Mechanochemical Effects of High-Intensity Ultrasound on Dual Starch Modification of Mango Cotyledons
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Ultrasonic (US) Modification
2.3. Dual Modification
2.4. Starch Modification Process Optimization
2.5. Apparent Amylose Content (AAC) and Particle Size of US Modified Starches
2.6. Degree of Substitution (DS) and Reaction Efficiency (RE) of Dual Modified Starch
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. X-Ray Diffraction (XRD) Analysis
2.9. Morphology Analysis
2.10. Thermal Properties
3. Results and Discussion
3.1. Optimization
3.1.1. Validity of the Models
3.1.2. Effects of US Modification on Starch
3.1.3. Effects of Dual Modification on Starch
3.1.4. Predictive Model Verification
3.2. Properties of Modified Starches
3.2.1. FTIR
3.2.2. X-Ray Diffraction (XRD)
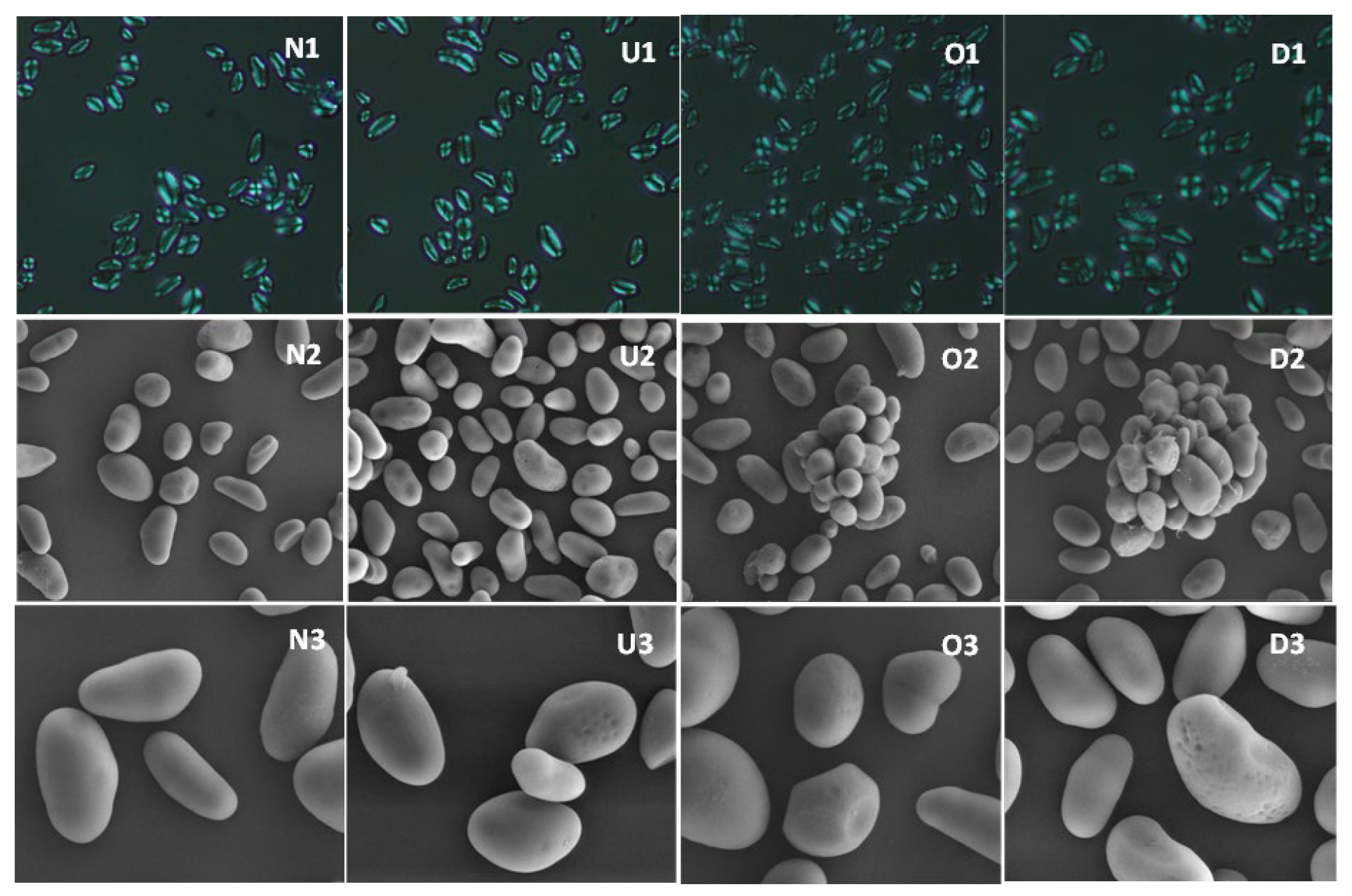
3.2.3. Morphological Characteristics
3.2.4. Thermal Properties
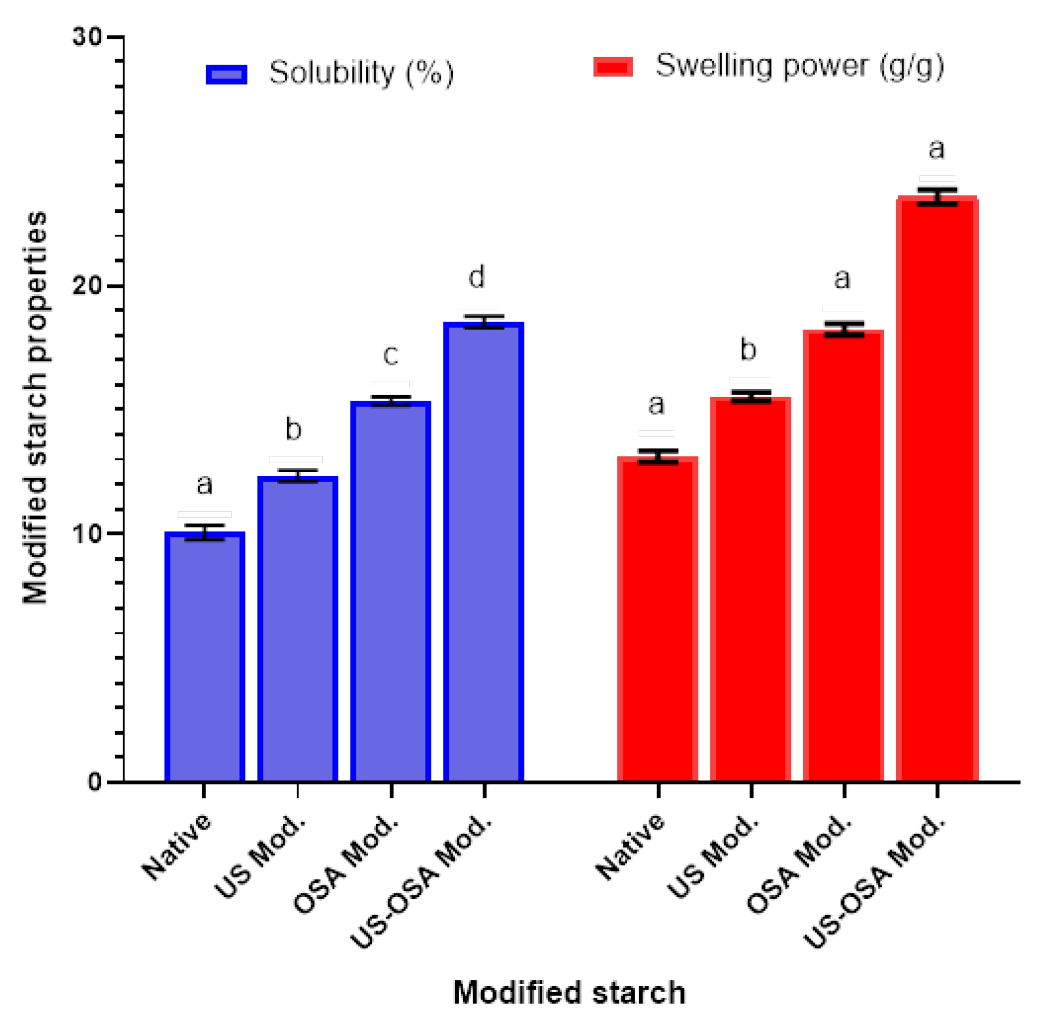
3.2.5. Solubility and Swelling Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. 2025. Available online: https://www.fao.org/faostat/es/#data/QCL/visualize (accessed on 14 April 2025).
- Musser, S.R.; Suhartini, S.; Melville, L. Methane from mango industrial waste: Influence of pH and co-anaerobic digestion. Bioresour. Technol. Rep. 2025, 29, 102056. [Google Scholar] [CrossRef]
- García, M.N.; Enríquez, A.J.B.; Escobar, J.L.G.; Cruz, O.d.J.C.; Pérez, V.P.; Becerril, M.S. Phenolic Compounds in Agro-Industrial Waste of Mango Fruit: Impact on Health and Its Prebiotic Effect—A Review. Pol. J. Food Nutr. Sci. 2023, 73, 5–23. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W.O. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2016, 5, 349–357. [Google Scholar] [CrossRef]
- Chen, H.; Su, Y.; Li, H.; Wang, Z.; Kan, J. Effects of synchronous intermissive multi-ultrasound and esterification dual modification on functionalities of starch and its emulsion stabilization ability. Food Chem. 2024, 450, 139412. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, H.; Zhang, H.; Ye, X.; Tian, J. Effect of ultrasonic pre-treatments on the octenyl succinic anhydride substitution of potato starch and its physicochemical and emulsifying properties. Int. J. Food Sci. Technol. 2022, 57, 7286–7295. [Google Scholar] [CrossRef]
- Vela, A.J.; Villanueva, M.; Ronda, F. Ultrasonication: An Efficient Alternative for the Physical Modification of Starches. Flours Grains Foods 2024, 13, 2325. [Google Scholar] [CrossRef]
- Chakraborty, G.; Kumar, Y.; Sharanagat, V.S. Effect of ultrasonication on OSA esterified surface modification of sorghum (Sorghum bicolor (L.) Moench) starch. Int. J. Biol. Macromol. 2025, 288, 138634. [Google Scholar] [CrossRef]
- Singh, R.; Sharanagat, V.S. Physico-functional and structural characterization of ultrasonic-assisted chemically modified elephant foot yam starch. Int. J. Biol. Macromol. 2020, 164, 1061–1069. [Google Scholar] [CrossRef]
- Núñez-Bretón, L.C.; Torres-González, C.E.; Del Ángel-Zumaya, J.A.; Peredo-Lovillo, A.; Rivera-Villanueva, J.M.; Perea-Flores, M.d.J.; Guzmán-Gerónimo, R.I.; Manero, O.; González-Jiménez, F.E. Functionalization of starches from Mexican Oxalis tuberosa using dual chemical modification. Food Hydrocoll. 2024, 149, 109500. [Google Scholar] [CrossRef]
- Torres-Gallo, R.; Bayuelo-Bonilla, S.; Carpio-Ortiz, L.; Barreto-Rodríguez, G.; Tirado, D.F. High-intensity ultrasound-assisted extraction of pectin from mango wastes at different maturity. Int. J. Food Sci. 2022, 2022, 4606024. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.J.; Villanueva, M.; Solaesa, Á.G.; Ronda, F. Impact of high-intensity ultrasound waves on structural, functional, thermal and rheological properties of rice flour and its biopolymers structural features. Food Hydrocoll. 2021, 113, 106480. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of ultrasonication on the aggregation structure and physicochemical characteristics of sweet potato starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Fontes, R.L.S.; Fontes-Sant’Ana, G.C.; Calado, V.V.; López, E.O.; Rocha-Leão, M.H.M. Extraction, Modification, and Chemical, Thermal and Morphological Characterization of Starch from the Agro-Industrial Residue of Mango (Mangifera indica L.) var. Ubá. Starch-Stärke 2019, 71, 1800023. [Google Scholar] [CrossRef]
- Gautam, G.; Talukdar, D.; Mahanta, C.L. Sonochemical effect on the degree of substitution of octenyl-succinic anhydride into waxy rice starch nanoparticles and study of gastro-intestinal hydrolysis using INFOGEST in vitro digestion method. Food Res. Int. 2023, 165, 112348. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Li, Z.; Luan, Y.; Gu, C.; Liu, R.; Ge, Q.; Yu, H.; Wu, M. Effects of octenyl succinylation on the properties of starches with distinct crystalline types and their Pickering emulsions. Int. J. Biol. Macromol. 2023, 230, 123183. [Google Scholar] [CrossRef]
- Silva, E.K.; Anthero, A.G.d.S.; Emerick, L.B.; Zabot, G.L.; Hubinger, M.D.; Meireles, M.A.A. Low-frequency ultrasound-assisted esterification of Bixa orellana L. seed starch with octenyl succinic anhydride. Int. J. Biol. Macromol. 2022, 207, 1–8. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Mounika, A.; Shanmugam, A.; Farzana, W. A comparative study on effect of succinylation on kodo millet and chickpea starch with ultrasound treatment. Food. Biosci. 2024, 62, 105104. [Google Scholar] [CrossRef]
- Torres, R.; Montes, E.J.; Pérez, O.A.; Andrade, R.D. Relación del Color y del Estado de Madurez con las Propiedades Fisicoquímicas de Frutas Tropicales. Inf. Tecnológica 2013, 24, 51–56. [Google Scholar] [CrossRef]
- Torres-Gallo, R.; Andrade-Pizarro, R.; Salcedo, J.; Chávez-Salazar, A.; Castellanos-Galeano, F.J. Optimization of ultrasound-assisted extraction of mango cotyledon starch: Physicochemical, structural, thermal and functional properties. Int. J. Biol. Macromol. 2025, 285, 138239. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Farooq, M.A.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Manzoor, M.F. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gamlath, C.J.; Pathak, R.; Martin, G.J.; Ashokkumar, M. Ultrasound—The Physical and Chemical Effects Integral to Food Processing. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–358. [Google Scholar] [CrossRef]
- Abiddin, N.Z.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Shi, S.-S.; He, G.-Q. Process optimization for cassava starch modified by octenyl succinic anhydride. Procedia Eng. 2012, 37, 255–259. [Google Scholar] [CrossRef]
- Morales-Trejo, F.; Trujillo-Ramírez, D.; Aguirre-Mandujano, E.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Ultrasound-assisted extraction of lychee (Litchi chinensis Sonn.) seed starch: Physicochemical and functional properties. Starch-Stärke 2022, 74, 2100092. [Google Scholar] [CrossRef]
- Zeng, F.; Zhu, S.; Chen, F.; Gao, Q.; Yu, S. Effect of different drying methods on the structure and digestibility of short chain amylose crystals. Food Hydrocoll. 2016, 52, 721–731. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Zheng, X.; Wang, H.; Wang, N. Determination of optimum experimental conditions for preparation and functional properties of hydroxypropylated, phosphorylated and hydroxypropyl-phosphorylated glutinous rice starch. Int. J. Biol. Macromol. 2017, 105, 317–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Hou, H.; Li, X.; Dong, H.; Wang, W.; Zhang, H. Ultrasound-assisted preparation of octenyl succinic anhydride modified starch and its influence mechanism on the quality. Food Chem. X 2020, 5, 100077. [Google Scholar] [CrossRef]
- Pesek, S.; Silaghi-Dumitrescu, R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2024, 29, 641. [Google Scholar] [CrossRef]
- Vela, A.J.; Villanueva, M.; Li, C.; Hamaker, B.; Ronda, F. Ultrasound treatments of tef [Eragrostis tef (Zucc.) Trotter] flour rupture starch α-(1,4) bonds and fragment amylose with modification of gelatinization properties. LWT 2023, 174, 114463. [Google Scholar] [CrossRef]
- Amarnath, M.; Muhammed, A.; Antony, A.K.; Malini, B.; Sunil, C. White finger millet starch: Physical modification (annealing and ultrasound), and its impact on physicochemical, functional, thermal and structural properties. Food Humanit. 2023, 1, 599–606. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; Lan, T.; Lei, Y.; Suo, J.; Zhao, Q.; Lei, J.; Sun, X.; Ma, T. Modification in structural, physicochemical, functional, and in vitro digestive properties of kiwi starch by high-power ultrasound treatment. Ultrason. Sonochem. 2022, 86, 106004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ran, C.; Jiang, X.; Dou, J. Impact of octenyl succinic anhydride (OSA) esterification on microstructure and physicochemical properties of sorghum starch. LWT 2021, 152, 112320. [Google Scholar] [CrossRef]
- Li, G.; Ge, X.; Guo, C.; Liu, B. Effect of ultrasonic treatment on structure and physicochemical properties of pea starch. Foods 2023, 12, 2620. [Google Scholar] [CrossRef]
- Amini, A.M.; Razavi, S.M.A.; Mortazavi, S.A. Morphological, physicochemical, and viscoelastic properties of sonicated corn starch. Carbohydr. Polym. 2015, 122, 282–292. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of physically modified oat starch with different sonication treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Zhang, H.; Zhu, X.; Wang, L.; Zhang, N.; Yu, D. Properties of OSA-modified starch and emulsion prepared with different materials: Glutinous rice starch, japonica rice starch, and indica rice starch. Food Res. Int. 2022, 161, 111845. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of amylose content in morphological, functional and emulsification properties of OSA modified corn starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Han, S.Y.; Zhu, X.; Zhang, B. Optimization of reaction conditions of octenyl succinic anhydride potato starch and its morphology, crystalline structure and thermal characterization. Adv. Mat. Res. 2011, 236–238, 2279–2289. [Google Scholar] [CrossRef]
- Song, X.; He, G.; Ruan, H.; Chen, Q. Preparation and properties of octenyl succinic anhydride modified early Indica rice starch. Starch-Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Synthesis and partial characterization of octenylsuccinic starch from Phaseolus lunatus. Food Hydrocoll. 2008, 22, 1467–1474. [Google Scholar] [CrossRef]
- Abiddin, Z.; Yusoff, A.; Ahmad, N. Optimisation of reaction conditions of octenyl succinic anhydride (OSA) modified sago starch using response surface methodology (RSM). Int. Food Res. J. 2015, 22, 930–935. [Google Scholar]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Jafari, M.; Koocheki, A. Impact of ultrasound treatment on the physicochemical and rheological properties of acid hydrolyzed sorghum starch. Int. J. Biol. Macromol. 2024, 256, 128521. [Google Scholar] [CrossRef]
- Wang, J.; Su, L.; Wang, S. Physicochemical properties of octenyl succinic anhydride-modified potato starch with different degrees of substitution. J. Sci. Food Agric. 2010, 90, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Li, Y.; Zheng, J. Dual-frequency ultrasonic effect on the structure and properties of starch with different size. LWT 2019, 106, 254–262. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef]
- Velásquez-Barreto, F.F.; Bello-Pérez, L.A.; Yee-Madeira, H.; Sánchez, C.E.V. Esterification and characterization of starch from Andean tubers. Starch-Stärke 2019, 71, 1800101. [Google Scholar] [CrossRef]
- Xu, L.; Bai, Z.; Feng, J.; He, L.; Ren, J.; Chai, S.; Chen, X. Effects of the degree of substitution of octenyl succinic anhydride on the physicochemical characteristics of adlay starch. Int. J. Biol. Macromol. 2023, 241, 124535. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Blennow, A.; Herburger, K.; Zhu, C.; Nurzikhan, S.; Wei, J.; Zhong, Y.; Guo, D. The location of octenyl succinate anhydride groups in high-amylose maize starch granules and its effect on stability of pickering emulsion stability. LWT 2022, 169, 113892. [Google Scholar] [CrossRef]
- Martins, A.; Beninca, C.; Bet, C.D.; Bisinella, R.Z.B.; de Oliveira, C.S.; Hornung, P.S.; Schnitzler, E. Ultrasonic modification of purple taro starch (Colocasia esculenta B. Tini): Structural, psychochemical and thermal properties. J. Therm. Anal. Calorim. 2020, 142, 819–828. [Google Scholar] [CrossRef]
- Zhou, F.; Dong, M.; Huang, J.; Lin, G.; Liang, J.; Deng, S.; Gu, C.; Yang, Q. Preparation and physico-chemical characterization of OSA-modified starches from different botanical origins and study on the properties of Pickering emulsions stabilized by these starches. Polymers 2023, 15, 706. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gill, B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019, 126, 367–375. [Google Scholar] [CrossRef]
- Bajaj, R.; Singh, N.; Kaur, A. Properties of octenyl succinic anhydride (OSA) modified starches and their application in low fat mayonnaise. Int. J. Biol. Macromol. 2019, 131, 147–157. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Lucas-Aguirre, J.C.; Bello-Pérez, L.A.; Rodríguez-Garcia, M.E. Starch from two unripe plantains and esterified with octenyl succinic anhydride (OSA): Partial characterization. Food Chem. 2020, 315, 126241. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Wu, X.; Yan, X.; Zhang, Q.; Zhang, B. Octenyl succinic anhydride tigernut starch: Structure, physicochemical properties and stability of curcumin-loaded Pickering emulsion. Int. J. Biol. Macromol. 2024, 275, 133475. [Google Scholar] [CrossRef]
- Prakruthi, L.Y.; Krishnan, H.; Medha, T.; Kumarakuru, K.; Kumari, P.V.; Surekha, C.; Alavilli, H.; Kaushik, D.; Hashem, A.; Alotaibi, N.H.; et al. Enhancing starch properties through dual modification: Ultrasonication and acetic acid treatment of non-conventional starches. Ultrason. Sonochem 2025, 115, 107301. [Google Scholar] [CrossRef]
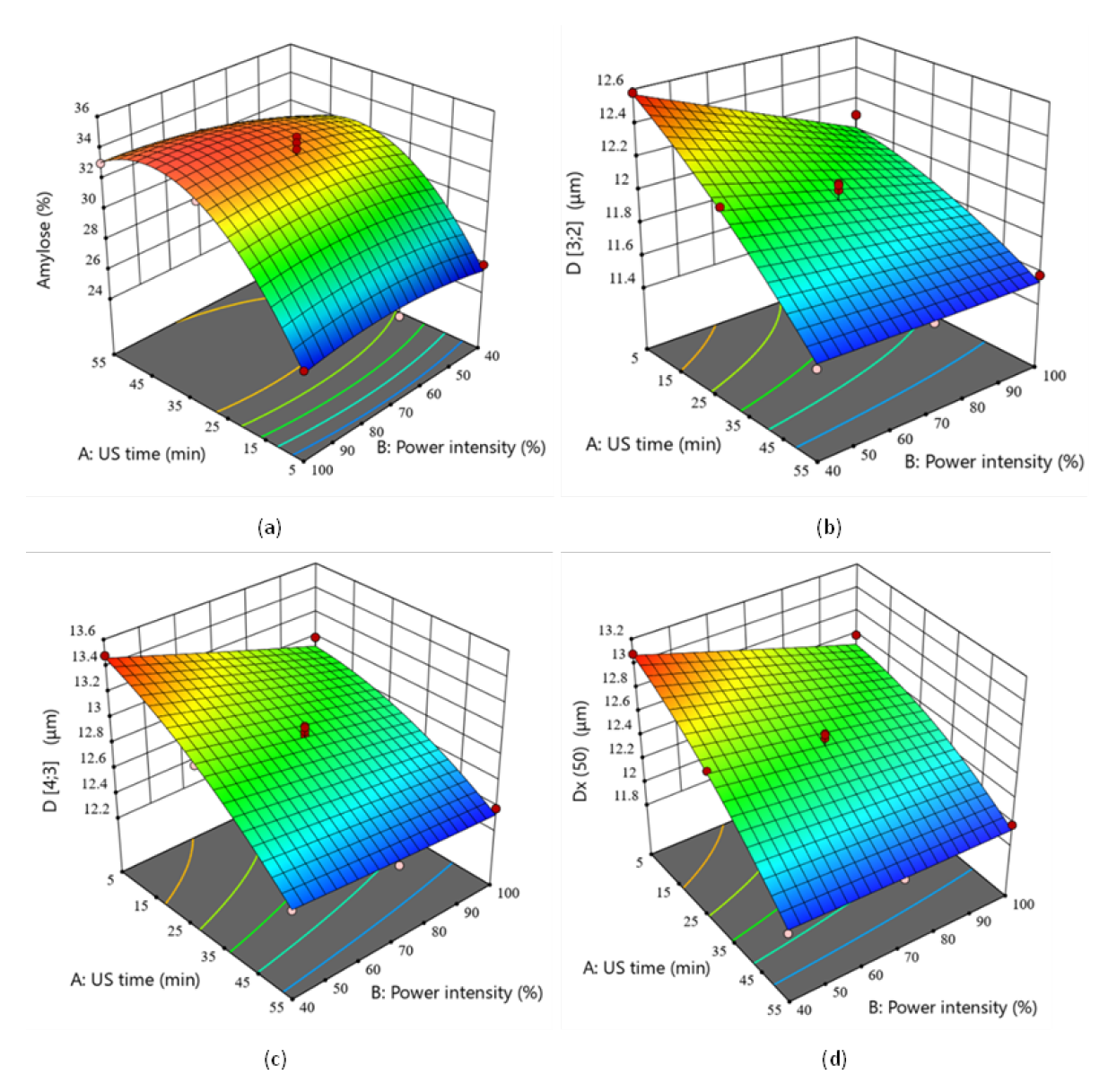
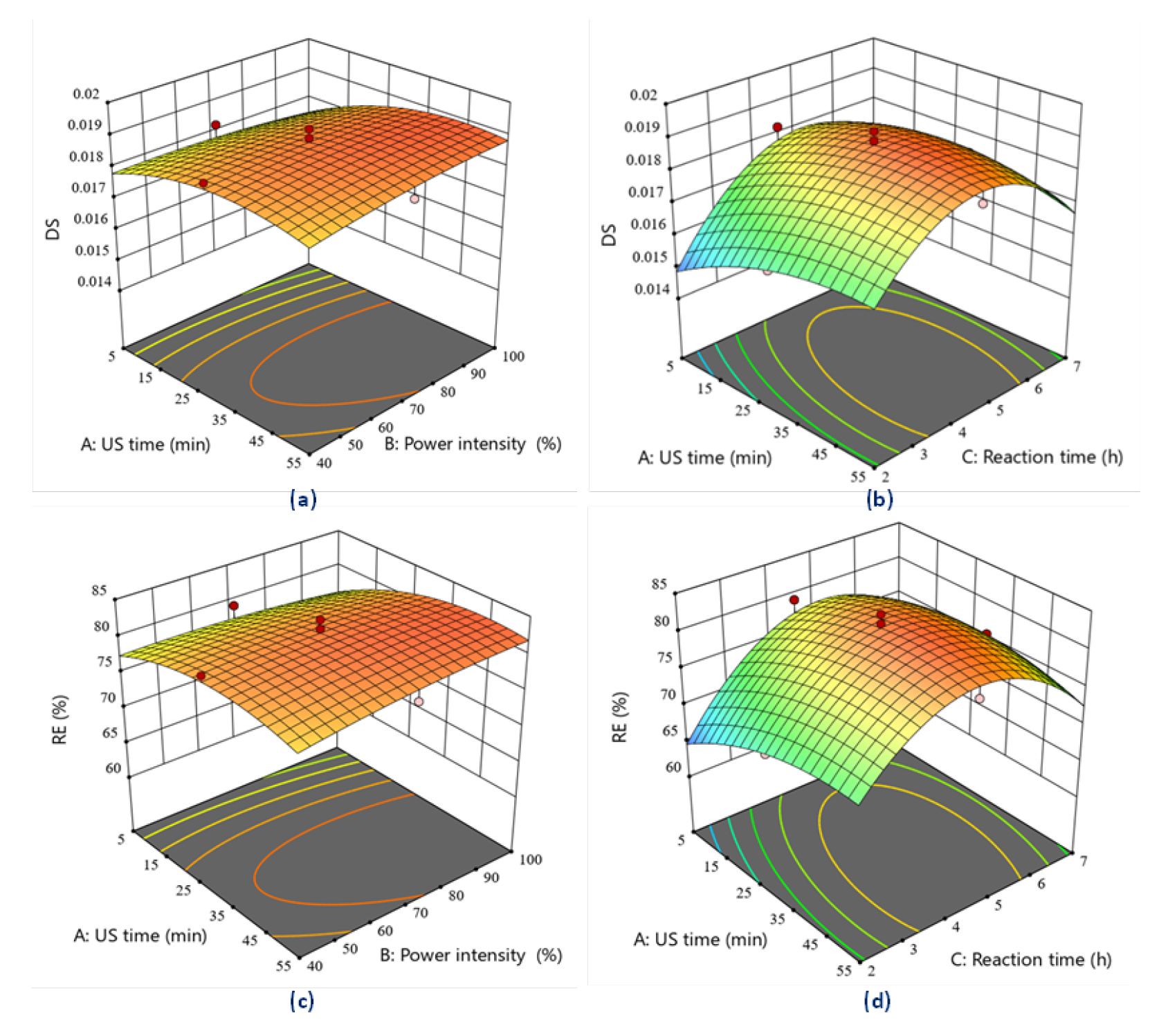
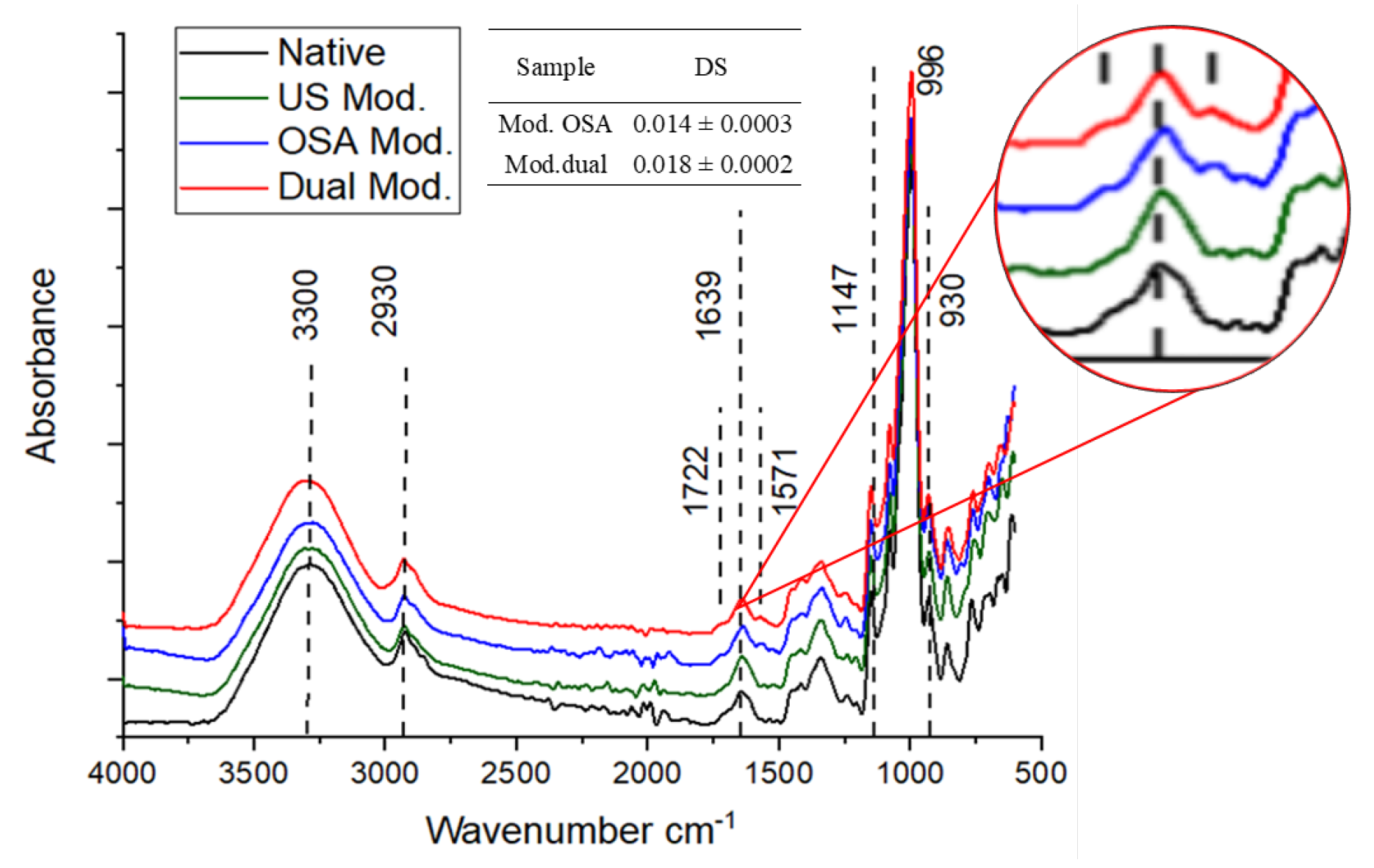
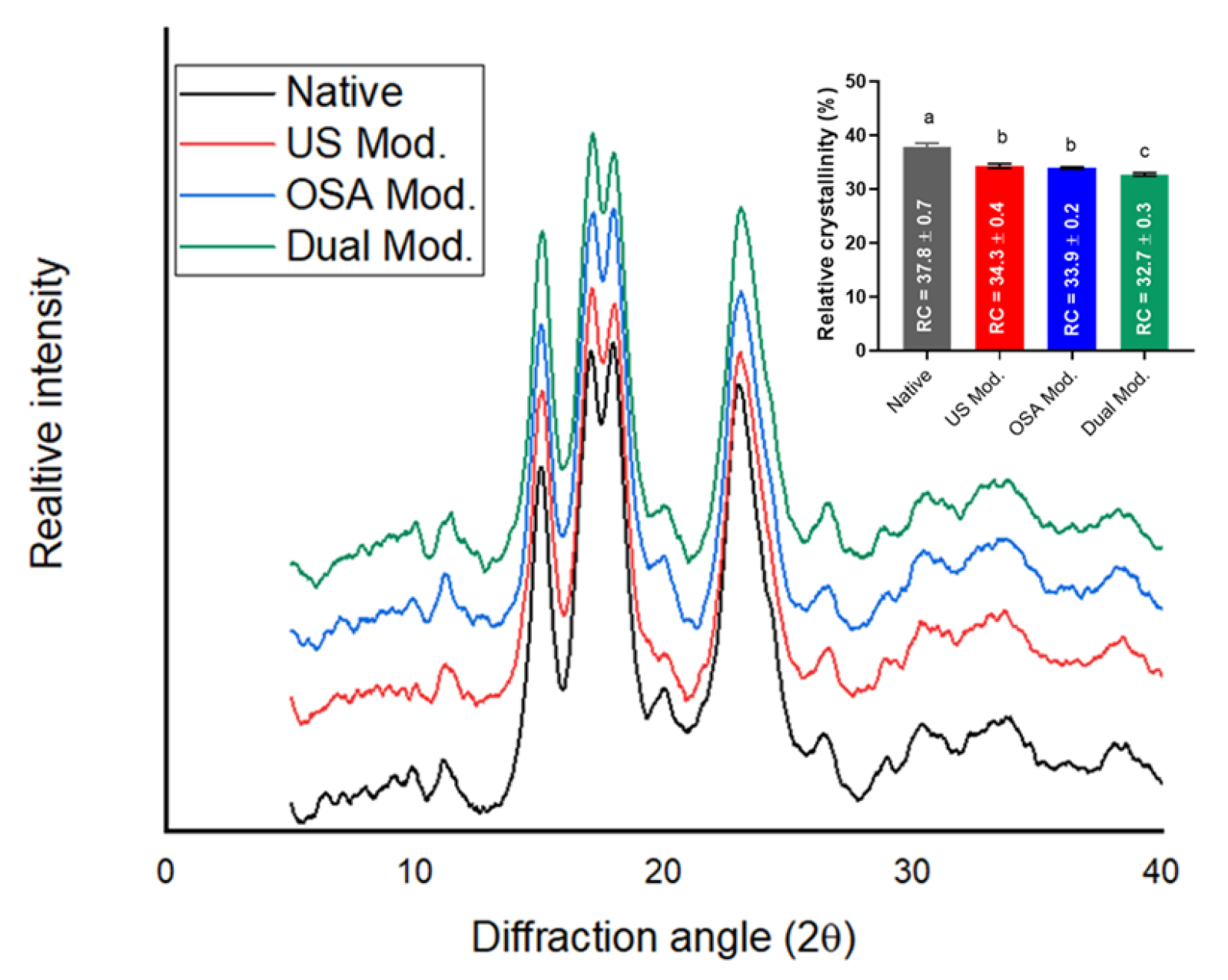
| Variable | Criteria | Impact | Weight | Theoretical Value | Experimental Value | RME (%) |
|---|---|---|---|---|---|---|
| Single US modification | ||||||
| AAC (%) | Maximize | 5 | 7 | 34.3 | 33.6 ± 1.9 | 2.29 |
| Dx50 (μm) | Minimize | 3 | 3 | 11.8 | 12.1 ± 0.2 | 1.23 |
| D[3;2] (μm) | Minimize | 3 | 3 | 12.6 | 11.6 ± 0.2 | 1.20 |
| D[4;3] (μm) | Minimize | 3 | 3 | 12.2 | 12.7 ± 0.1 | 1.23 |
| Dual modification | ||||||
| DS | Maximize | 3 | 3 | 0.0190 | 0.018 ± 0.0002 | 4.97 |
| RE (%) | Maximize | 3 | 3 | 82.3 | 80.7 ± 0.9 | 1.98 |
| Sample | IR Ratio 1047 cm−1/1022 cm−1 | IR Ratio 1022 cm−1/995 cm−1 |
|---|---|---|
| Native | 0.72 ± 0.01 a | 0.88 ± 0.03 a |
| US modification | 0.68 ± 0.01 b | 0.95 ± 0.02 b |
| OSA modification | 0.67 ± 0.01 b | 0.98 ± 0.02 b |
| Dual modification | 0.64 ± 0.01 c | 1.05 ± 0.03 c |
| Sample | T0 (°C) | TP (°C) | TC (°C) | ΔHG (J/g) | Range |
|---|---|---|---|---|---|
| Native | 71.7 ± 0.3 a | 76.5 ± 0.1 a | 81.4 ± 0.1 a | 14.1 ± 0.4 a | 9.7 ± 0.2 a |
| US modification | 69.7 ± 0.2 b | 75.8 ± 0.3 b | 78.3 ± 0.2 b | 11.1 ± 0.3 b | 8.6 ± 0.2 b |
| OSA modification | 69.3 ± 0.2 b | 76.3 ± 0.2 a | 77.7 ± 0.3 c | 10.8 ± 0.3 c | 7.9 ± 0.4 b |
| Dual modification | 67.6 ± 0.4 c | 73.5 ± 0.3 c | 75.5 ± 0.4 d | 9.7 ± 0.4 c | 7.8 ± 0.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Gallo, R.; Andrade-Pizarro, R.; Tirado, D.F.; Chávez-Salazar, A.; Castellanos-Galeano, F.J. Mechanochemical Effects of High-Intensity Ultrasound on Dual Starch Modification of Mango Cotyledons. AgriEngineering 2025, 7, 190. https://doi.org/10.3390/agriengineering7060190
Torres-Gallo R, Andrade-Pizarro R, Tirado DF, Chávez-Salazar A, Castellanos-Galeano FJ. Mechanochemical Effects of High-Intensity Ultrasound on Dual Starch Modification of Mango Cotyledons. AgriEngineering. 2025; 7(6):190. https://doi.org/10.3390/agriengineering7060190
Chicago/Turabian StyleTorres-Gallo, Ramiro, Ricardo Andrade-Pizarro, Diego F. Tirado, Andrés Chávez-Salazar, and Francisco J. Castellanos-Galeano. 2025. "Mechanochemical Effects of High-Intensity Ultrasound on Dual Starch Modification of Mango Cotyledons" AgriEngineering 7, no. 6: 190. https://doi.org/10.3390/agriengineering7060190
APA StyleTorres-Gallo, R., Andrade-Pizarro, R., Tirado, D. F., Chávez-Salazar, A., & Castellanos-Galeano, F. J. (2025). Mechanochemical Effects of High-Intensity Ultrasound on Dual Starch Modification of Mango Cotyledons. AgriEngineering, 7(6), 190. https://doi.org/10.3390/agriengineering7060190







