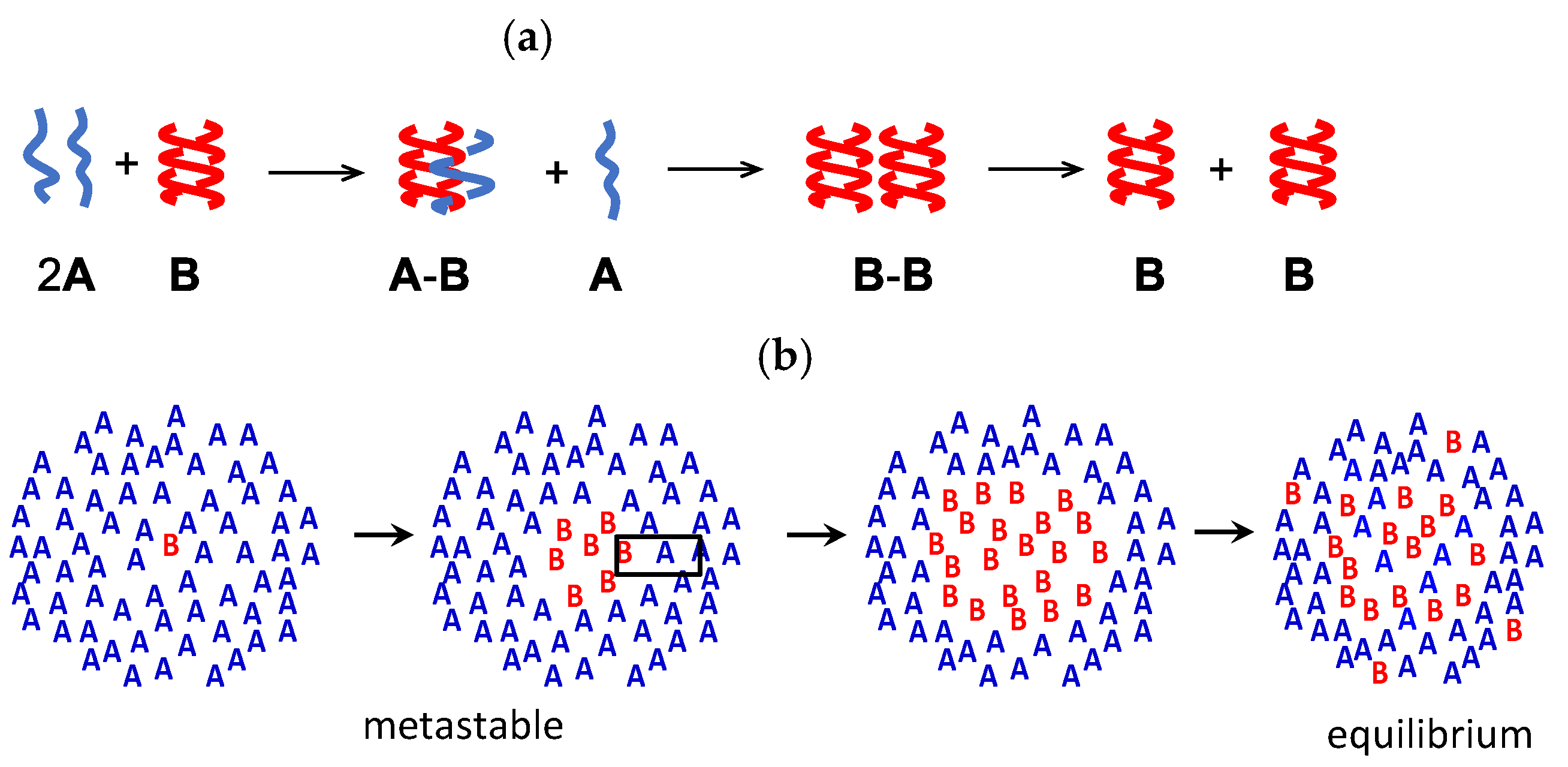
 B and distinguished from chemical equilibrium 2AB [29,30,31].
B and distinguished from chemical equilibrium 2AB [29,30,31].  B with different pathways during cooling and heating can occur in a closed system, which involves the addition and removal of thermal energy but not materials. Such reversible chemical reaction 2A
B with different pathways during cooling and heating can occur in a closed system, which involves the addition and removal of thermal energy but not materials. Such reversible chemical reaction 2A B might not be easy to consider. However, it can occur when the relative thermodynamic stability of 2A and B is inverted by cooling and heating and delay of the chemical reaction against the occurring temperature changes.
B might not be easy to consider. However, it can occur when the relative thermodynamic stability of 2A and B is inverted by cooling and heating and delay of the chemical reaction against the occurring temperature changes.  B in a closed system is described as follows. At a high temperature, 2A is thermodynamically stable, and dissociation reaction B→2A occurs, the process of which is out of chemical equilibrium when delay against the temperature changes is involved [32,33,34]. At a low temperature, the relative thermodynamic stability inverts, and B becomes thermodynamically stable. Then, association reaction 2A→B occurs with delay, which proceeds towards chemical equilibrium. The reversible chemical reaction 2A
B in a closed system is described as follows. At a high temperature, 2A is thermodynamically stable, and dissociation reaction B→2A occurs, the process of which is out of chemical equilibrium when delay against the temperature changes is involved [32,33,34]. At a low temperature, the relative thermodynamic stability inverts, and B becomes thermodynamically stable. Then, association reaction 2A→B occurs with delay, which proceeds towards chemical equilibrium. The reversible chemical reaction 2A B occurs in a different pathway in the formation of 2A and B by heating and cooling, respectively. Accordingly, two states appear at the same temperature, range which is bistability.
B occurs in a different pathway in the formation of 2A and B by heating and cooling, respectively. Accordingly, two states appear at the same temperature, range which is bistability. 3.2. Bistability by Thermal Hysteresis Involving Sigmoidal Relationship and Kinetics
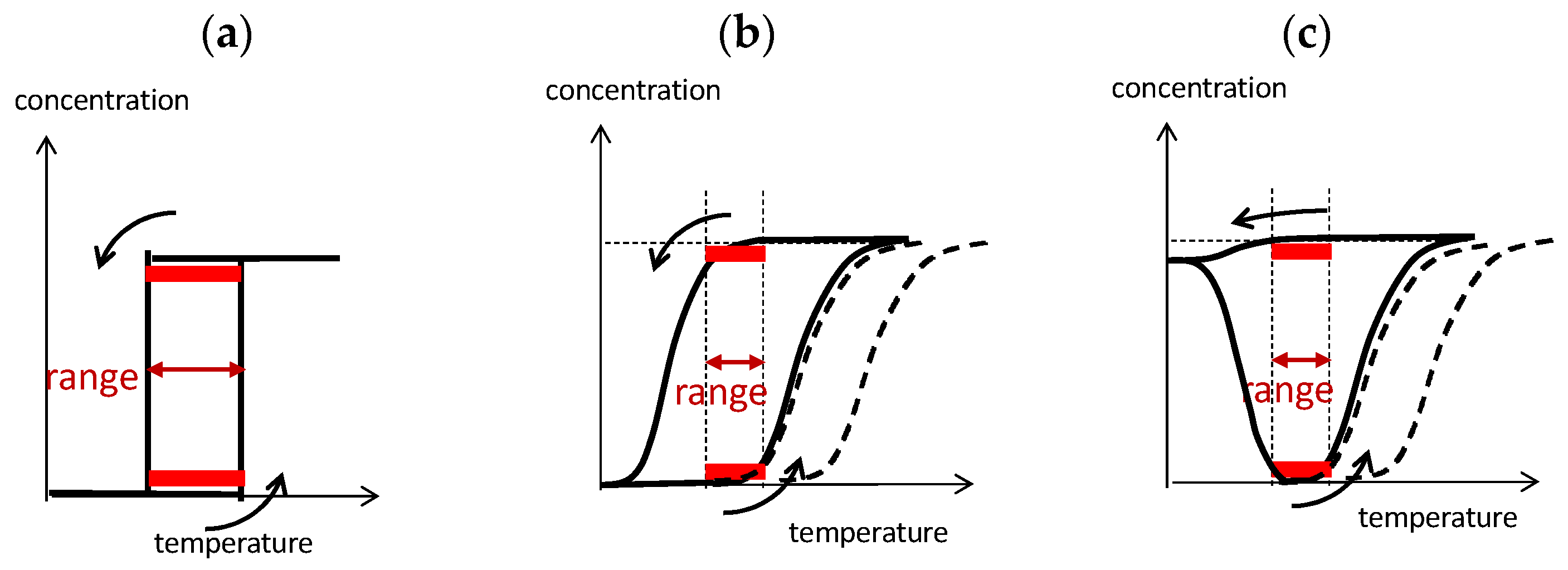
 B out of chemical equilibrium in a closed system. Here, the bistability is associated with thermal hysteresis involving sigmoidal relationship and kinetics. Such systems can convert various sophisticated thermal inputs into two different states of concentration outputs.
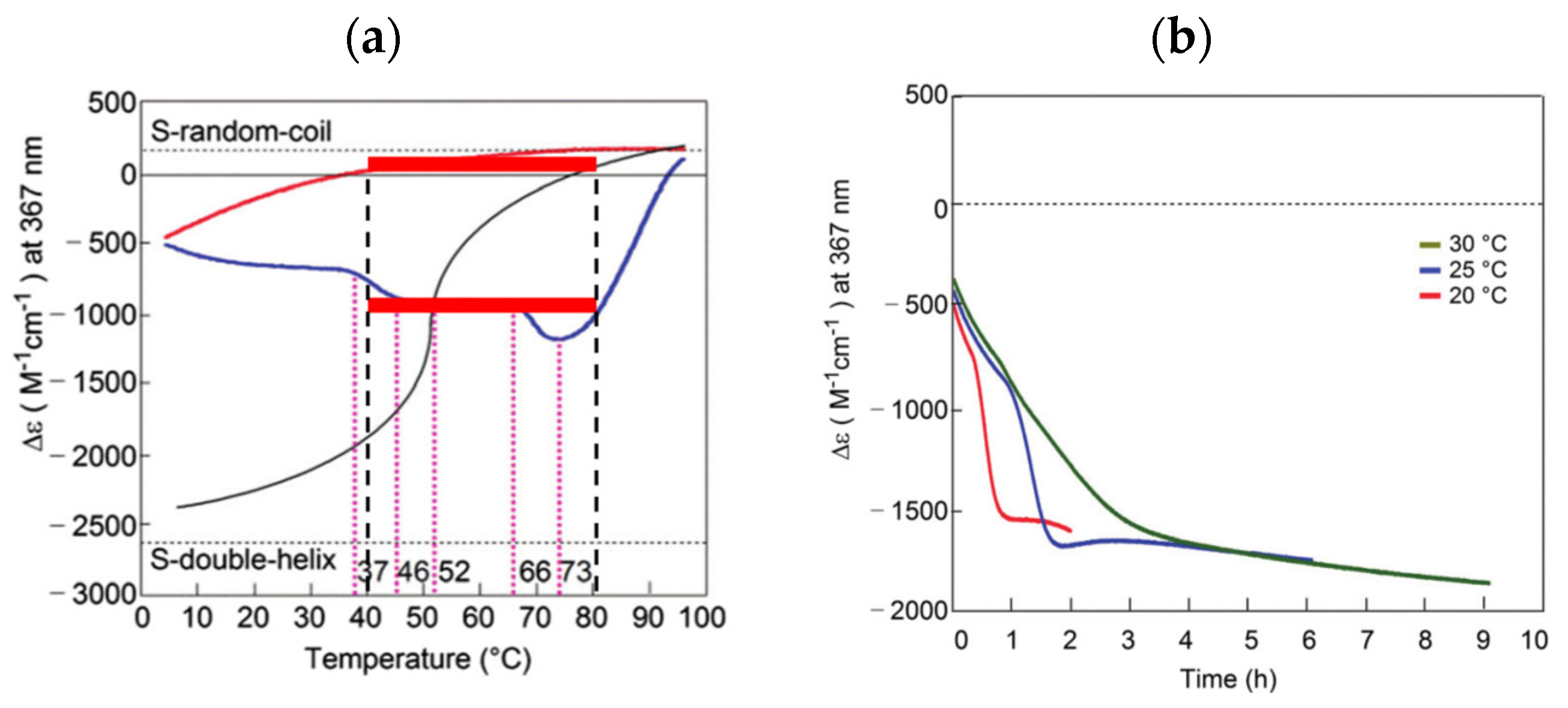
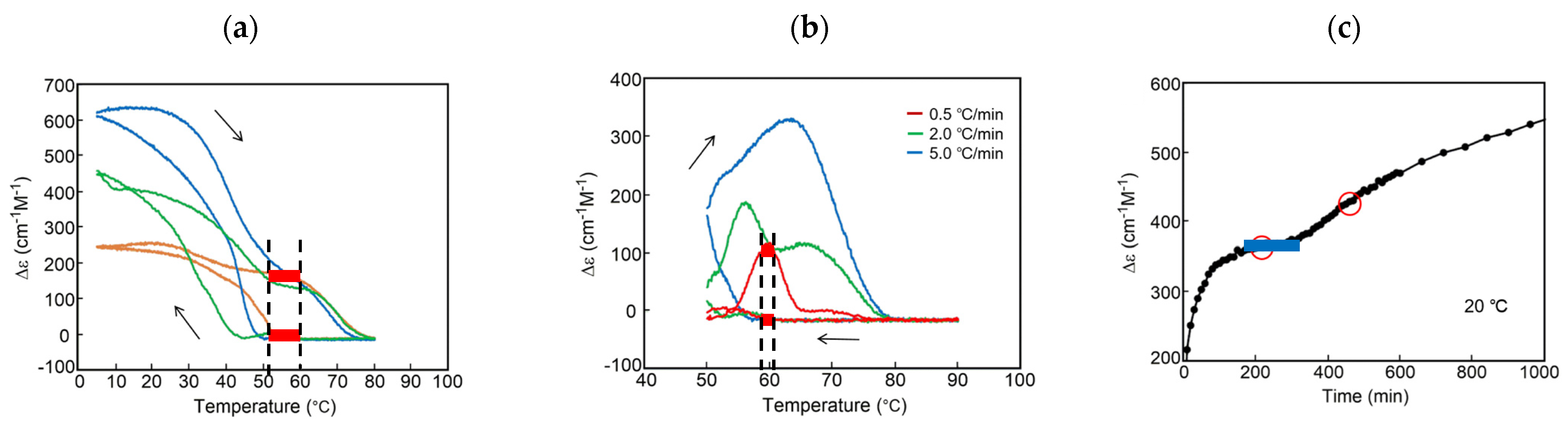
B out of chemical equilibrium in a closed system. Here, the bistability is associated with thermal hysteresis involving sigmoidal relationship and kinetics. Such systems can convert various sophisticated thermal inputs into two different states of concentration outputs.  B out of chemical equilibrium, which is expressed by two sigmoidal relationships during cooling and heating (Figure 6b). Note that two limit states at the same temperature range provide bistability (Figure 6b, red bold lines). Two sigmoidal curves exhibit bistability at the same temperature range during cooling and heating, which involves large differences in concentration and tolerances with stability against temperature perturbations. The temperature range of the bistability is a critical factor to be considered: A broad range exerts bistability in a broad temperature range, and a narrow range exerts bistability at a specific temperature. For the interfacing of the concentration output to other systems, large differences in concentrations are beneficial. Note that thermal hysteresis occurs by the delay of chemical reaction against temperature change and is a phenomenon out of chemical equilibrium (Figure 6b, compare dashed line).
B out of chemical equilibrium, which is expressed by two sigmoidal relationships during cooling and heating (Figure 6b). Note that two limit states at the same temperature range provide bistability (Figure 6b, red bold lines). Two sigmoidal curves exhibit bistability at the same temperature range during cooling and heating, which involves large differences in concentration and tolerances with stability against temperature perturbations. The temperature range of the bistability is a critical factor to be considered: A broad range exerts bistability in a broad temperature range, and a narrow range exerts bistability at a specific temperature. For the interfacing of the concentration output to other systems, large differences in concentrations are beneficial. Note that thermal hysteresis occurs by the delay of chemical reaction against temperature change and is a phenomenon out of chemical equilibrium (Figure 6b, compare dashed line).3.3. Positive Feedback by Self-Catalytic Chemical Reaction
3.4. Competitive Chemical Reactions
 2A
2A C, exhibit complex phenomena, especially when temperature changes are provided during the chemical reaction before reaching chemical equilibrium (Figure 4c). Depending on thermal inputs, complex increases and decreases in 2A, B, and C occur, which involve two or more metastable states. Note that metastable states can be beneficial for interfacing when the states have sufficiently long lifetime.
C, exhibit complex phenomena, especially when temperature changes are provided during the chemical reaction before reaching chemical equilibrium (Figure 4c). Depending on thermal inputs, complex increases and decreases in 2A, B, and C occur, which involve two or more metastable states. Note that metastable states can be beneficial for interfacing when the states have sufficiently long lifetime. B
B C occur in a closed system. When self-catalytic reactions 2A + B→2B and B + C→2C are involved, complex phenomena due to competitive acceleration occur.
C occur in a closed system. When self-catalytic reactions 2A + B→2B and B + C→2C are involved, complex phenomena due to competitive acceleration occur. 3.5. Fine-Tuning for Parallel Processing
4. Scope of This Article
5. Thermal Input/Concentration Output Systems Processed by the Chemical Reactions of Helicene Oligomers
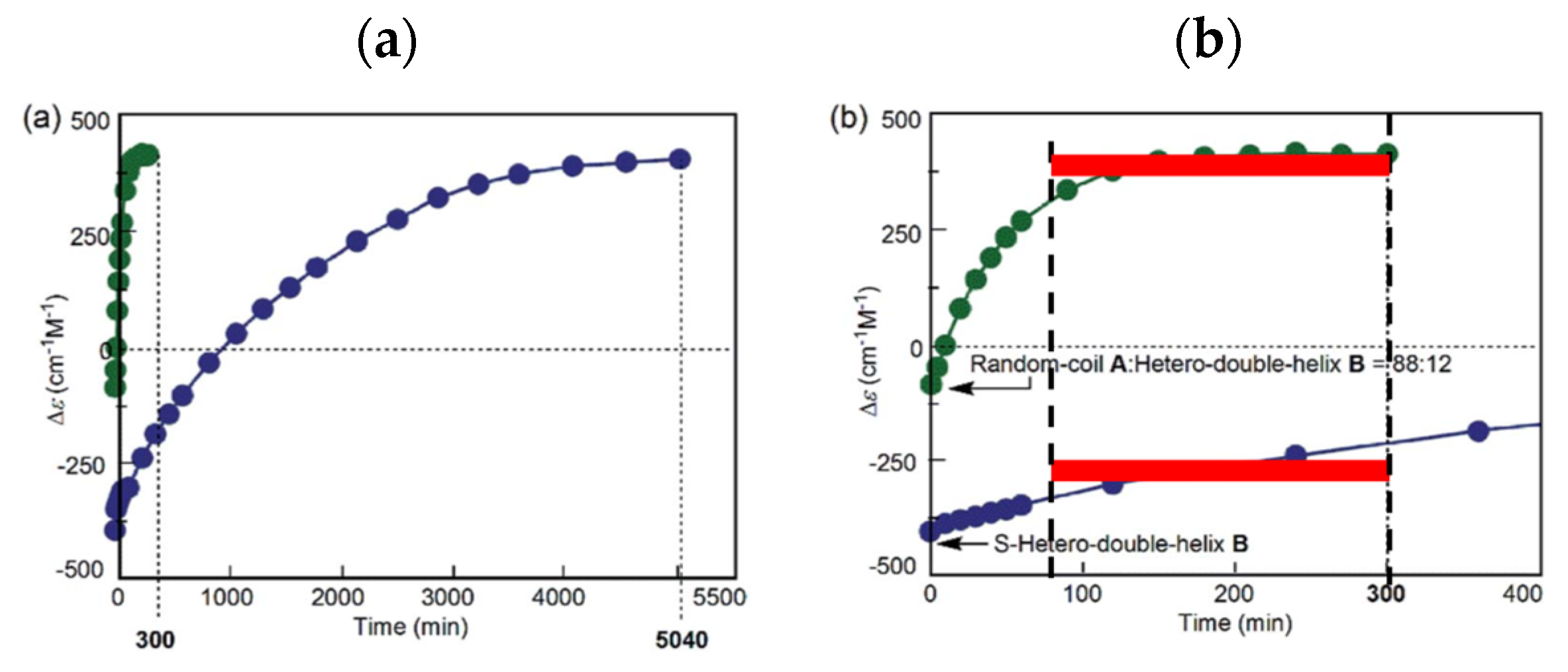
 B between the two oligomers of random coils 2A and a homo-double-helix B, and 2A and B form upon heating and cooling, respectively. These chemical reactions proceed out of chemical equilibrium in different pathways during heating and cooling, which is thermal hysteresis, while positive feedback by the self-catalytic reaction 2A + B→2B is involved and the bistability occurs at different concentrations of 2A and B at the same temperature. Outputs are shown by concentration/temperature and concentration/time profiles, in which Δε obtained by circular dichroism (CD) spectroscopy is considered an equivalent of concentration.
B between the two oligomers of random coils 2A and a homo-double-helix B, and 2A and B form upon heating and cooling, respectively. These chemical reactions proceed out of chemical equilibrium in different pathways during heating and cooling, which is thermal hysteresis, while positive feedback by the self-catalytic reaction 2A + B→2B is involved and the bistability occurs at different concentrations of 2A and B at the same temperature. Outputs are shown by concentration/temperature and concentration/time profiles, in which Δε obtained by circular dichroism (CD) spectroscopy is considered an equivalent of concentration. 2A
2A C, which involve two different structures of oligomers of random-coils 2A containing enantiomeric helicenes and two hetero-double-helices B and C with inverted right-handed and left-handed helix senses, are described as follows. Here, 2A and B/C form upon heating and cooling, respectively, positive feedback by competitive self-catalytic reactions 2A + B→2B and 2A + C→2C is involved and bistability among 2A/B, 2A/C, and B/C occurs at the same temperature.
C, which involve two different structures of oligomers of random-coils 2A containing enantiomeric helicenes and two hetero-double-helices B and C with inverted right-handed and left-handed helix senses, are described as follows. Here, 2A and B/C form upon heating and cooling, respectively, positive feedback by competitive self-catalytic reactions 2A + B→2B and 2A + C→2C is involved and bistability among 2A/B, 2A/C, and B/C occurs at the same temperature.5.1. High or Low Temperature States
5.2. Cooling or Heating States
5.2.1. Thermal Hysteresis
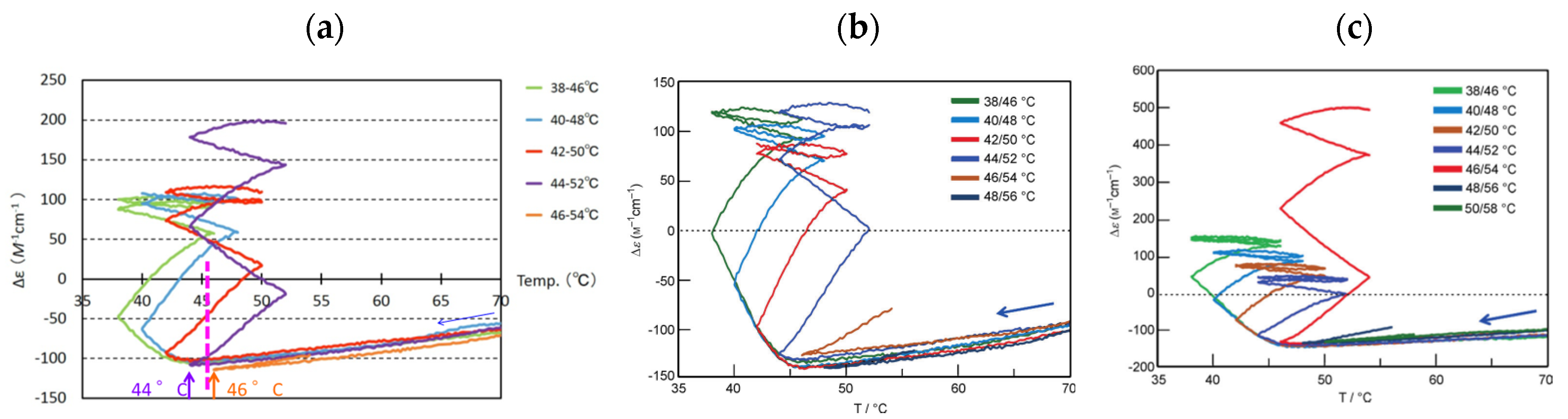
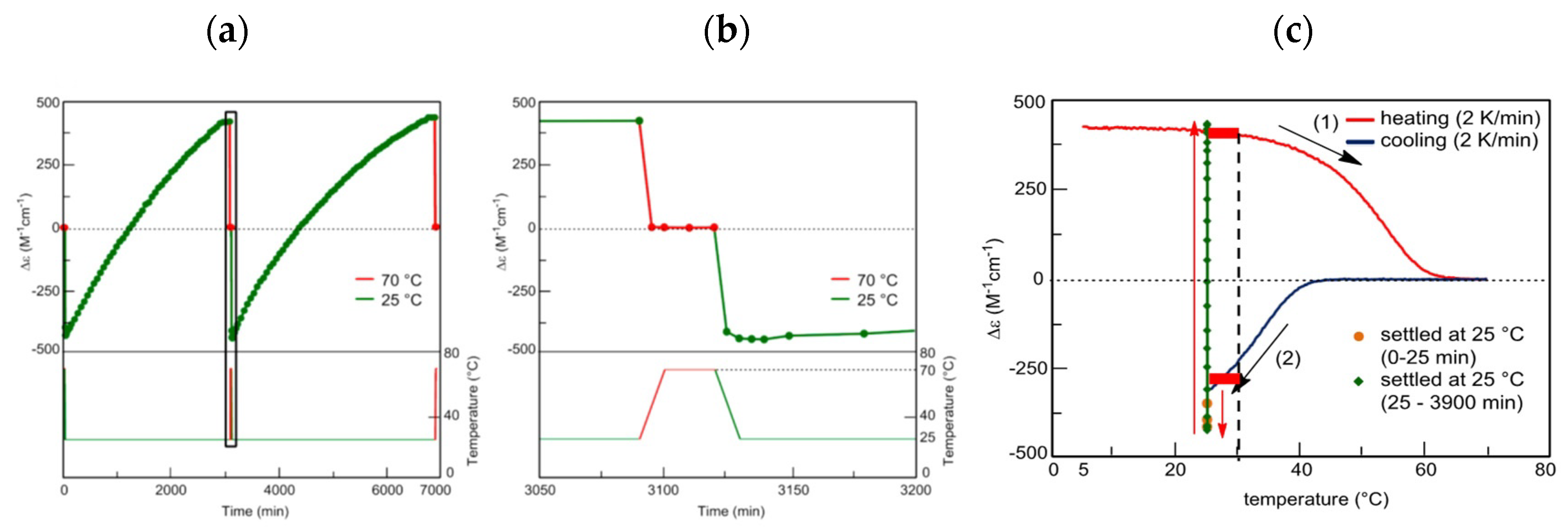
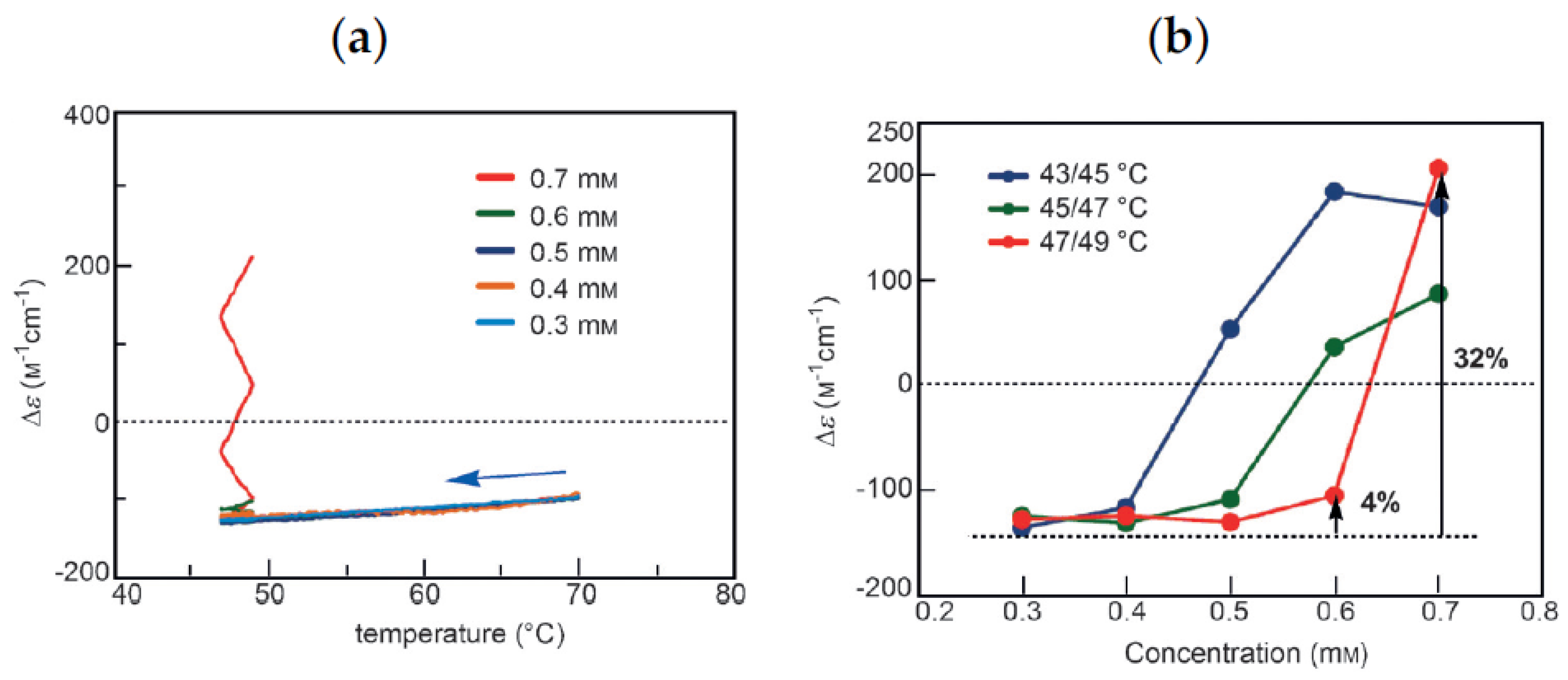
 B can be used in determining cooling or heating states. During cooling between 40 °C and 50 °C, Δε value of −140 cm−1 M−1 indicates fully dissociated 2A(S). During heating between 40 °C and 50 °C, Δε of approximately +400 cm−1 M−1 indicates the formation of approximately 50% B(A). Bistability with a considerable difference in the concentrations of 2A(S) and B(S) appears during cooling and heating between 40 °C and 50 °C (Figure 12a, red bold lines). In other words, when significant amounts of 2A(S) are formed between 40 °C and 50 °C, it shows the cooling state of (P)-1. When approximately 50% B(S) is formed, it shows the heating state. Both states are beyond chemical equilibrium (Figure 12a).
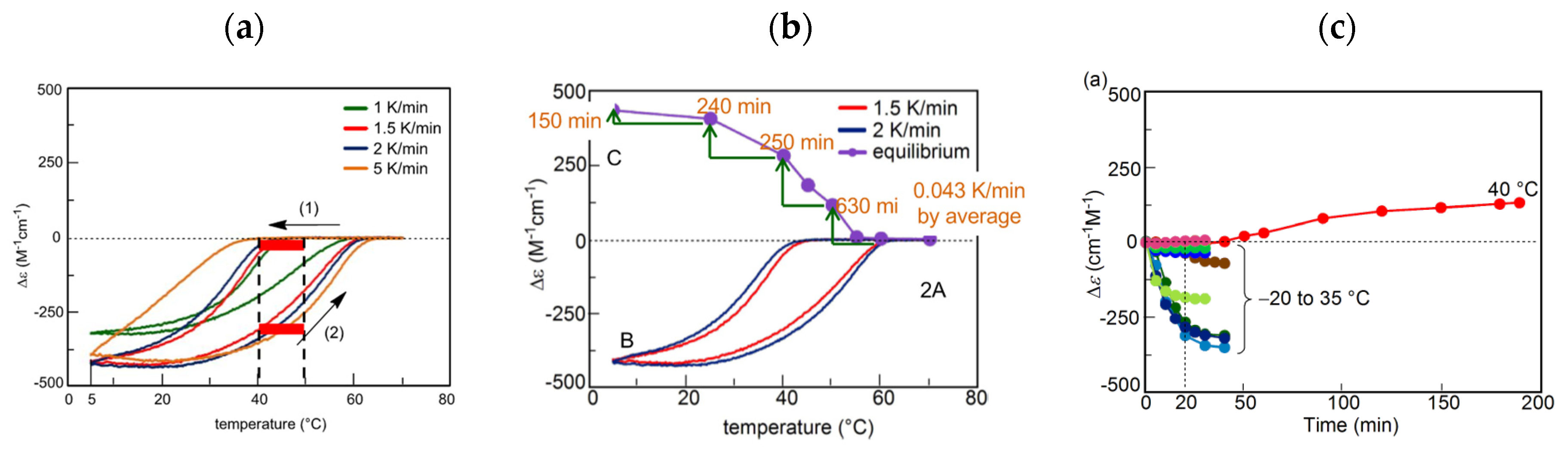
B can be used in determining cooling or heating states. During cooling between 40 °C and 50 °C, Δε value of −140 cm−1 M−1 indicates fully dissociated 2A(S). During heating between 40 °C and 50 °C, Δε of approximately +400 cm−1 M−1 indicates the formation of approximately 50% B(A). Bistability with a considerable difference in the concentrations of 2A(S) and B(S) appears during cooling and heating between 40 °C and 50 °C (Figure 12a, red bold lines). In other words, when significant amounts of 2A(S) are formed between 40 °C and 50 °C, it shows the cooling state of (P)-1. When approximately 50% B(S) is formed, it shows the heating state. Both states are beyond chemical equilibrium (Figure 12a). B. Much slower temperature change provides an identical cooling and heating curve, which coincides with the equilibrium curve.
B. Much slower temperature change provides an identical cooling and heating curve, which coincides with the equilibrium curve. 5.2.2. Equilibrium Crossing
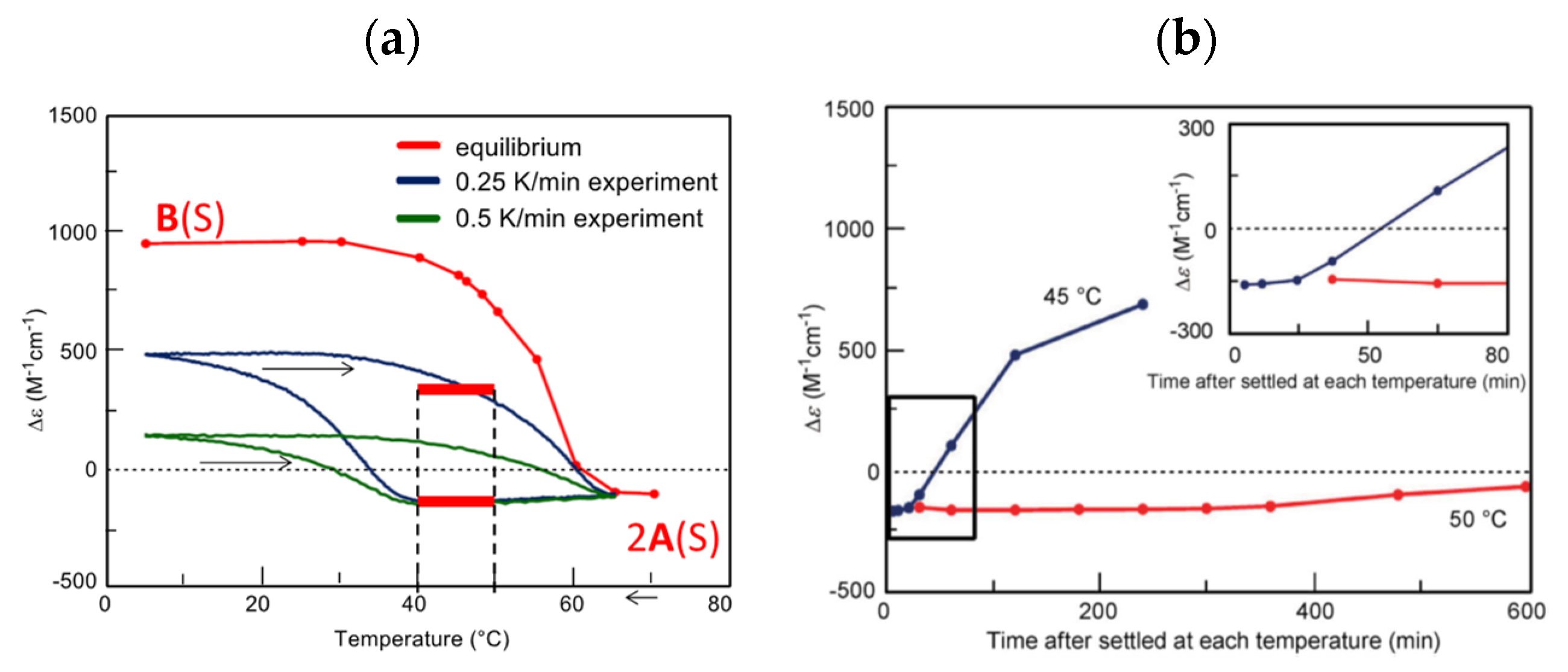
 B(E). Notably, heating increases B(E) (Figure 6c), which is due to the self-catalytic chemical reaction 2A(E) + B(E)→2B(E). Thus, the heating curve crosses the equilibrium curve upon heating, and an energetically uphill chemical reaction 2A(E)→B(E) occurs. The phenomenon is compared with (P)-1, by which self-catalytic reaction 2A(S) + B(S)→2B(S) occurs during cooling (Figure 6b and Figure 12a).
B(E). Notably, heating increases B(E) (Figure 6c), which is due to the self-catalytic chemical reaction 2A(E) + B(E)→2B(E). Thus, the heating curve crosses the equilibrium curve upon heating, and an energetically uphill chemical reaction 2A(E)→B(E) occurs. The phenomenon is compared with (P)-1, by which self-catalytic reaction 2A(S) + B(S)→2B(S) occurs during cooling (Figure 6b and Figure 12a). 5.2.3. Aggregation to Form Organogels and Vesicles
 B
B Bn, in which Bn indicates aggregates with ordered structures. Such systems provide concentration outputs by the reversible chemical reaction 2A
Bn, in which Bn indicates aggregates with ordered structures. Such systems provide concentration outputs by the reversible chemical reaction 2A B and subsequent reversible aggregation to form Bn, which exhibit thermal hysteresis.
B and subsequent reversible aggregation to form Bn, which exhibit thermal hysteresis. B(O)
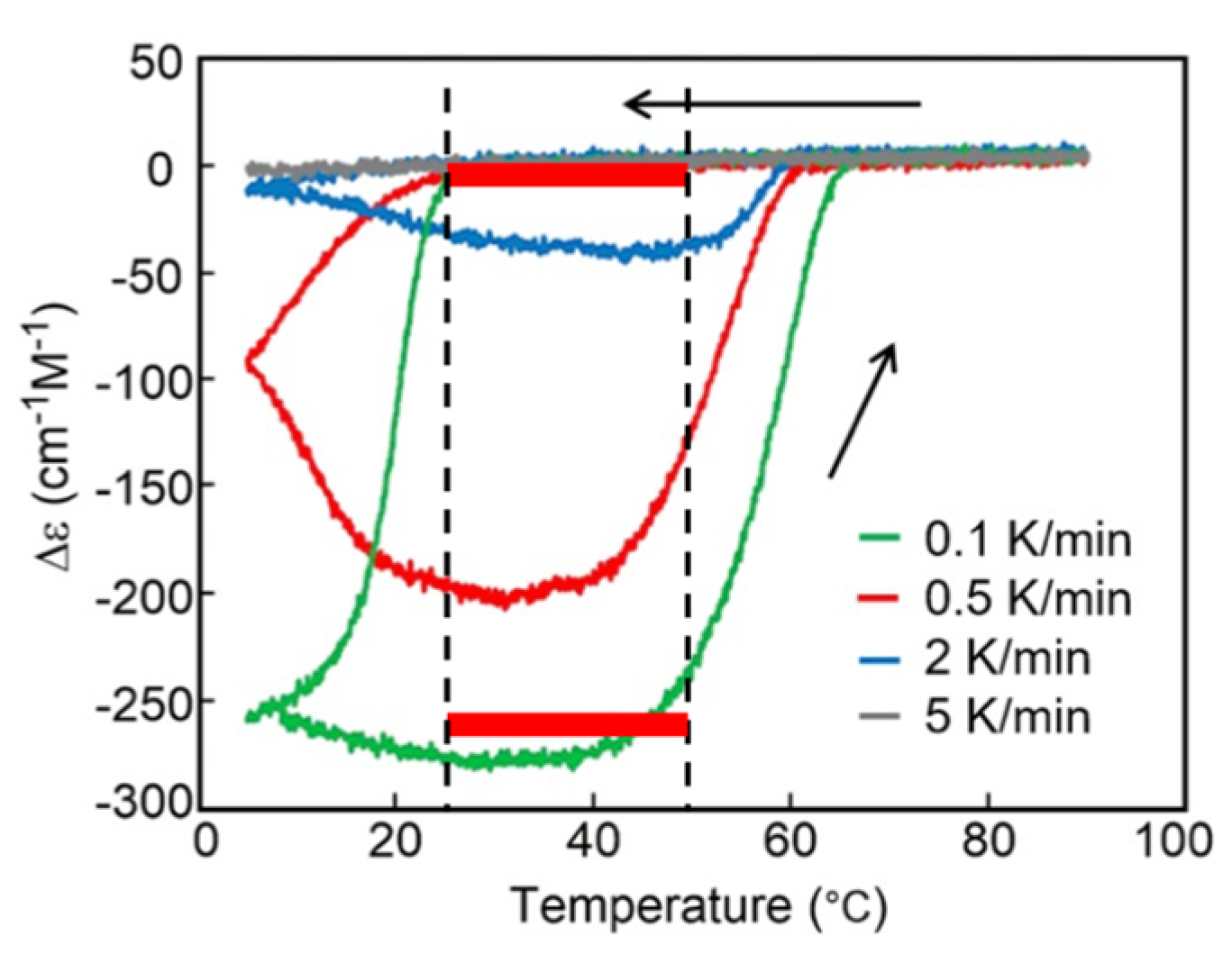
B(O) B(O)n, which is different from the modest increment in the decrease and increase of (P)-1 (Figure 12a). Bistability appears with large differences in the concentrations of B(O) between 25 °C and 50 °C at the same temperature range (Figure 6b). In addition, the three-dimensional molecular and molecular assembly structures of the organogels and concentric vesicles B(O)n are considerably different from those of 2A(O), which may be beneficial for interfacing. Thermal hysteresis does not appear at a rate of 5 K min−1 because the temperature change is much faster than the chemical reactions.
B(O)n, which is different from the modest increment in the decrease and increase of (P)-1 (Figure 12a). Bistability appears with large differences in the concentrations of B(O) between 25 °C and 50 °C at the same temperature range (Figure 6b). In addition, the three-dimensional molecular and molecular assembly structures of the organogels and concentric vesicles B(O)n are considerably different from those of 2A(O), which may be beneficial for interfacing. Thermal hysteresis does not appear at a rate of 5 K min−1 because the temperature change is much faster than the chemical reactions.5.2.4. Aggregation to Form Organogels with Stepwise Relationship and Kinetics
 B
B Bn provides different concentration outputs in the formation of organogels, exhibiting stepwise sigmoidal relationships and kinetics with an intermediate metastable state [63].
Bn provides different concentration outputs in the formation of organogels, exhibiting stepwise sigmoidal relationships and kinetics with an intermediate metastable state [63].  B(E)
B(E) B(E)n.
B(E)n.5.3. Fast or Slow Cooling States
 B(A)
B(A) C(A) involving two competitive self-catalytic reactions 2A(E) + B(E)→2B(E) and 2A(E) + C(E)→2C(E). At high temperatures, 2A(A) is thermodynamically the most stable. At low temperatures, C(A) is the most stable. B(A) is metastable at both temperatures. The thermal input/concentration output system exhibits complex bistability phenomena. The system provides either structured B(A) or C(A) as the major product at the same temperature because of the difference in temperature dependency among the competitive self-catalytic chemical reactions. Ordinary bimolecular competitive reactions do not generally switch major products through temperature changes unless the mechanisms change, as noted in Section 3.4.
C(A) involving two competitive self-catalytic reactions 2A(E) + B(E)→2B(E) and 2A(E) + C(E)→2C(E). At high temperatures, 2A(A) is thermodynamically the most stable. At low temperatures, C(A) is the most stable. B(A) is metastable at both temperatures. The thermal input/concentration output system exhibits complex bistability phenomena. The system provides either structured B(A) or C(A) as the major product at the same temperature because of the difference in temperature dependency among the competitive self-catalytic chemical reactions. Ordinary bimolecular competitive reactions do not generally switch major products through temperature changes unless the mechanisms change, as noted in Section 3.4.  B(A)
B(A) C(A) out of chemical equilibrium.
C(A) out of chemical equilibrium. B(A)
B(A) C(A) involving two competitive self-catalytic chemical reactions 2A(A) + C(A)→2C(A) and 2A(A) + B(A)→2B(A) can exhibit diverse bistability phenomena depending on thermal inputs (Figure 8). The thermal inputs described in this section include high or low temperature, cooling or heating states, and slow or fast cooling and heating states. The system can convert the analog thermal signals into digital signals of different concentrations of B(A) and C(A). The right-handed and left-handed helix senses of B(A) and C(A) with a large difference in the three-dimensional molecular structures may be beneficial for interfacing with other systems. Memory effect and future predicting also appear.
C(A) involving two competitive self-catalytic chemical reactions 2A(A) + C(A)→2C(A) and 2A(A) + B(A)→2B(A) can exhibit diverse bistability phenomena depending on thermal inputs (Figure 8). The thermal inputs described in this section include high or low temperature, cooling or heating states, and slow or fast cooling and heating states. The system can convert the analog thermal signals into digital signals of different concentrations of B(A) and C(A). The right-handed and left-handed helix senses of B(A) and C(A) with a large difference in the three-dimensional molecular structures may be beneficial for interfacing with other systems. Memory effect and future predicting also appear. 5.4. Cooling History
6. Concentration Threshold
7. Interfacing of Concentration Outputs with Other Input/Output Systems
7.1. Processing by Chemical Reactions and Effective Interfacing
7.2. Increasing the Efficiency of Interfacing
 B and receptor molecules or allosteric catalysts P in another system, which interacts with B and exert chemical reaction to form X and/or Y, should be considered. The reversible chemical reaction 2A
B and receptor molecules or allosteric catalysts P in another system, which interacts with B and exert chemical reaction to form X and/or Y, should be considered. The reversible chemical reaction 2A B occurs between unstructured random-coil 2A and structured homo-double-helix B. When the concentration of B is considerably larger than 2A in the output, interfacing through the selective binding of B with P over A can occur. Then, the resulting BP complex exerts chemical reaction that forms X and Y (Figure 21a). Such a phenomenon can be termed as an activation mode. A related binding phenomenon has been observed in nanoparticle precipitation, and helicene-grafted nanoparticles selectively remove B but not 2A from solution through molecular recognition and precipitation [68].
B occurs between unstructured random-coil 2A and structured homo-double-helix B. When the concentration of B is considerably larger than 2A in the output, interfacing through the selective binding of B with P over A can occur. Then, the resulting BP complex exerts chemical reaction that forms X and Y (Figure 21a). Such a phenomenon can be termed as an activation mode. A related binding phenomenon has been observed in nanoparticle precipitation, and helicene-grafted nanoparticles selectively remove B but not 2A from solution through molecular recognition and precipitation [68]. 2A
2A C is also considered, which facilitates the binding of structured B or C with P in other systems (Figure 21c). In such a case, the concentration differences between B and C can be enhanced by the selective binding of B over C. For instance, when binding constant K for P and B is 104 times larger than that of C, the apparent concentration difference can be increased 104 times, and chemical reaction by BP complex to form X and Y is predominant compared with that of the CP complex (activation mode).
C is also considered, which facilitates the binding of structured B or C with P in other systems (Figure 21c). In such a case, the concentration differences between B and C can be enhanced by the selective binding of B over C. For instance, when binding constant K for P and B is 104 times larger than that of C, the apparent concentration difference can be increased 104 times, and chemical reaction by BP complex to form X and Y is predominant compared with that of the CP complex (activation mode). 8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Boell, S.; Cecez-Kecmanov, D. Conceptualizing Information Systems: From Input-Processing-Output Devices to Sociomaterial Apparatuses. ECIS 2012 Proc. 2012. Paper 20. Available online: http://aisel.aisnet.org/ecis2012/20 (accessed on 1 November 2021).
- Input/Output. Available online: https://en.wikipedia.org/wiki/Input/output (accessed on 1 November 2021).
- Input Output Model; Six Sigma Daily. Available online: https://www.sixsigmadaily.com/input-output-model/ (accessed on 1 November 2021).
- Input Interfacing Circuits; Electronic Tutorials. Available online: https://www.electronics-tutorials.ws/io/input-interfacing-circuits.html (accessed on 1 November 2021).
- Input Output Interface. Available online: https://www.sciencedirect.com/topics/engineering/input-output-interface (accessed on 1 November 2021).
- Introduction to Input-Output Interface; GeeksforGeeks. Available online: https://www.geeksforgeeks.org/introduction-to-input-output-interface/ (accessed on 1 November 2021).
- Electronic Systems. Available online: https://www.electronics-tutorials.ws/systems/electronic-system.html (accessed on 1 November 2021).
- Digital I/O and Analog I/O; Electrical Classroom. Available online: https://www.electricalclassroom.com/digital-i-o-and-analog-i-o/ (accessed on 1 November 2021).
- Digital Signal Processing. Available online: https://en.wikipedia.org/wiki/Digital_signal_processing (accessed on 1 November 2021).
- Analog Signal Processing. Available online: https://en.wikipedia.org/wiki/Analog_signal_processing (accessed on 1 November 2021).
- Electronic Circuit. Available online: https://en.wikipedia.org/wiki/Electronic_circuit (accessed on 1 November 2021).
- Zwass, V. Information System; Encyclopedia Britannica. Available online: https://www.britannica.com/topic/information-system (accessed on 1 November 2021).
- Data Processing; Encyclopedia Britannica. Available online: https://www.britannica.com/technology/data-processing (accessed on 1 November 2021).
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Amon, A.; Martin, K.C. Molecular Cell Biology, 8th ed.; W.H. Freeman & Company: New York, NY, USA, 2016. [Google Scholar]
- Signal Transduction. Available online: https://en.wikipedia.org/wiki/Signal_transduction (accessed on 1 November 2021).
- Otero-Muras, I.; Banga, J.R.; Alonso, A.A. Characterizing multistationarity regimes in biochemical reaction networks. PLoS ONE 2012, 7, e39194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrell, J.E., Jr.; Xiong, W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos 2001, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Rubens, J.R.; Sarpeshkar, R.; Lu, T.K. Synthetic analog computation in living cells. Nature 2013, 497, 619–624. [Google Scholar] [CrossRef]
- Bradley, R.W.; Wang, B. Designer cell signal processing circuits for biotechnology. New Biotechnol. 2015, 32, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Sarpeshkar, R. Analog synthetic biology. Phil. Trans. Royal. Soc. A 2014, 372, 20130110. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.J.; Chen, K.C.; Novak, B. Sniffers, buzzers, toggles and blinkers: Dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 2003, 15, 221–231. [Google Scholar] [CrossRef]
- Signal Processing. Available online: https://en.wikipedia.org/wiki/Signal_processing (accessed on 1 November 2021).
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience: Exploring the Brain, 4th ed.; Jones & Bartlett Learning, LLC: Burlington, VT, USA, 2016. [Google Scholar]
- Systems Biology. Available online: https://en.wikipedia.org/wiki/Systems_biology (accessed on 1 November 2021).
- Systems Biology as Defined by NIH; National Institutes of Health Webpage. Available online: https://irp.nih.gov/catalyst/v19i6/systems-biology-as-defined-by-nih (accessed on 1 November 2021).
- Cosentino, C.; Bates, D. Feedback Control in Systems Biology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Angeli, D. A Tutorial on Chemical Reaction Network Dynamics. Eur. J. Control. 2009, 3–4, 398–406. [Google Scholar] [CrossRef]
- Afroz, A.; Beisel, C.L. Understanding and exploiting feedback in synthetic biology. Chem. Eng. Sci. 2013, 103, 79–90. [Google Scholar] [CrossRef]
- Yamaguchi, M. Thermal hysteresis involving reversible self-catalytic reactions. Acc. Chem. Res. 2021, 54, 2603–2613. [Google Scholar] [CrossRef]
- Shigeno, M.; Kushida, Y.; Yamaguchi, M. Molecular switching involving metastable states: Molecular thermal hysteresis and sensing of environmental changes by chiral helicene oligomeric foldamers. Chem. Commun. 2016, 52, 4955–4970. [Google Scholar] [CrossRef] [PubMed]
- Shigeno, M.; Kushida, Y.; Yamaguchi, M. Energy aspects of thermal molecular switching: Molecular thermal hysteresis of helicene oligomers. Chem. Phys. Chem. 2015, 16, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.D.; Bernstein, R.B. Molecular Reaction Dynamics; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wright, M.R. Fundamental Chemical Kinetics; Horwood Publishing Limited: Woodgate, UK, 1999. [Google Scholar]
- Steinfeld, J.I.; Francisco, J.S.; Hase, W.L. Chemical Kinetics and Dynamics; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1989. [Google Scholar]
- Atkins, P.; de Paula, J. Physical Chemistry, 10th ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Várhegyi, G. Empirical models with constant and variable activation energy for biomass pyrolysis. Energy Fuels 2019, 33, 2348–2358. [Google Scholar] [CrossRef]
- Smith, I.W.M. The temperature-dependence of elementary reaction rates: Beyond Arrhenius. Chem. Soc. Rev. 2008, 37, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Silva, V.H.; Coutinho, N.D.; Aquilanti, V. Temperature dependence of rate processes beyond Arrhenius and Eyring: Activation and transitivity. Front. Chem. 2019, 7, 380. [Google Scholar] [CrossRef]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Bistability. Available online: https://en.wikipedia.org/wiki/Bistability (accessed on 1 November 2021).
- Wilhelm, T. A definition of bistability in systems biology. BMC Syst. Biol. 2009, 3, 90. [Google Scholar]
- Heaviside Step Function. Available online: https://en.wikipedia.org/wiki/Heaviside_step_function (accessed on 1 November 2021).
- Sigmoid Function. Available online: https://en.wikipedia.org/wiki/Sigmoid_function (accessed on 1 November 2021).
- Ueno, H.; Tsuruyama, T.; Nowakowski, B.; Gorecki, J.; Yoshikawa, K. Discrimination of time-dependent inflow properties with a cooperative dynamical system. Chaos 2015, 25, 103115. [Google Scholar] [CrossRef]
- Frank, S.A. Input-output relations in biological systems: Measurement, information and the Hill equation. Biol. Direct 2013, 8, 31. [Google Scholar] [CrossRef]
- Qian, H. Cooperativity in Cellular Biochemical Processes. Ann. Rev. Biophys. 2012, 41, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Positive Feedback; Biology Dictionary. Available online: https://biologydictionary.net/positive-feedback/ (accessed on 1 November 2021).
- Feedback Control in Biological Interactions; MCB111: Mathematics in Biology (Fall 2021). Available online: http://mcb111.org/w11/w11-lecture.html (accessed on 1 November 2021).
- Zeron, E.S. Positive and negative feedback in engineering and biology. Math. Model. Nat. Phenom. 2008, 3, 67–84. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Sawato, T.; Saito, N.; Yamaguchi, M. Chemical systems involving two competitive self-catalytic reactions. ACS Omega 2019, 4, 5879–5899. [Google Scholar] [CrossRef] [PubMed]
- Sawato, T.; Yamaguchi, M. Synthetic chemical systems involving self-catalytic reactions of helicene oligomer foldamers. Chem. Plus Chem. 2020, 85, 2017–2038. [Google Scholar] [CrossRef]
- Rovelli, C. Memory and entropy. arXiv 2020, arXiv:2003.06687. Available online: https://arxiv.org/abs/2003.06687 (accessed on 1 November 2021).
- Nakasone, Y.; Ono, T.; Ishii, A.; Masuda, S.; Terazima, M. Temperature-sensitive reaction of a photosensor protein YcgF: Possibility of a role of temperature sensor. Biochemistry 2010, 49, 2288–2296. [Google Scholar] [CrossRef]
- Enoki, S.; Watanabe, R.; Iino, R.; Noji, H. Single-molecule study on the temperature-sensitive reaction of F1-ATPase with a hybrid F1 carrying a single b(E190D). J. Biol. Chem. 2009, 284, 23169–23176. [Google Scholar] [CrossRef]
- García-Ávila, M.; Islas, L.D. What is new about mild temperature sensing? A review of recent findings. Temperature 2019, 6, 132–141. [Google Scholar] [CrossRef]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. TRP ion channels and temperature sensation. Ann. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Shigeno, M.; Kushida, Y.; Kobayashi, Y.; Yamaguchi, M. Molecular function of counting the numbers 1 and 2 exhibited by a sulfoneamidohelicene tetramer. Chem. Eur. J. 2014, 20, 12759–12762. [Google Scholar] [CrossRef]
- Shigeno, M.; Kushida, Y.; Yamaguchi, M. Molecular thermal hysteresis in helix dimer formation of sulfonamidohelicene oligomer in solution. Chem. Eur. J. 2013, 19, 10226–10234. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, M.; Yagi, A.; Shigeno, M.; Yamaguchi, M. Equilibrium crossing exhibited by an ethynylhelicene (M)-nonamer during random-coil- to-double-helix thermal transition in solution. Chem. Commun. 2014, 50, 14447–14450. [Google Scholar] [CrossRef] [PubMed]
- Sawato, T.; Arisawa, M.; Yamaguchi, M. Reversible formation of self-assembly gels containing giant vesicles in trifluoromethylbenzene using oxymethylenehelicene oligomers with terminal C16 alkyl groups. Bull. Chem. Soc. Jpn. 2020, 93, 1497–1503. [Google Scholar] [CrossRef]
- Auer, S.; Kashchiev, D. Insight into the correlation between lag time and aggregation rate in the kinetics of protein aggregation. Proteins 2010, 78, 2412–2416. [Google Scholar] [CrossRef]
- Sawato, T.; Masahiko Yamaguchi, M. Sequential self-catalytic reactions in the formation of hetero-double-helix and their self-assembled gels by pseudoenantiomer mixtures of ethynylhelicene oligomers. Chirality 2020, 32, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Shigeno, M.; Kushida, Y.; Yamaguchi, M. Heating/cooling stimulus induces three-state molecular switching of pseudoenantiomeric aminomethylenehelicene oligomers: Reversible nonequilibrium thermodynamic processes. J. Am. Chem. Soc. 2014, 136, 7972–7980. [Google Scholar] [CrossRef]
- Kushida, Y.; Saito, N.; Shigeno, M.; Yamaguchi, M. Multiple competing pathways for chemical reaction: Drastic reaction shortcut for the self-catalytic double-helix formation of helicene oligomers. Chem. Sci. 2017, 8, 1414–1421. [Google Scholar] [CrossRef]
- Kushida, Y.; Shigeno, M.; Yamaguchi, M. Concentration threshold and amplification exhibited by a helicene oligomer during helix-dimer formation: A proposal on how a cell senses concentration changes of a chemical. Chem. Eur. J. 2015, 21, 13788–13792. [Google Scholar] [CrossRef]
- Miyagawa, M.; Yamaguchi, M. Material clocking by silica nanoparticle precipitation in solution phase that is tunable by organic molecules. ChemPlusChem 2015, 80, 1502–1507. [Google Scholar] [CrossRef]
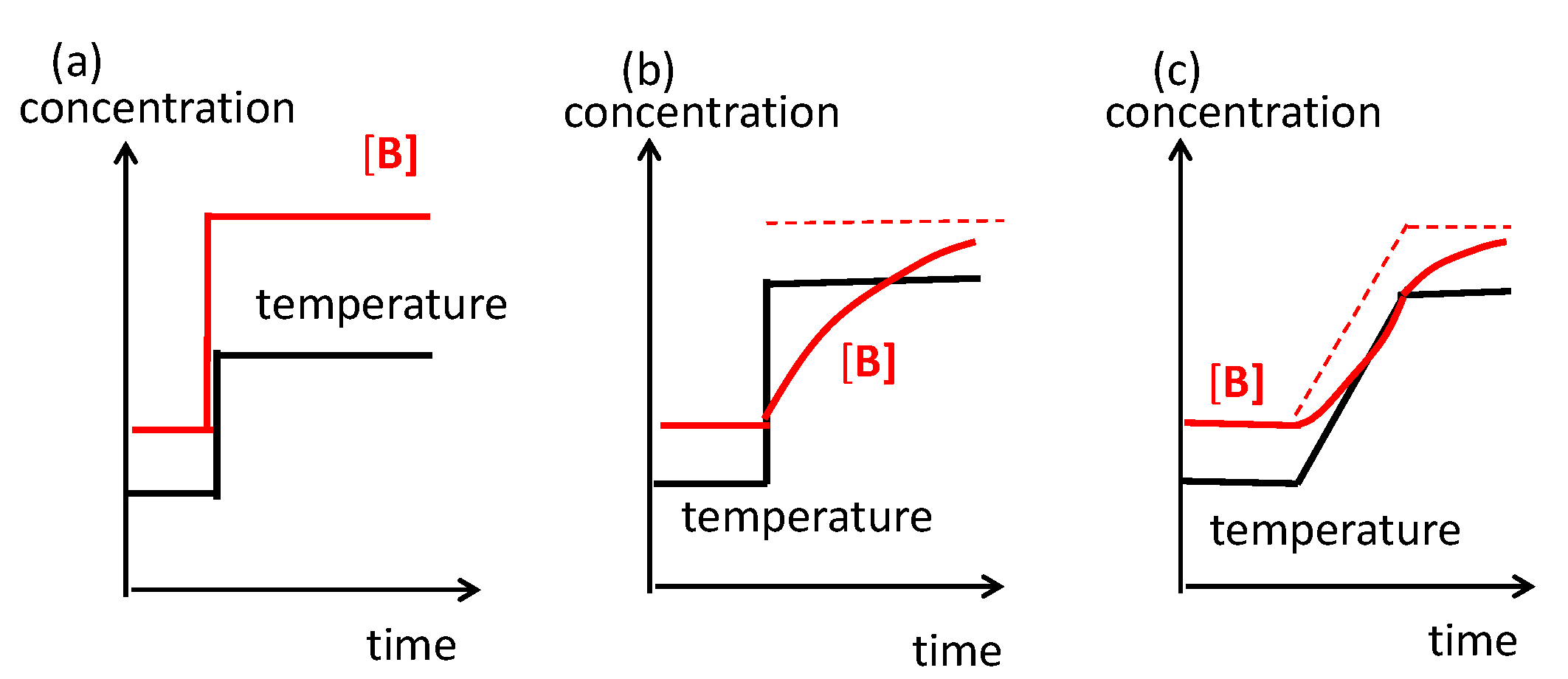
 B by different pathways, in which external energy is supplied to regenerate 2A from B. (c) Conditions are changed before the completion of reversible chemical reaction 2A
B by different pathways, in which external energy is supplied to regenerate 2A from B. (c) Conditions are changed before the completion of reversible chemical reaction 2A B, which inverts the direction.
B, which inverts the direction.
 B by different pathways, in which external energy is supplied to regenerate 2A from B. (c) Conditions are changed before the completion of reversible chemical reaction 2A
B by different pathways, in which external energy is supplied to regenerate 2A from B. (c) Conditions are changed before the completion of reversible chemical reaction 2A B, which inverts the direction.
B, which inverts the direction.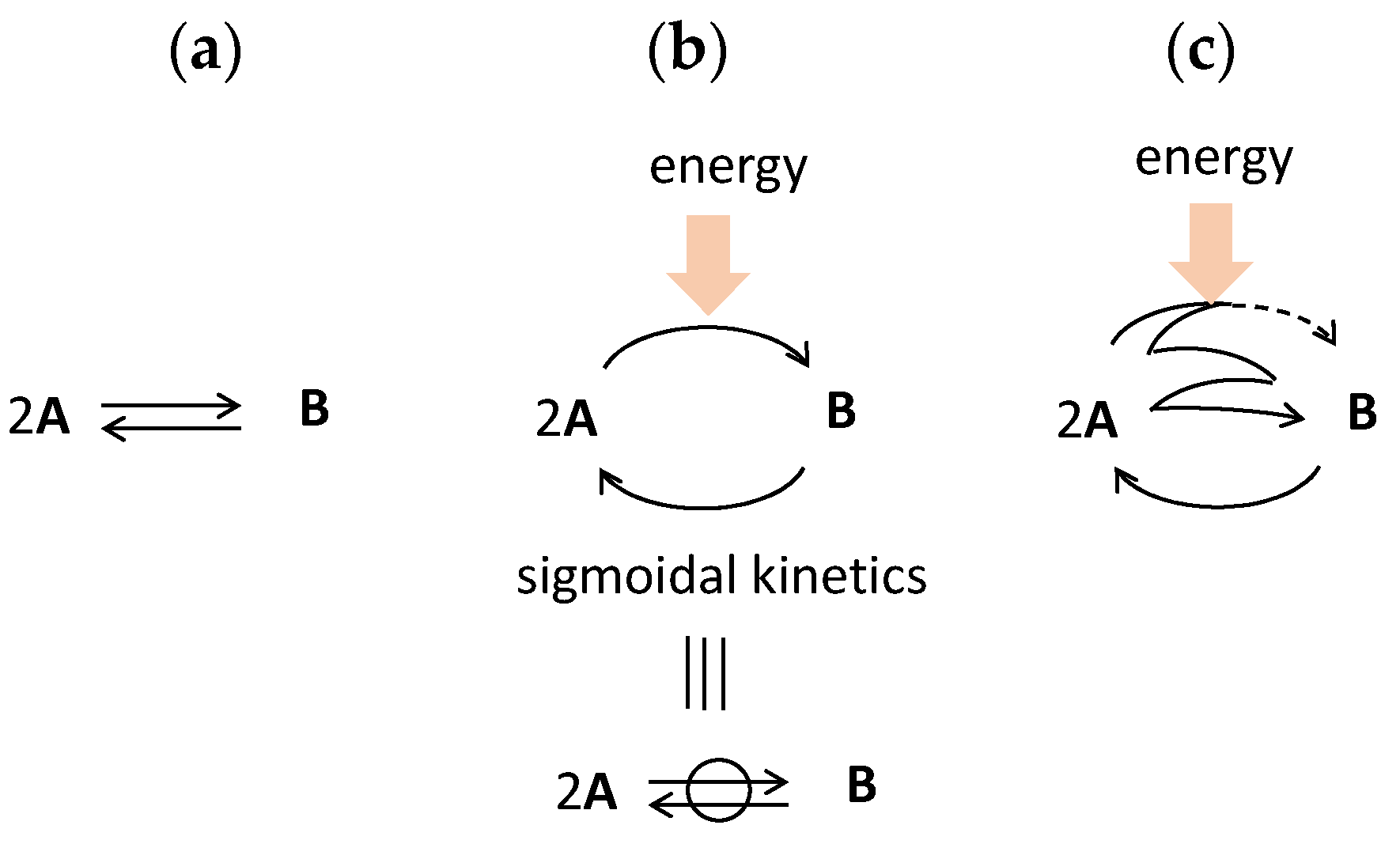

 2A
2A C involving self-catalytic chemical reactions 2A + B→2B and 2A + C→2C (red square), which are compared with chemical reaction 2A→B.
C involving self-catalytic chemical reactions 2A + B→2B and 2A + C→2C (red square), which are compared with chemical reaction 2A→B.
 2A
2A C involving self-catalytic chemical reactions 2A + B→2B and 2A + C→2C (red square), which are compared with chemical reaction 2A→B.
C involving self-catalytic chemical reactions 2A + B→2B and 2A + C→2C (red square), which are compared with chemical reaction 2A→B.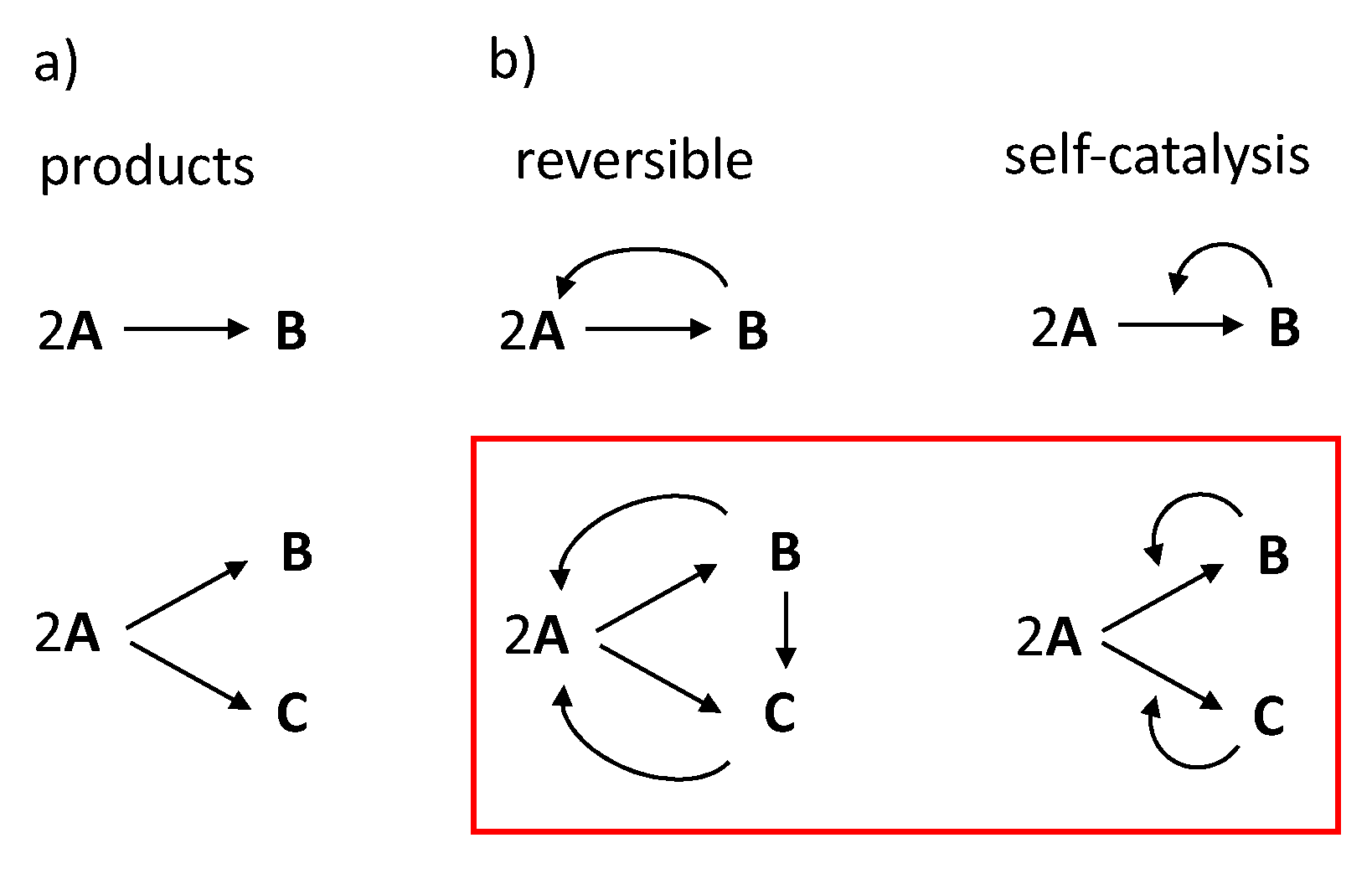



 B, in which 2A is unstructured random-coil and B is structured homo-double-helix. (a) Activation mode and (b) deactivation mode in the binding of B over A with a receptor or allosteric catalyst P, which exerts the formation of X and/or Y. (c) Interfacing of output derived from reversible competitive chemical reactions B
B, in which 2A is unstructured random-coil and B is structured homo-double-helix. (a) Activation mode and (b) deactivation mode in the binding of B over A with a receptor or allosteric catalyst P, which exerts the formation of X and/or Y. (c) Interfacing of output derived from reversible competitive chemical reactions B 2A
2A C by activation mode. Orange figure indicates active P and blue inactive P.
C by activation mode. Orange figure indicates active P and blue inactive P.
 B, in which 2A is unstructured random-coil and B is structured homo-double-helix. (a) Activation mode and (b) deactivation mode in the binding of B over A with a receptor or allosteric catalyst P, which exerts the formation of X and/or Y. (c) Interfacing of output derived from reversible competitive chemical reactions B
B, in which 2A is unstructured random-coil and B is structured homo-double-helix. (a) Activation mode and (b) deactivation mode in the binding of B over A with a receptor or allosteric catalyst P, which exerts the formation of X and/or Y. (c) Interfacing of output derived from reversible competitive chemical reactions B 2A
2A C by activation mode. Orange figure indicates active P and blue inactive P.
C by activation mode. Orange figure indicates active P and blue inactive P.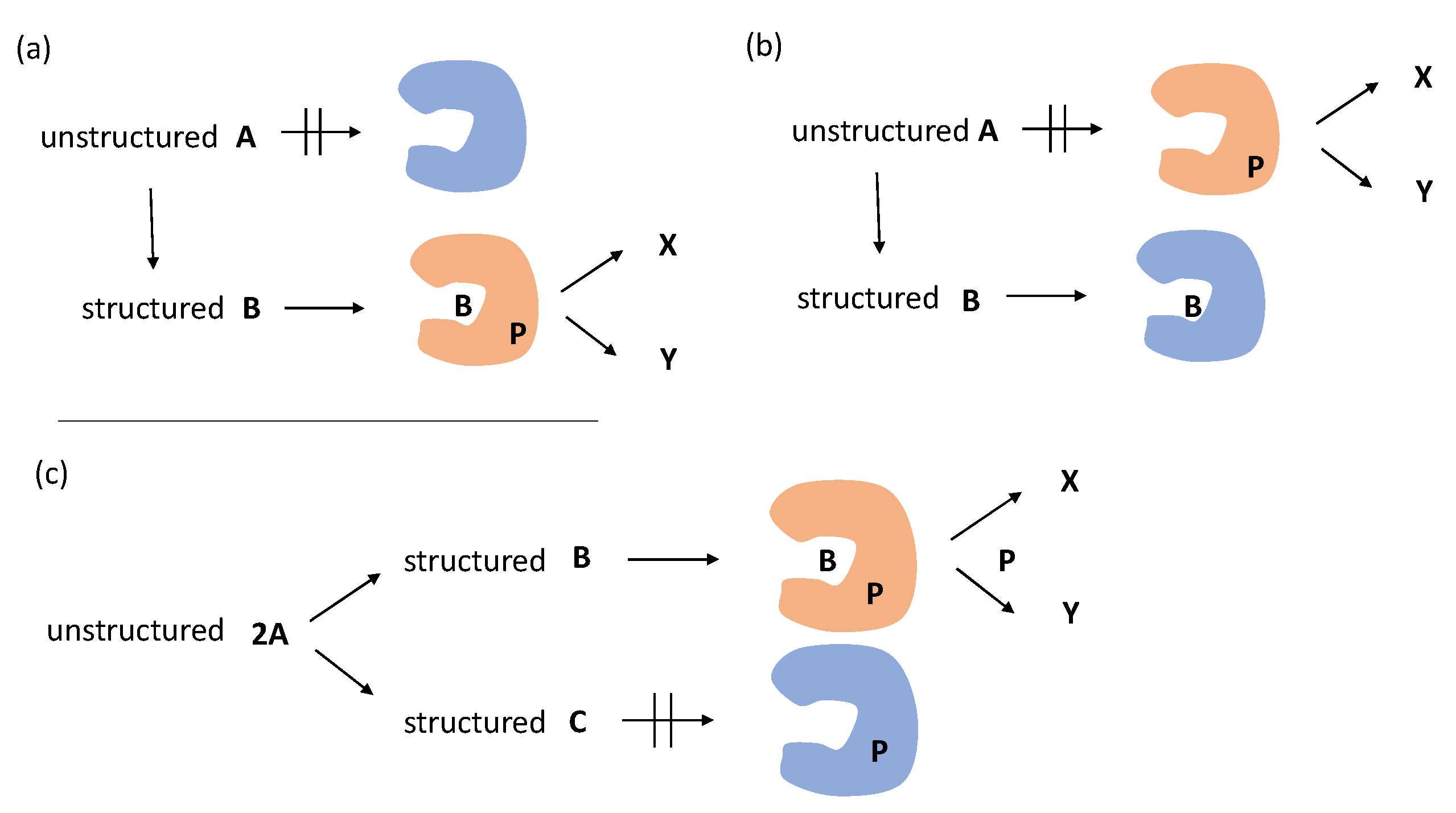
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Bao, M.; Yamaguchi, M. Thermal Input/Concentration Output Systems Processed by Chemical Reactions of Helicene Oligomers. Reactions 2022, 3, 89-117. https://doi.org/10.3390/reactions3010008
Zhang S, Bao M, Yamaguchi M. Thermal Input/Concentration Output Systems Processed by Chemical Reactions of Helicene Oligomers. Reactions. 2022; 3(1):89-117. https://doi.org/10.3390/reactions3010008
Chicago/Turabian StyleZhang, Sheng, Ming Bao, and Masahiko Yamaguchi. 2022. "Thermal Input/Concentration Output Systems Processed by Chemical Reactions of Helicene Oligomers" Reactions 3, no. 1: 89-117. https://doi.org/10.3390/reactions3010008
APA StyleZhang, S., Bao, M., & Yamaguchi, M. (2022). Thermal Input/Concentration Output Systems Processed by Chemical Reactions of Helicene Oligomers. Reactions, 3(1), 89-117. https://doi.org/10.3390/reactions3010008




