Determination of Olive Maturity Stage and Optimal Harvest Interval of ‘Kalinjot’ Cultivar Using Destructive and Non-Destructive Methods
Abstract
1. Introduction
2. Materials and Methods
- –
- L is the lightness component (ranging from 0 = black to 100 = white),
- –
- a is the red–green component (positive values indicate red, negative values indicate green),
- –
- b is the yellow–blue component (positive values indicate yellow, negative values indicate blue).
- –
- DI is the Detachment Index, expressed in Newton per gram (N/g);
- –
- F is the detachment force measured in Newtons (N);
- –
- FW is the fresh weight of the fruit in grams (g).
3. Results and Discussion
3.1. Physicochemical Evolution of Olive Fruit During Ripening
3.2. Chemical Composition Changes During Ripening
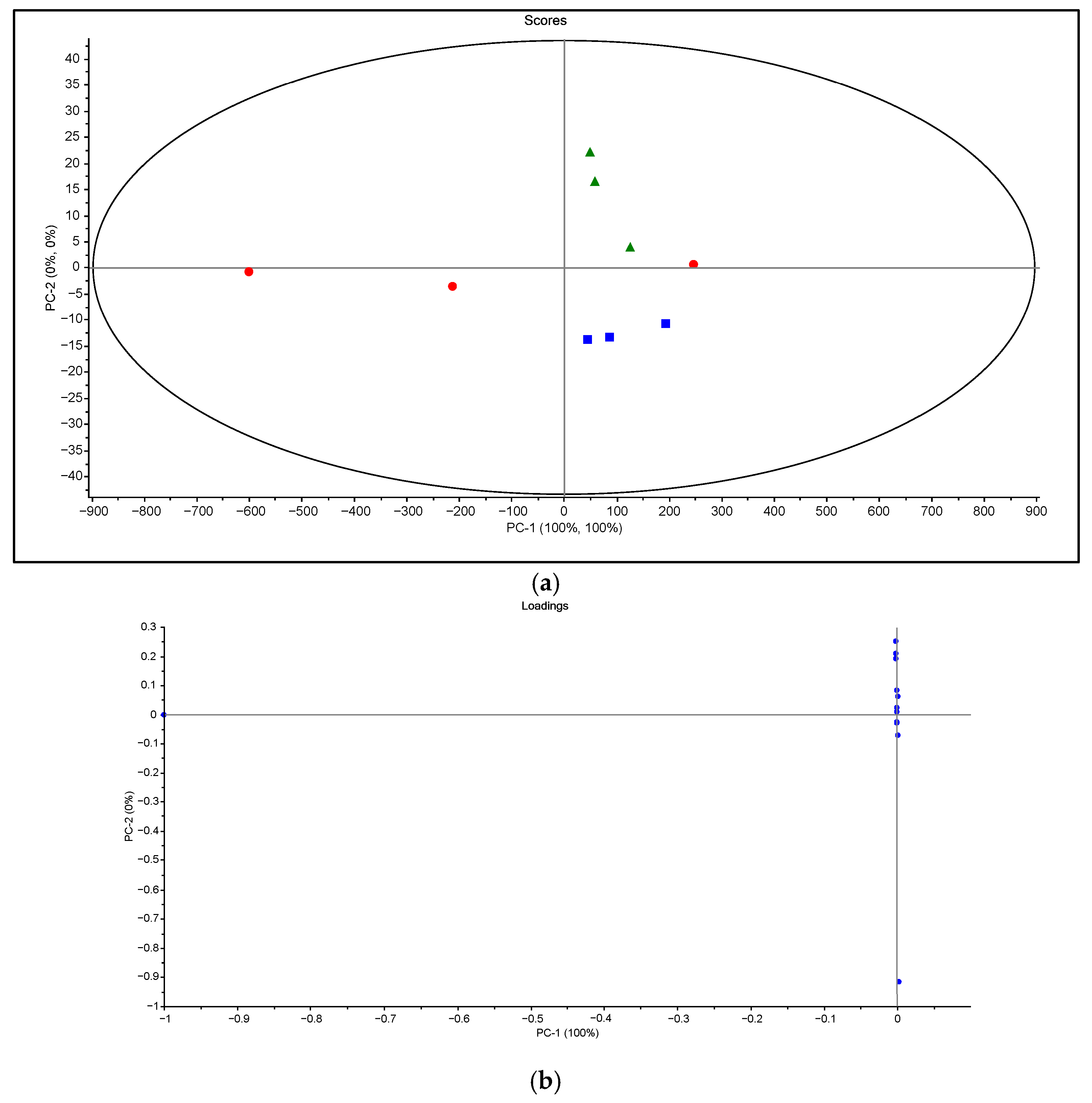
3.3. Statistical Interpretation of Ripening Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mairech, H.; López-Bernal, A.; Moriondo, M.; Dibari, C.; Regni, L.; Proietti, P.; Villalobos, F.J.; Testi, L. Sustainability of olive growing in the Mediterranean area under future climate scenarios: Exploring the effects of intensification and deficit irrigation. Eur. J. Agron. 2021, 129, 126319. [Google Scholar] [CrossRef]
- Ismaili, H. Kultura e Ullirit; Streha: Tiranë, Albania, 2018; p. 92. ISBN 978-9928-4486-5-1. [Google Scholar]
- Famiani, F.; Gucci, R. La raccoltadelle olive. In EdizioniAgricole di New Business Media; Edagricole: Bologna, Italy, 2022; pp. 478–509. [Google Scholar]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. Effects of malaxation temperature and harvest time on the chemical characteristics of olive oils. Food Chem. 2016, 211, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Yousfi, K. Non-destructive and objective methods for the evaluation of the maturation level of olive fruit. Eur. Food Res. Technol. 2005, 221, 538–541. [Google Scholar] [CrossRef]
- Tarquini, G.; Bellincontro, A.; Mencarelli, F. Non-destructive techniques to evaluate olive ripening and oil content: A review. Sci. Hortic. 2022, 299, 111004. [Google Scholar] [CrossRef]
- Grassi, S.; Jolayemi, O.S.; Giovenzana, V.; Tugnolo, A.; Squeo, G.; Conte, P.; De Bruno, A.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives. Foods 2021, 10, 1042. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Camino, M.d.C.P. Prediction of quality of intact olives by near infrared spectroscopy. Eur. J. Lipid Sci. Technol. 2010, 112, 1209–1217. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Moradi, A.; Esfahani, M. Application of hyperspectral imaging for non-destructive assessment of olive ripeness and oil content. Biosyst. Eng. 2021, 200, 289–299. [Google Scholar] [CrossRef]
- Cinosi, N.; Portarena, S.; Almadi, L.; Berrettini, A.; Torres, M.; Pierantozzi, P.; Villa, F.; Galletti, A.; Famiani, F.; Farinelli, D. Use of portable devices and an innovative and non-destructive index for in-field monitoring of olive fruit ripeness. Agriculture 2023, 13, 194. [Google Scholar] [CrossRef]
- Rodríguez de la Borbolla, J.M.; Alcalá, M.J.; Fernández Díez, M.; González Pellissó, F. Cambios en la composición de la aceituna durante su desarrollo. I. Grasas Aceites 1955, 6, 5. [Google Scholar]
- Uceda, M.; Frias, L. Harvest Dates. Evolution of the Fruit Oil Content, Oil Composition and Oil Quality. In Proceedings of the II Seminario Oleícola Internacional, International Olive Oil Council, Cordoba, Spain, 6–17 October 1975; p. 125130. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Method, 934.01, 920.39; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Gamli, O.F.; Eker, T. Determination of harvest time of Gemlik olive cultivars by using physical chemical properties. J. Food Meas. Charact. 2017, 11, 2022–2030. [Google Scholar] [CrossRef]
- Romero, C.; García, P.; Brenes, M.; García, A.; Garrido, A. Phenolic compounds in natural black Spanish olive varieties. Eur. Food Res. Technol. 2002, 215, 489–496. [Google Scholar] [CrossRef]
- Garofalo, S.P.; Maldera, F.; Nicol, F.; Vivaldi, G.A.; Camposeo, S. Physical ripening indices improve the assessment of mechanical harvesting time for olive cultivars resistant to Xylella fastidiosa subsp. pauca. Horticulturae 2024, 10, 1108. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of VIS/NIR spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. Fresh Prod. 2009, 1, 35–41. [Google Scholar]
- Farinelli, D.; Boco, M.; Tombesi, A. Intensity and growth period of the fruit components of olive varieties. Acta Hortic. 2002, 586, 607–610. [Google Scholar] [CrossRef]
- Vázquez-Roncero, A.; Janer del Valle, C.; Janer del Valle, M.L. Determinación de polifenoles totales del aceite de oliva. Grasas Aceites 1973, 24, 350. [Google Scholar]
- Baccouri, O.; Guerfel, M.; Baccouri, B.; Cerretani, L.; Bendini, A.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008, 109, 743–754. [Google Scholar] [CrossRef]
- Beltrán, G.; Aguilera, M.P.; Del Rio, C.; Sanchez, S.; Martinez, L. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem. 2005, 89, 207–215. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of Cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality: A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on VIS spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening indices and harvesting times of different olive cultivars for continuous harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- Beltrán, G.; Del Río, C.; Sánchez, S.; Martínez, L. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric. 2004, 84, 1783–1790. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Rivas, A. Influence of the malaxation time and olive ripening stage on oil quality and phenolic compounds of virgin olive oils. Int. J. Food Sci. Technol. 2014, 49, 2521–2527. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Phenolic compounds in virgin olive oil: Influence of agricultural practices, processing and storage. Food Res. Int. 2002, 33, 457–464. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterization of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Lü, X.; Ma, J.; Yan, H.; Yang, L.; Ren, X.; Guo, J. Effect of degree of ripening on the quality of virgin olive oils produced in Longnan, China. J. Am. Oil Chem. Soc. 2021, 98, 1045–1056. [Google Scholar] [CrossRef]
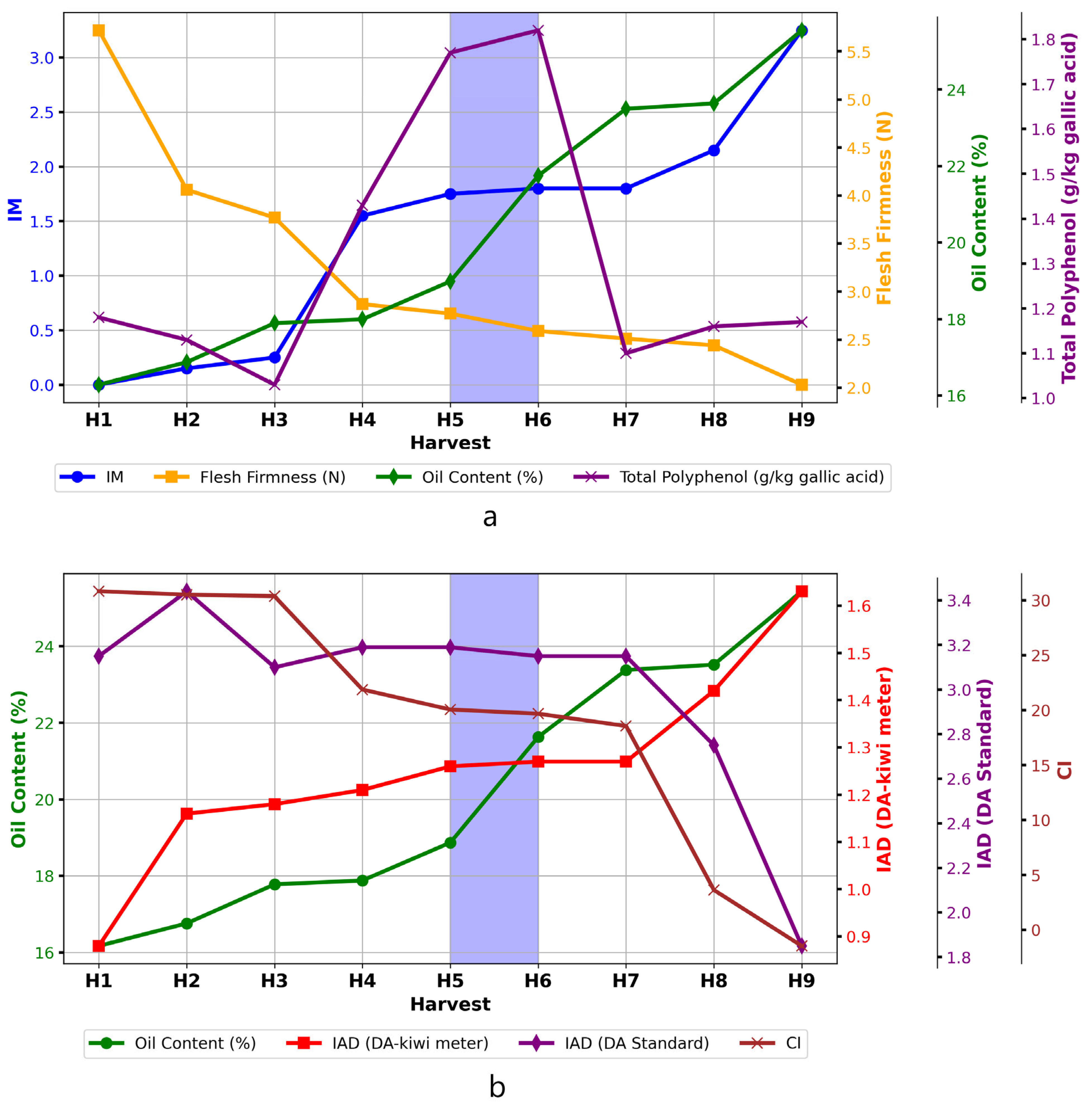
| Harvest | Diameter (mm) | Fresh Weight (g) | Maturity Index (MI) | Color Index (CI) | Flesh Firmness (N) | Detachment Index (DI) (N/g) | IAD (Kiwi-Meter®) | IAD (DA-Meter®) |
|---|---|---|---|---|---|---|---|---|
| H1 | 15.96 ± 0.8 c | 2.82 ± 0.3 d | 0.0 ± 0.0 d | 29.90 ± 2.8 abc | 5.73 ± 0.4 a | 2.09 ± 0.3 a | 0.83 ± 0.3 d | 2.99 ± 0.7 bc |
| H2 | 17.31 ± 0.9 b | 3.06 ± 0.4 c | 0.14 ± 0.3 d | 31.73 ± 2.9 ab | 4.06 ± 0.3 b | 1.75 ± 0.3 b | 1.16 ± 0.2 c | 3.42 ± 0.2 a |
| H3 | 17.31 ± 1.1 b | 3.16 ± 0.3 bc | 0.25 ± 0.4 d | 30.17 ± 3.1 abc | 3.77 ± 0.3 b | 1.69 ± 0.3 b | 1.19 ± 0.2 c | 3.08 ± 0.3 abc |
| H4 | 14.22 ± 0.8 d | 3.36 ± 0.4 b | 1.55 ± 0.9 c | 23.23 ±16.3 c | 2.87 ± 0.4 c | 2.16 ± 0.6 a | 1.21 ± 0.2 c | 3.17 ± 0.4 ab |
| H5 | 17.98 ± 0.7 ab | 3.57 ± 0.2 ab | 1.75 ± 0.6 bc | 34.47 ± 6.3 ab | 2.76 ± 0.2 cd | 1.17 ± 0.2 c | 1.26 ± 0.2 bc | 3.17 ± 0.2 ab |
| H6 | 18.25 ± 1.1 ab | 3.59 ± 0.6 ab | 1.80 ± 0.6 bc | 36.43 ± 5.5 a | 2.60 ± 0.3 cde | 1.20 ± 0.2 c | 1.26 ± 0.1 bc | 3.13 ± 0.3 ab |
| H7 | 18.25 ± 1.1 ab | 3.57 ± 0.4 ab | 1.80 ± 0.6 bc | 36.84 ± 4.7 a | 2.51 ± 0.4 de | 1.20 ± 0.2 c | 1.27 ± 0.1 bc | 3.13 ± 0.3 ab |
| H8 | 18.54 ± 1.0 a | 3.70 ± 0.4 a | 2.15 ± 0.5 b | 26.80 ± 14.1 bc | 2.44 ± 0.3 e | 1.08 ± 0.1 c | 1.42 ± 0.1 b | 2.74 ± 0.4 c |
| H9 | 18.52 ± 1.1 a | 3.64 ± 0.4 a | 3.25 ± 0.4 a | −0.85 ± 5.6 d | 2.03 ± 0.2 f | 0.99 ± 0.2 c | 1.62 ± 0.3 a | 1.83 ± 0.3 d |
| Harvest | Oil Content (%) | Water Content (%) | Total Phenols (mg GAE/kg) |
|---|---|---|---|
| H1 | 16.17 ± 0.6 f | 50.83 ± 0.5 h | 1175.20 ± 1.6 d |
| H2 | 16.77 ± 0.0 ef | 48.48 ± 0.1 i | 1134.08 ± 1.2 f |
| H3 | 17.78 ± 0.0 e | 56.20 ± 0.0 g | 1027.66 ± 0.0 h |
| H4 | 17.88 ± 0.1 de | 58.59 ± 0.3 d | 1433.10 ± 0.9 c |
| H5 | 18.88 ± 0.2 d | 61.08 ± 0.1 b | 1722.31 ± 1.1 b |
| H6 | 21.63 ± 0.6 c | 59.24 ± 0.2 c | 1820.89 ± 1.6 a |
| H7 | 23.38 ± 0.1 b | 62.82 ± 0.0 a | 1095.48 ± 3.5 g |
| H8 | 23.52 ± 0.0 b | 57.17 ± 0.1 e | 1161.75 ± 0.7 e |
| H9 | 25.42 ± 0.1 a | 57.73 ± 0.1 f | 1171.49 ± 0.9 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuksani, G.; Vuksani, A.; Kyçyk, O.; Pazari, F.; Thomaj, T. Determination of Olive Maturity Stage and Optimal Harvest Interval of ‘Kalinjot’ Cultivar Using Destructive and Non-Destructive Methods. AgriEngineering 2025, 7, 253. https://doi.org/10.3390/agriengineering7080253
Vuksani G, Vuksani A, Kyçyk O, Pazari F, Thomaj T. Determination of Olive Maturity Stage and Optimal Harvest Interval of ‘Kalinjot’ Cultivar Using Destructive and Non-Destructive Methods. AgriEngineering. 2025; 7(8):253. https://doi.org/10.3390/agriengineering7080253
Chicago/Turabian StyleVuksani, Gjoke, Angjelina Vuksani, Onejda Kyçyk, Florina Pazari, and Tokli Thomaj. 2025. "Determination of Olive Maturity Stage and Optimal Harvest Interval of ‘Kalinjot’ Cultivar Using Destructive and Non-Destructive Methods" AgriEngineering 7, no. 8: 253. https://doi.org/10.3390/agriengineering7080253
APA StyleVuksani, G., Vuksani, A., Kyçyk, O., Pazari, F., & Thomaj, T. (2025). Determination of Olive Maturity Stage and Optimal Harvest Interval of ‘Kalinjot’ Cultivar Using Destructive and Non-Destructive Methods. AgriEngineering, 7(8), 253. https://doi.org/10.3390/agriengineering7080253







