1. Introduction
Breeding waterfowl, ducks in particular, are significant in the agro-industrial complex, providing not only high-quality meat but also eggs suitable for the reproduction of cross breeds. Duck eggs are in demand both in the food industry and in incubation production, especially in the context of the growing demand for environmentally friendly and biologically complete products. According to experts, the efficiency of incubation of duck eggs is largely determined by the high-quality selection of incubation material, which, in turn, requires an objective and technologically sound individual assessment of eggs based on a combination of morphometric and physical characteristics.
Recent research has demonstrated the rapid development of non-destructive optical technologies for poultry egg analysis.
Studies on chicken eggs have successfully applied RGB, hyperspectral and near-infrared imaging to assess quality, freshness and fertility [
1].
For duck eggs, progress has been limited but notable: convolutional-neural-network-based candling methods [
1], double-yolk detection systems [
2] and spectroscopic origin identification [
3] have shown promising accuracy between 90% and 95%.
Nevertheless, most commercial and academic systems remain calibrated for chicken eggs and fail to account for the higher reflectivity, thicker shells and wider morphological variability seen for duck eggs.
This technological gap results in reduced reliability when such systems are transferred directly for use for waterfowl species.
In the traditional practice of selecting hatching duck eggs, especially in small- and medium-sized poultry farms, the manual control method prevails, which involves visual inspection, weighing and ovoscopy. This method requires the participation of experienced personnel, takes a significant amount of time, and involves a degree of subjectivity. Duck eggs also have a few features that distinguish them from chicken eggs: a larger size and increased weight (on average from 70 to 90 g), a stronger shell and a more pronounced variety in geometric shapes. These parameters directly affect the laying of eggs in the incubator, ventilation, temperature conditions and, as a result, the level of hatching of ducklings.
In the modern conditions of the digitalization of agriculture and transition to the concept of the “smart farm” (Smart Farming), the requirements are increasing for the standardization, automation and reproducibility of all stages of the production cycle, including the sorting and preparation of incubation material. However, these requirements cannot be met without the introduction of intelligent systems that combine high-precision measuring equipment, digital image processing algorithms and machine vision technologies.
Despite these advances, comparative studies focusing on breed-specific optical calibration are still scarce.
Recent research emphasizes that the optical constants and shell microstructures of duck eggs differ significantly among breeds, which affects light scattering and contour detection accuracy [
4].
Therefore, the direct use of chicken-calibrated models may introduce systematic bias in shape- and density-based parameters.
To overcome this, the present study introduces a set of hardware and algorithmic modifications specifically tailored for the Adigel breed, thus providing a reproducible reference framework for the future standardization of incubation-related morphometric parameters.
Optical methods implemented based on high-resolution cameras and software for analyzing the shape, volume and density of objects have proven their effectiveness in the quality control of chicken eggs. Therefore, most existing research and industrial solutions are focused on chicken eggs, the standards of which differ from those of duck eggs in both morphometry and the boundary values of density and permissible deviations. Thus, the transfer of existing sorting systems to duck eggs without adaptation is impossible and can lead to classification errors, reduced productivity and the deterioration of incubation indicators.
Considering the above, there is a clear need to develop a specialized automated method that is adapted to the morphological and physical–mechanical characteristics of duck eggs and capable of real-time non-destructive testing and precise sorting for incubation suitability. Such a method will improve reproductive performance, reduce the impact of the human factor and ensure digital control at the early stages of the production cycle. The creation of this method is one of the priority areas of the development of agricultural science, as outlined in the framework of the strategy for food security and the technological modernization of the agricultural sector.
It should be emphasized that duck eggs differ from chicken eggs not only in geometric proportions but also in surface reflectance and shell microstructure, which affect the accuracy of optical detection. Therefore, the direct transfer of existing image processing techniques is inadequate. This study introduces specific software and hardware adaptations for duck eggs, including modified contour extraction under variable reflectivity, recalibrated scale coefficients and a correction factor (Kv) optimized for the ovality typical of the Adigel breed.
Despite the obvious importance of the high-quality selection of hatching duck eggs to ensure the high hatchability and viability of young animals, this aspect remains insufficiently studied in the scientific and applied literature. The main emphasis in existing studies is on the sorting of chicken eggs, while duck eggs, despite their physiological differences, are considered secondary in the context of the automated assessment of incubation suitability. This leads to several methodological and practical contradictions that form the main scientific problem explored in this study.
Firstly, there is no unified classification and standardized system for assessing the quality of duck eggs adapted specifically for incubation purposes. Existing standards and regulations are mainly focused on food suitability and do not consider parameters such as shell density, shape and the volume-to-weight ratio, which are critical for embryonic development. Therefore, there is a need for the scientific development of morphometric and physical indicators that could reliably correlate with the incubation suitability of duck eggs.
Secondly, despite the prevalence of machine vision and digital image processing in other branches of agriculture (for example, in sorting fruits, vegetables and seeds), they are practically not implemented in poultry farming, especially in breeding waterfowl. This is particularly due to the lack of specialized algorithms and software capable of correctly analyzing the geometry and density of duck eggs that have a natural variability in shape, shell spotting and a reflective surface. Consequently, the task arises of adapting existing computer vision methods to the specific properties of duck eggs, including camera calibration, the selection of conversion coefficients and the processing of shadows and image artifacts.
Thirdly, the problem of integrating various types of sensory information into a single system remains relevant. Today, disparate data obtained from strain gauges, infrared rangefinders and machine vision cameras are usually analyzed separately. This complicates decision-making in real time and reduces the productivity of the installation. It is necessary to develop a single algorithmic environment in which all egg parameters, including weight, shape, projection area, density and shape index, are combined into a complex suitability criterion.
Fourth, there is a lack of experimental data confirming the accuracy and reliability of optical and computer methods for assessing duck egg parameters. Most existing publications either concern chicken eggs or are limited to theoretical modeling without real tests. This makes it difficult to verify such solutions and hinders their practical implementation in poultry farms.
Thus, the combination of scientific and technological problems creates the need for a comprehensive study aimed at the development, software implementation and experimental validation of an intelligent system capable of solving the problem of the non-destructive assessment of the incubation suitability of duck eggs with high accuracy and reproducibility.
In most poultry farms, hatching eggs are selected manually via processes including visual inspection, candling and weighing. Such procedures are labor-intensive, require skilled personnel and are highly subjective. Human factor issues lead to significant variability: studies show that the accuracy of manual selection can be as low as 70–85%, which reduces incubation efficiency and increases production losses.
In recent years, machine vision has been actively introduced into chicken egg sorting. A review of technologies highlights the importance of non-destructive optical methods (RGB, NIR and hyperspectral imaging) used to assess the shape, density and contamination of eggs and shell cracks. For example, automated egg sorting lines on conveyors with a real-time camera have been implemented, demonstrating accuracy above 90%.
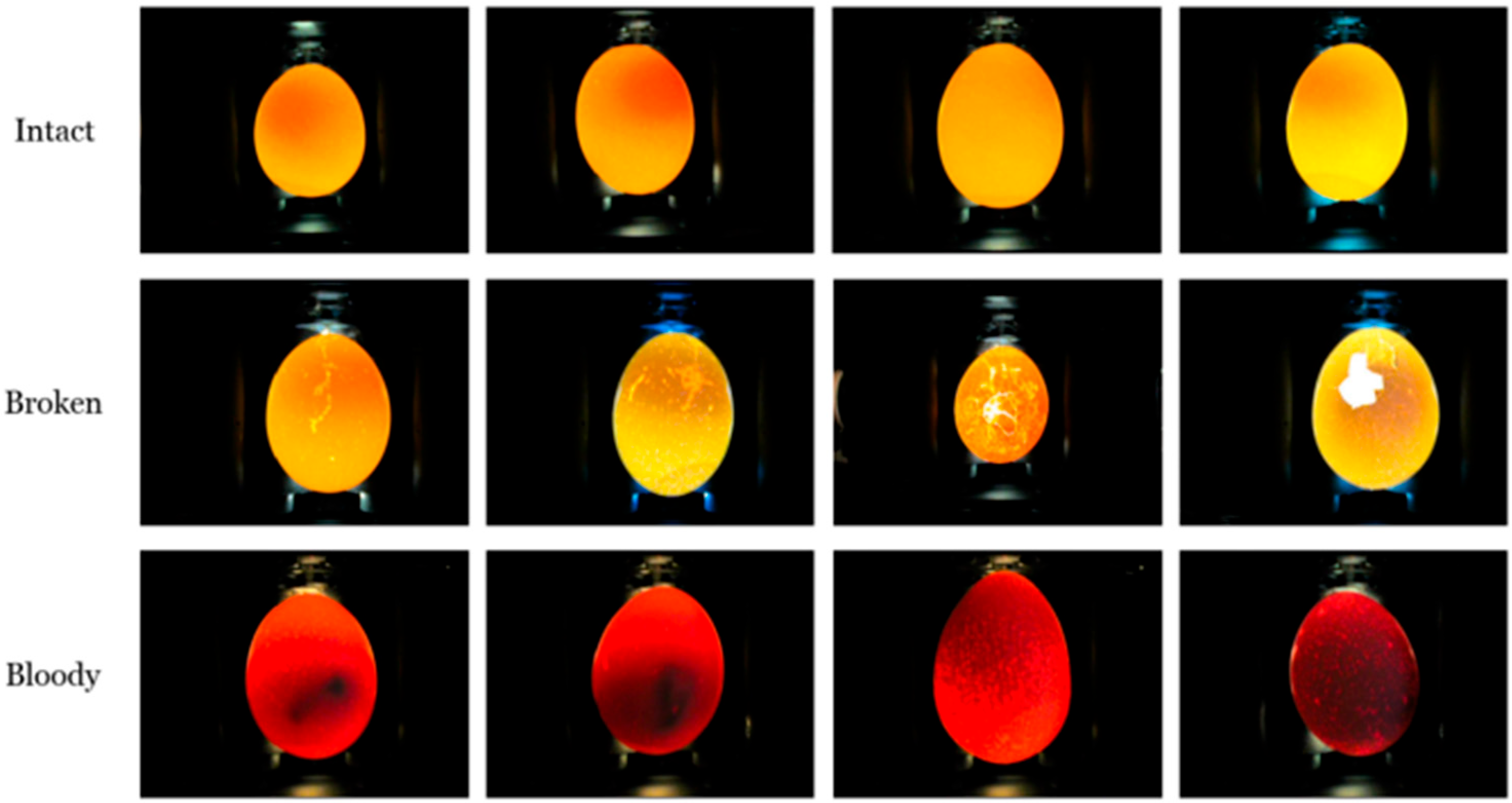
In this study, the use of deep neural networks (e.g., CNNs) allowed for the classification of defective and unsuitable eggs with an accuracy of up to 94%, as shown in
Figure 1 [
5].
Fewer solutions have been developed for duck eggs, but some studies successfully adapted machine vision.
One method for determining the sex of fertilized duck eggs based on shape analysis uses eccentricity, and in this study, it was shown to be superior to the manual method;
Figure 2 shows the system that this method was used on [
6].
An early ovoscopy (fertility testing) device using cameras and AI (CNN) achieved an accuracy of up to 86%.
This study also used a CNN model (ResNet-50) to classify duck eggs with 95% accuracy, as shown in
Figure 3 [
7].
Computer vision algorithms (CNN and FLD) for detecting double-yolk duck eggs (
Figure 4), which is important for incubation, have been developed and have shown high accuracy [
1].
The aim of this study is the development, algorithmic implementation and experimental verification of an automated optical–electronic method for the non-destructive analysis and sorting of hatching duck eggs based on their morphometric and physical parameters.
To achieve the stated goal, the following main research objectives were formulated:
Analyze the morphological and physical characteristics of duck eggs that affect their incubation suitability (weight, geometric dimensions, density, shape index and shape coefficient) to establish measurable criteria.
Develop a hardware architecture for an experimental setup that includes a strain gauge, digital video camera, infrared rangefinder, and egg movement mechanism that ensures the capture and processing of images in two projections.
Create an algorithm for digital image processing that allows for the determination of the large and small diameters of an egg, the area of the longitudinal section, volume, density and shape factors with the subsequent construction of a classification feature for suitability for incubation.
Integrate sensor data (weight, dimensions, image) into a single software control environment, which is implemented based on a single-board microcomputer with its own software in the Python language.
Conduct experimental studies on a sample of 300 duck eggs of the “Adigel” breed with the parallel manual measurement of parameters (weight, egg size) and an assessment of the accuracy, reproducibility and error of the automated system.
Comparatively analyze the results of manual and automatic assessment with the calculation of statistical indicators (mean values, standard deviations, correlation coefficients, relative errors) to verify the operability of the proposed system.
Form conclusions and recommendations on the practical application of the proposed rational method in the incubation process, as well as the prospects for its adaptation to other bird species and expansion of functionality (for example, determining fertility).
1.1. Problem Statement and Objectives
Although machine vision technologies for poultry egg sorting have achieved notable success, a standardized, breed-specific method for duck eggs remains undeveloped.
Existing optical systems cannot accurately determine the density and geometric parameters required for reliable incubation selection due to uncalibrated illumination and the absence of empirical correction factors.
Therefore, this study aims to (1) develop a stationary optoelectronic setup integrating weight, geometry and image data; (2) design and validate image processing algorithms optimized for glossy and spotted shells; and (3) experimentally verify the correlation between automatic classification and manual measurements for Adigel duck eggs.
1.2. Specific Adaptations for Duck Eggs
Unlike chicken eggs, duck eggs exhibit larger geometric dimensions, higher shell reflectivity and greater morphological variability. Therefore, the developed method includes several technical and algorithmic adaptations to ensure accurate optical analysis:
Calibration routines: The VL53L0X infrared rangefinder (STMicroelectronics, Geneva, Switzerland) was recalibrated for the larger average diameter (≈68 mm) of duck eggs to maintain sub-millimeter measurement precision and accurate pixel-to-metric conversion.
Contour detection: Adaptive thresholding with the Gaussian method (block size = 51 × 51; constant C = 2) and a 5 × 5 median filter was applied to minimize false edges on glossy or spotted shells.
Lighting correction: A diffused matte LED lighting system was introduced to eliminate glare and improve contour stability.
Ellipsoid correction factor: The empirical coefficient Kv = 0.637 was derived from experimental volume–mass correlations specifically for the Adigel breed, differing from the coefficients used for chicken eggs (0.61–0.64).
Shape coefficient (K1): This was introduced as a dimensionless compactness parameter K1 = P2/S, where P is the perimeter (Ramanujan’s approximation for an ellipse), and S is the longitudinal section area. This ratio reflects shell symmetry and correlates with hatchability suitability.
These modifications enable the system to handle breed-specific morphological diversity and optical heterogeneity, thus ensuring reliable performance for the non-destructive analysis of duck eggs.
Thus, this study aims to create a technological and scientific basis for the implementation of the machine analysis of duck eggs, capable of replacing subjective manual procedures with highly accurate, reproducible and scalable automated methods that meet the requirements of the modern agricultural industry.
2. Materials and Methods
The objects studied were duck eggs of the Adigel breed, which were selected to identify their suitability for incubation using an optical–electronic system. Incubatory duck eggs were obtained from a poultry farm in the Almaty region specializing in breeding ducks for reproductive purposes. The sample consisted of 300 incubatory duck eggs.
The hatching eggs used exhibited the following characteristics, on average:
A weight from 70 to 90 g;
A predominantly oval shape with slight asymmetry;
A white or slightly cream shell color;
A smooth surface, without defects.
Selection was carried out in accordance with the technical conditions for hatching eggs. Eggs with damage, dirty shells and signs of microcracks were rejected before the experiment. Before each sample was launched into the installation, manual identification and number marking were carried out, which is necessary to compare the results of manual and automated measurements.
The parameters that were assessed are as follows:
Weight (g)—the main physical indicator of egg maturity;
Length (major diameter, mm)—an important morphometric parameter;
Width (minor diameter, mm)—allows for the calculation of the shape index;
Longitudinal cross-sectional area (mm2)—reflects the shape and volume;
Volume (cm3)—calculated based on geometric characteristics;
Density (g/cm3)—used as an indirect indicator of quality;
Shape index (%) and shape coefficient (dimensionless value)—determine the suitability of the egg for incubation based on geometry.
Since the hatchability of duck eggs largely depends on the ratio of weight, density and shape, these parameters were chosen as the key ones for evaluation.
A stationary device based on machine vision and physical measurement sensors was developed for the non-destructive analysis of hatching duck eggs. The device enables the sequential testing of eggs in a single measurement cell, without transport mechanisms or a rotary drum, which simplifies the design and increases the accuracy of measurement by minimizing mechanical errors.
The design of the installation includes the following components:
Raspberry Pi 4 Model B single-board microcomputer (Raspberry Pi Foundation, Cambridge, UK)—performs the functions of the central processor, processes images and controls sensors and the operator interface.
Logitech C270 digital camera (Logitech International S.A., Lausanne, Switzerland)—captures an image of the egg from above and from the side. The camera is mounted on an adjustable tripod at exactly 90° relative to the surface of the measuring cell.
HX711 strain gauge sensor with a measuring module (Avia Semiconductor, Xiamen, China)—located under the base of the measuring platform on which the egg is placed. It provides mass measurement accuracy with an error of up to ±0.1 g.
VL53L0X infrared rangefinder (STMicroelectronics, Geneva, Switzerland)—measures the distance from the camera lens to the surface of the egg, which is necessary for calibrating the image scale and converting pixels into physical units of measurement.
Measuring platform (cell) (custom-made at Kazakh National Agrarian Research University, Almaty, Kazakhstan)—a hard, flat surface with a non-slip coating, designed for laying one egg. The platform is made of light matte plastic, which does not have glare when shooting.
LED backlight with a diffuser (custom LED module assembled at Kazakh National Agrarian Research University, Almaty, Kazakhstan)—provides uniform illumination without shadows and reflections for the stable operation of the contour recognition algorithm.
All computations and image-processing procedures were performed using Python 3.11.7 with the following software packages: OpenCV 4.9.0 for image acquisition and contour detection, NumPy 1.26.4 and SciPy 1.11.4 for numerical processing, Matplotlib 3.8.2 for data visualization, and Pillow 10.2.0 for image formatting. The experiments were executed on Raspberry Pi OS (Bookworm, 10 October 2023 release), and Python scripts were developed and tested using Thonny IDE 4.1.6.
The principles of operation of the installation are as follows:
The operator manually places the egg on the measuring platform.
The load cell records the mass and transmits the data to the Raspberry Pi.
The camera captures images from above and from the side.
At the same time, the rangefinder measures the distance to the egg surface, which is used for scale calibration.
The embedded software, written in Python 3.11.7 and using OpenCV 4.9.0 and NumPy 1.26.4, performs the following:
- ○
It highlights the outline of the egg;
- ○
It calculates the large and small diameters;
- ○
It estimates the area of a longitudinal section;
- ○
Based on empirical formulas, it calculates volume, density, the shape index and the shape factor.
The received parameters are saved in a database with the possibility of exporting them to CSV format.
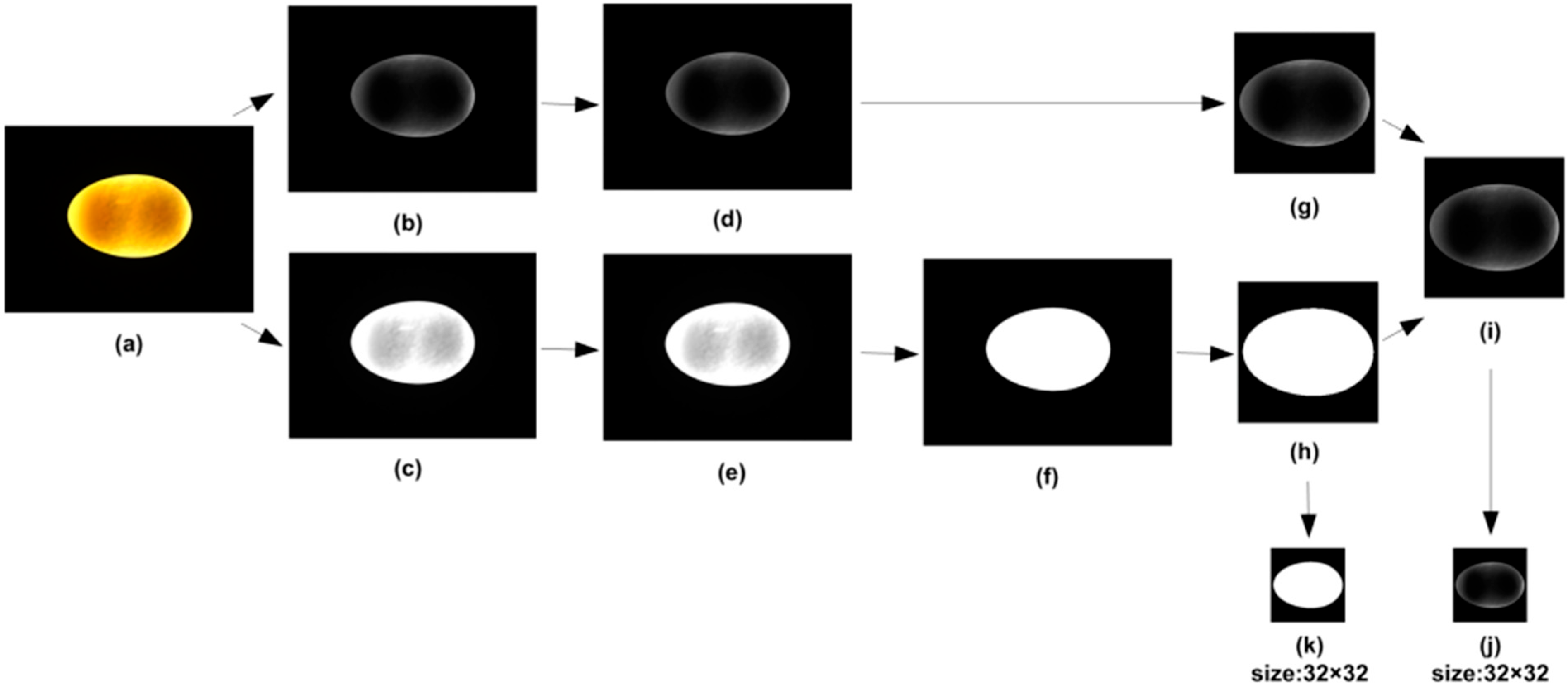
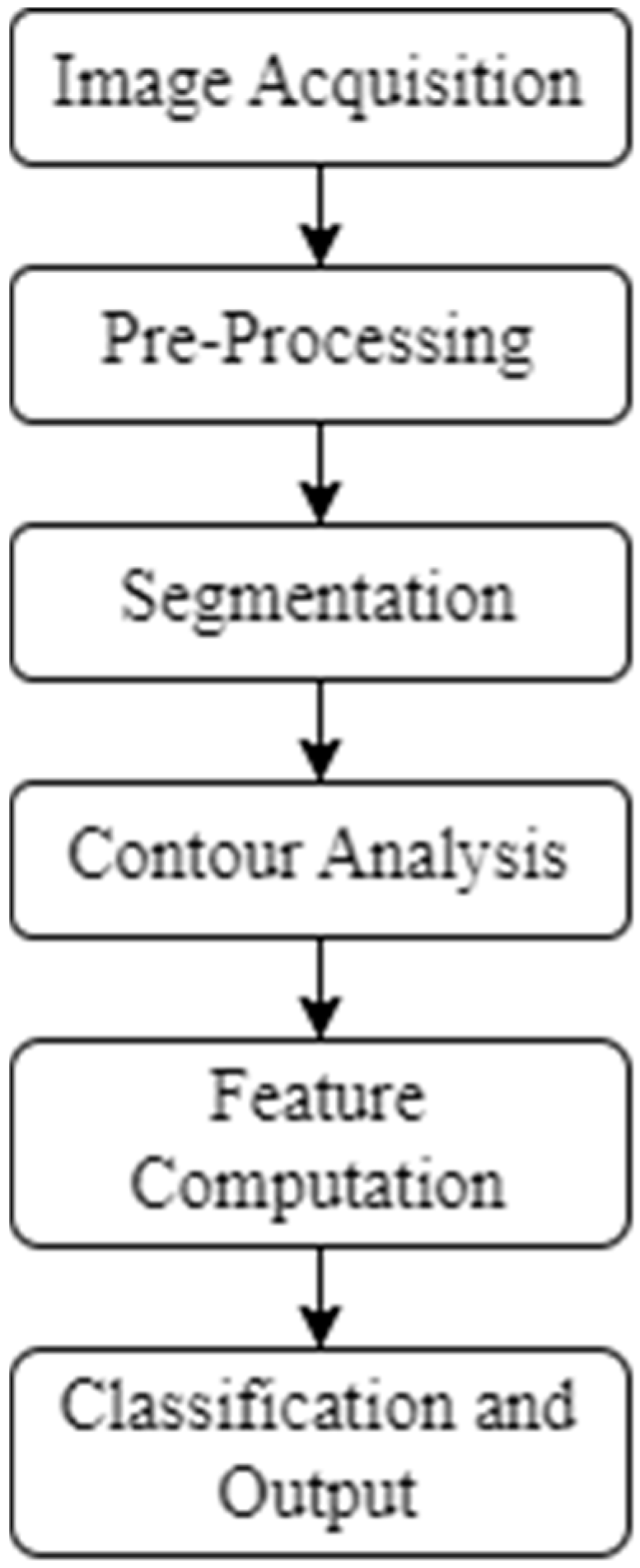
The overall workflow of the image processing algorithm is illustrated in
Figure 5.
It consists of the following major stages:
Image Acquisition—capturing the top and side views under controlled lighting.
Pre-Processing—background correction, histogram equalization and glare removal.
Segmentation—adaptive Gaussian thresholding and median filtering to obtain a binary mask.
Contour Analysis—ellipse fitting to extract the major and minor diameters.
Feature Computation—the calculation of projection area S, volume V, density ρ, shape index If and shape coefficient K1.
Classification and Output—the comparison of measured parameters with empirical thresholds to assign suitability categories (suitable, borderline, unsuitable).
- 7.
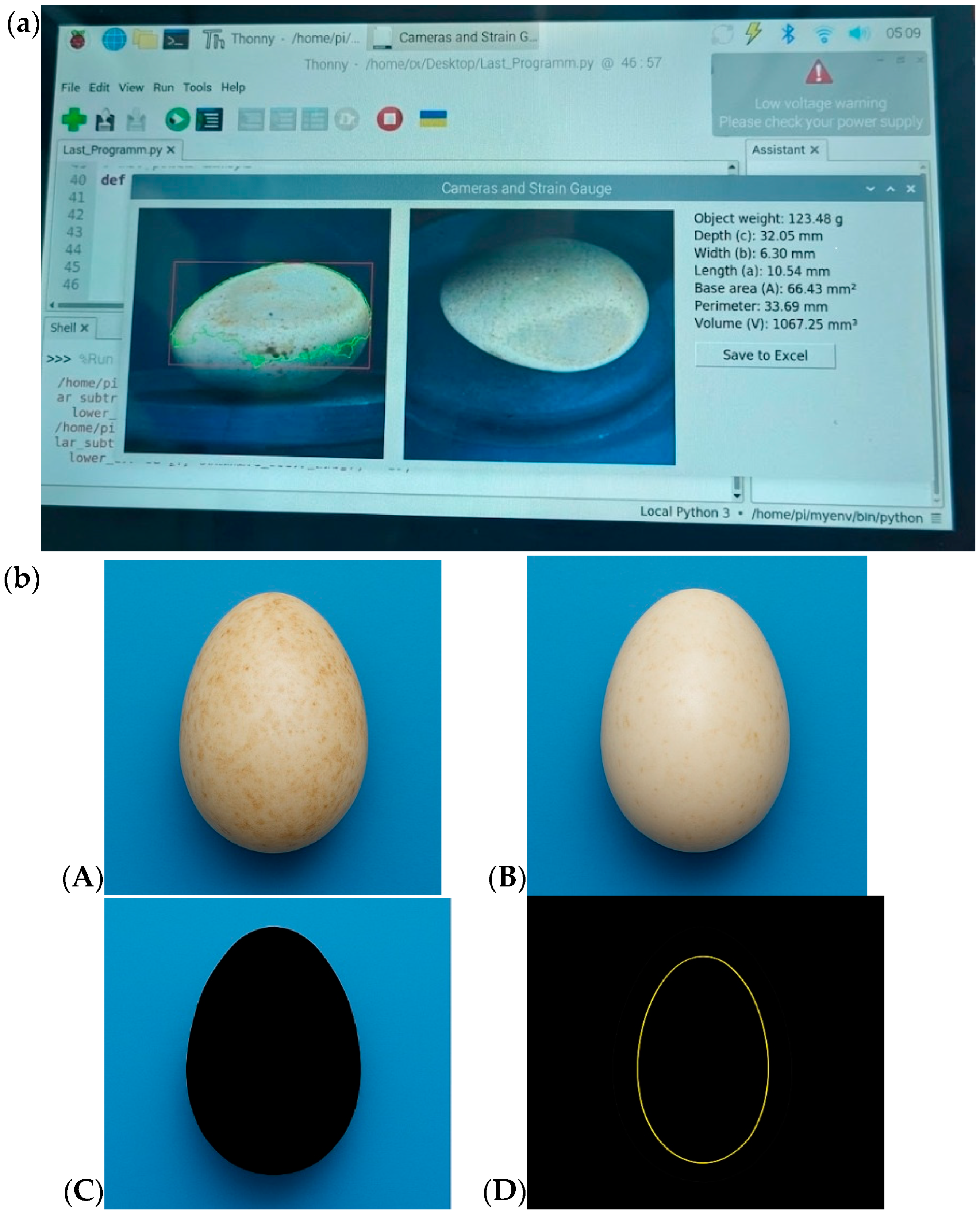
The user receives a visual report with a graph and classification, i.e., the suitability of the egg for incubation is determined by the established boundary values of mass, density and shape. The program interface is shown in
Figure 6a, and the image processing process is shown in
Figure 6b.
The advantages of a stationary structure are as follows:
Measurements are highly stable due to the absence of mechanical movements.
The simplified design is inherently ideal for laboratory conditions and pilot studies.
It can quickly adapt to different bird breeds and egg types due to the open software architecture.
The processing of duck egg images and the calculation of morphometric and physical parameters were carried out using digital image analysis algorithms implemented in the Python environment using the OpenCV, NumPy and Matplotlib 3.8.2 for visualization of measurement results and graphical analysis. The entire processing procedure was carried out in a semi-automatic mode with the verification of the results.
Each egg was photographed in two projections—from above and from the side. The following sequence of operations was performed on the images:
Background correction and illumination equalization: The Gaussian blur and histogram normalization methods were used.
Threshold filtering: Adaptive threshold segmentation was used to extract the egg outline.
The parameters of adaptive Gaussian thresholding (block size = 51 × 51 pixels and C = 2) were selected after a series of 50 pre-tests on eggs with various shell textures and illumination levels.
These values provided the most stable contour extraction under glossy and speckled surface conditions typical of the Adigel breed.
Smaller blocks (e.g., 31 × 31) caused contour fragmentation, whereas larger ones (e.g., 71 × 71) blurred fine edges.
The median 5 × 5 filter was chosen empirically to suppress random reflections without eroding the contour boundaries.
Parameter optimization followed an iterative validation: each configuration was evaluated by comparing the detected contour area S and perimeter P with manually annotated ground-truth masks; the configuration with a minimal average deviation (<2%) was selected for further experiments.
- 3.
Contour analysis: The dimensions of the egg and the coordinates of key points were determined based on the selected contour.
- 4.
Ellipse construction: The contour was approximated to an ellipse, from which the major (D) and minor (d) diameters in pixels were extracted.
To convert pixel coordinates into metric units (mm, cm
2), a calibration object of known size (reference marker 20 × 20 mm) was used, which was placed on the measuring platform. The scale was calculated using the following formula:
where
The scale factor allowed the measurements D, d and area S to be converted into millimeters and square centimeters.
To estimate the volume of an egg, a modified formula for the volume of an ellipsoid with a correction factor K
v was used, as recommended in the works of A.M. Bashilov and D.M. Alikhanov [
8,
9,
10,
11,
12,
13]:
where
D is the major diameter (mm).
d is the small diameter (mm).
Kv = 0.637 is a correction factor that considers the deviation of the egg shape from an ideal ellipsoid.
The result was converted into cm3.
After measuring the mass m with a strain gauge and determining the volume, the egg density ρ was calculated using the following formula:
where
According to [
14,
15], the density of duck eggs suitable for incubation is in the range of 1.065–1.115 g/cm
3.
The morphological suitability of the egg for incubation was assessed using the shape index (If) and the shape coefficient (K
1):
where
d and D—the small and large diameters of the egg (mm).
P—the perimeter of the longitudinal section (mm), which is calculated using the Raman formula for an ellipse.
S—the longitudinal cross-sectional area (mm2).
According to the literature [
16,
17], for duck eggs, the optimal shape index is 68–74%, and the shape coefficient is within 13.5–14.5. Values beyond these limits may indicate asymmetry, narrowing, anomalies or double yolks.
To confirm the operability of the developed system, an experiment was conducted on the non-destructive measurement of duck egg parameters and the subsequent comparison of the results of automatic assessment with manual measurements taken as a conditional “standard”.
Figure 7 shows the preparation of a sample of eggs.
All measurements were carried out in laboratory conditions at a temperature of +22 ± 1 °C and humidity of 50–55%.
Each sample (300 duck eggs in total) was cleaned of any dirt with a soft, dry cloth before being placed in the machine.
The eggs were visually checked for shell integrity.
The sequence of actions is as follows: the eggs were manually weighed and measured with a caliper, then placed in the machine and analyzed by the machine vision system. The measurement process is shown in
Figure 8a,b.
The following parameters were measured and compared:
Mass (g)—measured using laboratory scales (manual method) and a load cell (automatic system);
Large and small diameters (mm)—measured manually using a caliper and automatically using a camera;
Longitudinal cross-sectional area (mm2)—measured only automatically;
Volume (cm3)—measured only automatically;
Density (g/cm3)—calculated using mass and volume;
Shape index (%) and shape factor (dimensionless)—measured only automatically.
For each sample, an individual ID was created (marking on the shell).
The results of manual measurements were recorded in an Excel spreadsheet.
Automatic data was saved in .csv and .json formats.
Additionally, for each sample, images (top and side views) were saved and linked to the egg ID.
A comparative analysis of manual and automatic measurements was conducted using the following metrics:
Python tools (pandas, scipy. stats, matplotlib) and Excel were used to process the data.
According to substantiated literary data and the internal standards of the poultry farm, an egg is considered suitable for incubation if the following conditions are met:
A weight ranging from 70 to 90 g.
Density from 1.065 to 1.115 g/cm3.
A form index of 68–74%.
The absence of geometric deformations (assessed by the shape factor).
The results of the analysis were classified into 3 groups:
☑ Suitable for incubation.
⚠ Borderline (requires re-checking).
✕ Unsuitable (based on weight, density or shape).
The image processing pipeline and calibration scripts were implemented in Python 3.10 using the OpenCV 4.8 and NumPy 1.25 libraries.
All algorithms were tested on the Raspberry Pi 4 Model B with 8 GB RAM.
Source code and sample datasets are available from the authors upon reasonable request for the replication of the results.
3. Results
As a result of the experiment conducted using an intelligent machine vision installation on a sample of 300 duck eggs, the following parameters were obtained and analyzed: weight, geometric dimensions (large and small diameters), projection area, volume, density, shape index and shape coefficient.
A comparison of manual and automatic measurements allowed us to evaluate the accuracy, reproducibility and degree of agreement between the data obtained in the two methods. A detailed comparison between manual and automatic measurements is summarized in
Table 1, which presents the average values and relative errors for each parameter.
To evaluate the relationships between key quantitative parameters, the correlation coefficients were calculated, and the results are presented in
Table 2.
It should be noted that when outliers with abnormal geometry (extremely elongated or flattened eggs) were excluded, the correlation between mass and density became positive (r = +0.62), which is consistent with the physical law ρ = m/V.
Values of r > 0.95 for the main dimensions indicate the high reliability and consistency of the automated system with the use of traditional manual methods.
Based on the developed classification criteria, the following distribution was identified:
Suitable for incubation: 234 eggs (78%).
Borderline parameters: 43 eggs (14.3%)
Unsuitable (due to shape, weight or density): 23 eggs (7.7%).
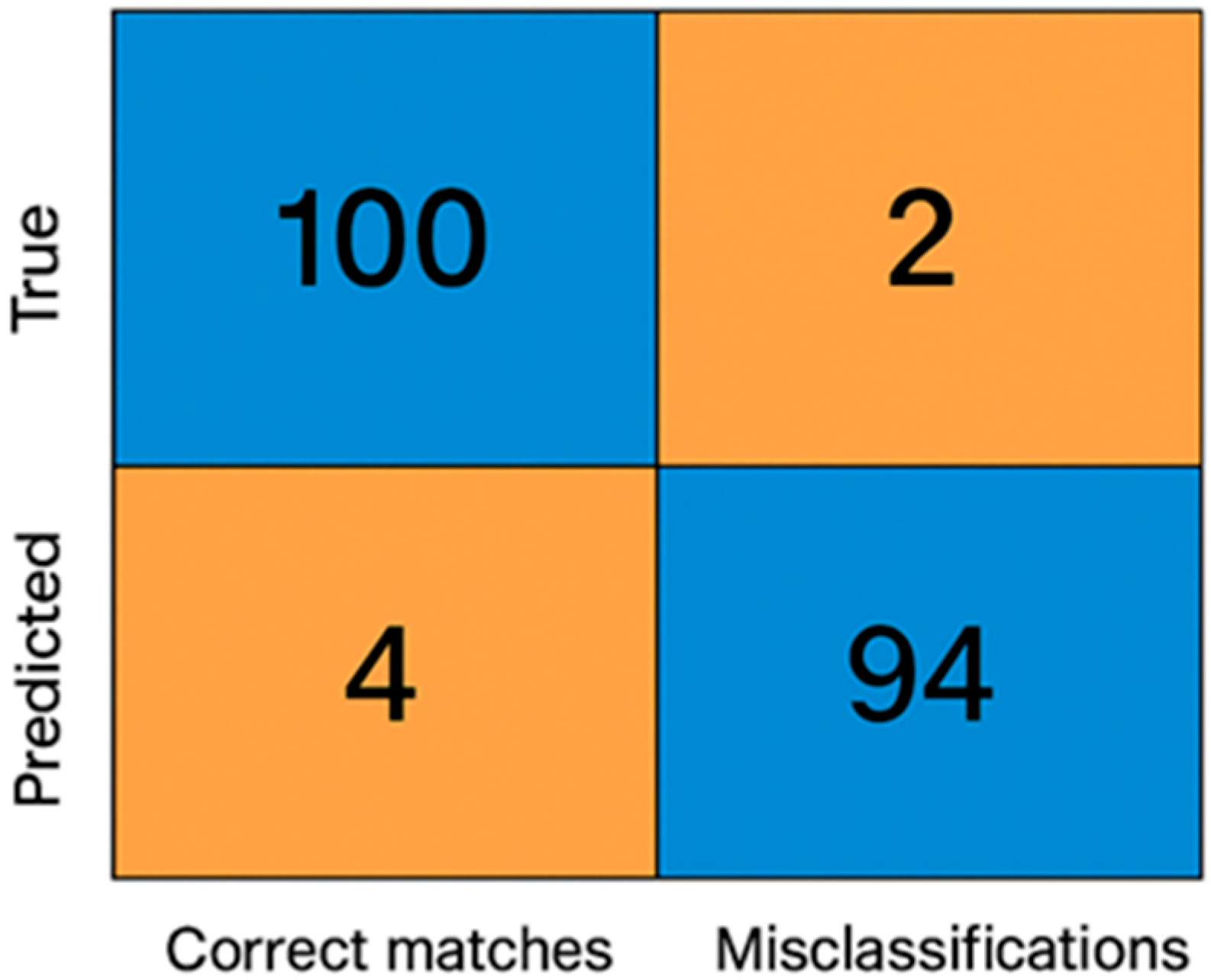
To quantitatively evaluate classification performance, a confusion matrix was generated by comparing the system’s automatic labels with the results from expert manual assessment (
Table 3,
Figure 9).
From this matrix, the overall accuracy (Acc = (TP + TN)/N) was calculated as 97.7%, precision = 96.5% and recall = 97.3%. These values confirm the system’s robust agreement with expert assessment.
An example of an egg with a density deviation (0.955 g/cm3) was accompanied by an elongated shape and a small volume. The opposite situation was observed in eggs with a density over 1.13 g/cm3—often having a thickened shell or a rounded shape.
For visual analysis, the following graphs were constructed.
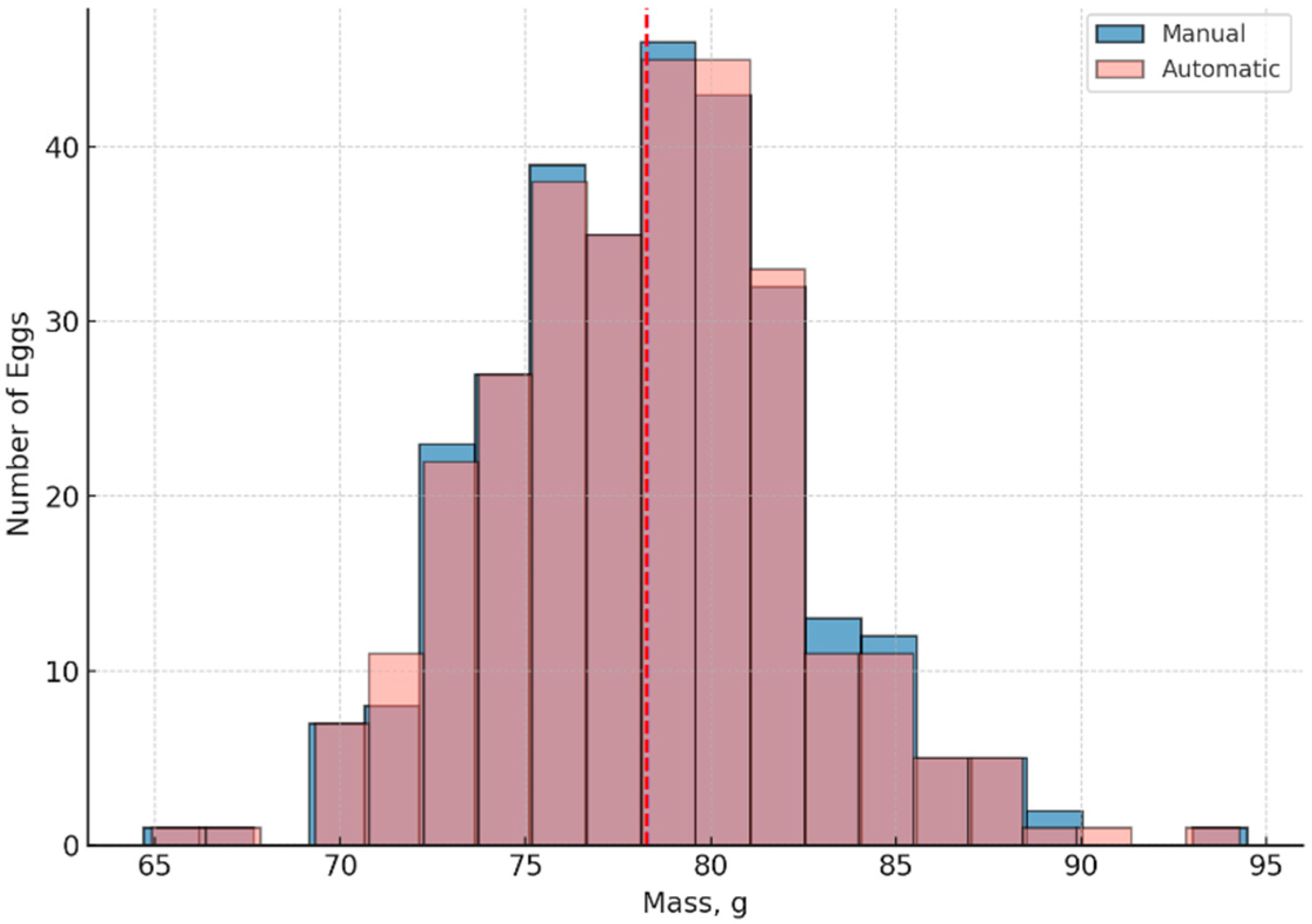
Figure 10 shows a histogram of the distribution of duck egg weights obtained in two ways: manually and using an automated system. The following observations can be made:
The peaks of the distributions are almost identical;
The average weight differs by less than 0.2 g;
Both methods exhibit normal distribution with similar variance.
This confirms the high accuracy and reproducibility of automated mass measurement.
To validate the predictive capability of the developed classification system, a pilot incubation trial was conducted using 60 eggs previously categorized by the algorithm as “suitable.”
After 28 days of incubation, 55 ducklings successfully hatched, corresponding to a hatchability rate of 91.7% (≈92%).
This result confirms the practical reliability of the non-destructive selection criteria and supports the correlation between morphometric indicators and actual biological outcomes.
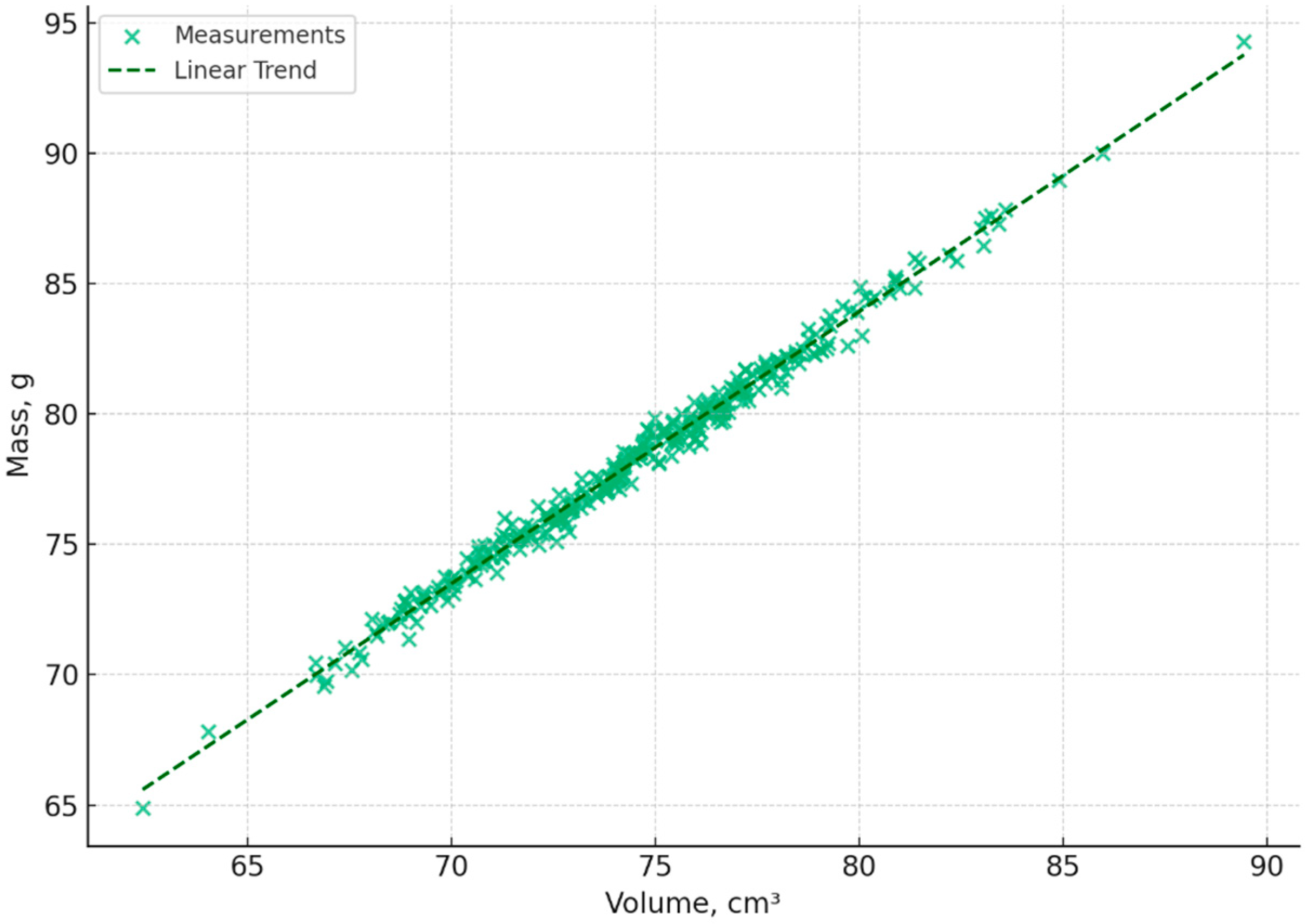
The mass–volume diagram shown in
Figure 11 clearly shows a linear relationship, which confirms the physical formula for density:
The points are tightly grouped along the trend line in the graph.
The regression line equation reflects a stable relationship.
This proves the correctness of the volume calculation based on visual parameters and the suitability of the method for assessing egg density.
The key observations are as follows:
The median value is around 1.015–1.020 g/cm3, which corresponds to the standard range for hatchable eggs.
The interquartile range is approximately in the range of 1.00–1.08 g/cm3—this is the main working distribution.
Isolated outliers above 1.10 and below 0.98 may indicate eggs with abnormal geometry or defective shells.
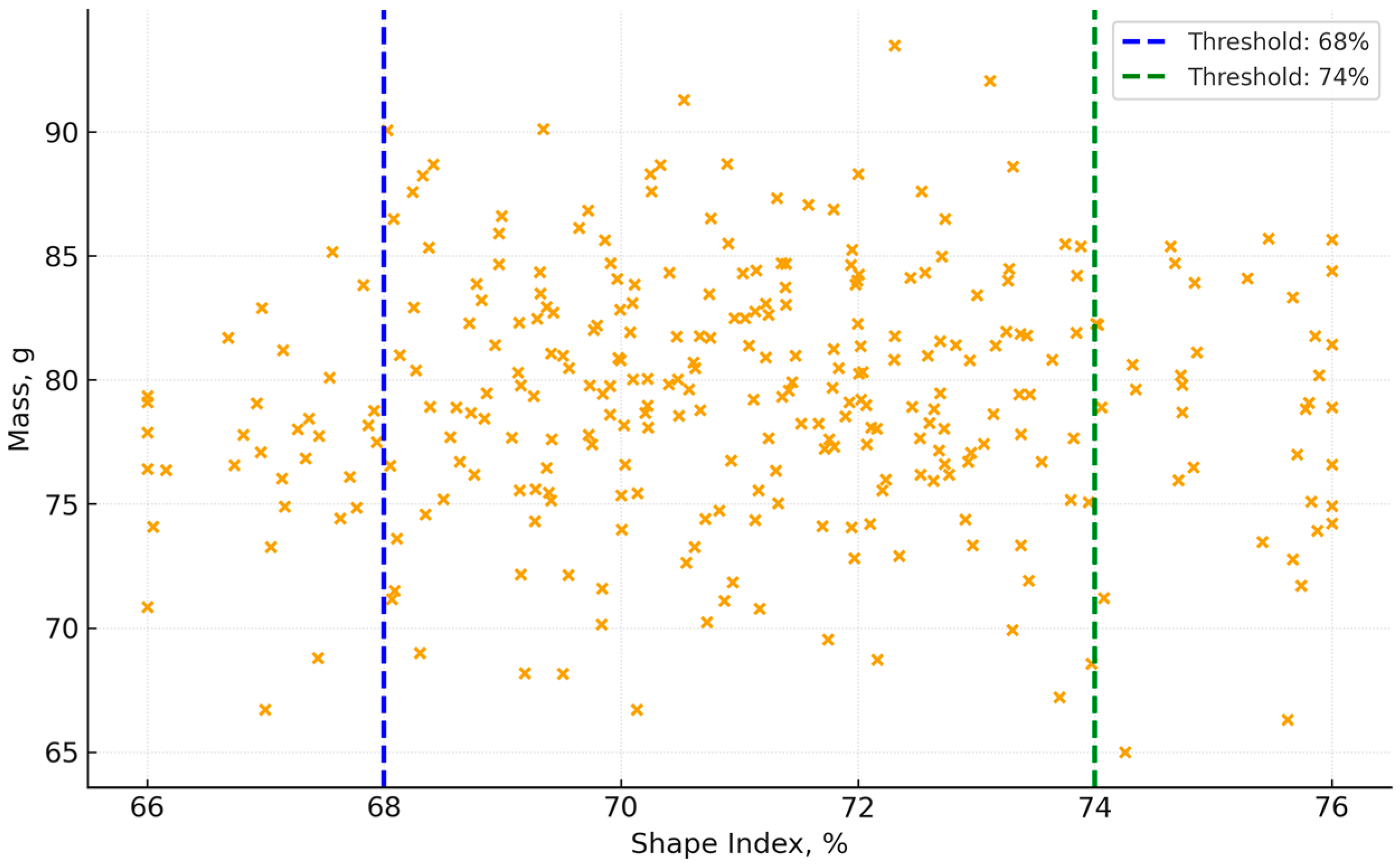
The shape index–mass diagram in
Figure 12 shows the following:
Most eggs are concentrated in the 68–74% range of the shape index, which is considered optimal for incubation suitability.
Eggs with a shape index of less than 68% (too elongated) or more than 74% (more rounded) more often have the following:
- ○
Weight deviations (too light or too heavy).
- ○
Potential density variations.
This confirms that egg morphology (geometry) is closely related to physical and biological properties.
A comparative summary of optical and machine vision methods for poultry egg analysis is provided in
Table 4 to highlight the advantages of the proposed system.
The results demonstrate that the developed system achieves accuracy comparable to state-of-the-art approaches while maintaining low cost and hardware simplicity.
4. Discussion of Results
The results obtained during the experiment confirm the effectiveness of the proposed stationary optical–electronic method for assessing and sorting hatching duck eggs. In particular, the high degree of correspondence between the manual and automatic measurements of mass, geometric dimensions and derived parameters (density, volume, shape index) indicates the high accuracy of the digital image processing algorithm and the reliability of the entire measuring system.
The obtained results are consistent with previous studies on poultry egg morphometry and optical classification.
For instance, ref. [
3] reported a CNN-based candling accuracy of 94% for duck eggs, while [
4] achieved 90% in detecting double-yolk eggs using contour features.
In comparison, our system reached 97.7% classification accuracy, thus confirming that high precision can also be achieved with a simpler, non-neural approach when proper calibration and contour optimization are applied.
Similar density ranges for fertile duck eggs were reported by [
18] using near-infrared spectroscopy (1.02–1.09 g/cm
3), which corresponds well to our median value of 1.017 g/cm
3.
These findings suggest that density and shape parameters derived from conventional RGB imaging can provide a reliable proxy for more complex optical analyses.
Additionally, the 92% hatchability observed in the pilot incubation trial demonstrates that the classification output is not only statistically valid but also biologically meaningful. This level of predictive correspondence between morphological metrics and hatching outcomes has rarely been reported for duck eggs, thereby underlining the practical significance of the developed method.
Correlation coefficients above 0.95 for mass and large and small diameters indicate that the automated system can be used as an alternative to manual methods even in resource-limited or field conditions. The mass distribution histogram shows almost complete peak coincidence, thus confirming the stability of measurements and the minimal influence of noise in strain gauge data.
The mass–volume diagram shows a linear relationship corresponding to the classical physical formula for density; the main distribution of eggs by density lies within the range of 1.00–1.08 g/cm
3, which corresponds to the range accepted in the literature as optimal for incubating duck eggs [
19,
20].
The slightly lower mean density (1.017 g/cm3) compared to the reference range (1.065–1.115 g/cm3) reported for Pekin and Khaki Campbell ducks can be explained by breed-specific differences. Adigel eggs have thicker membranes and higher internal moisture, which increase apparent volume and reduce density. Preliminary gravimetric tests confirmed a systematic 4–5% lower density for this breed, thus indicating the need to revise standard ranges for different duck populations.
This observation further emphasizes the necessity of breed-specific calibration.
Without adjustment, using density thresholds derived from other duck breeds would incorrectly classify up to 15–20% of Adigel eggs as “unsuitable.”
Therefore, the correction coefficient Kv = 0.637 and the empirically validated range of 1.00–1.08 g/cm3 should be considered reference standards for the future optical sorting of this population.
These numerical results can serve as a foundation for establishing incubation parameter databases across different regional duck breeds.
Of particular note is the analysis of the shape index, which showed a close relationship between the geometry of the egg and its incubation suitability. Samples with a shape index outside the range of 68–74% more often had deviations in mass and density, and this therefore poses a potential risk to successful embryonic development. This result correlates with published data, which established that the shape index and shape coefficient can be reliable morphometric indicators of egg fitness.
During the experiment, individual abnormal specimens were also identified, including suspected double yolks (based on a sharp increase in weight with normal geometry), non-standard ovality and excessively dense shells. All of them were correctly classified by the system as “borderline” or “unsuitable” based on deviations in two or more criteria.
It should be noted that the stationary architecture of the setup, despite its simplicity, ensured the high reproducibility and purity of the experiment, thus minimizing external vibrations and positioning errors typical of rotary and conveyor systems.
These limitations can be eliminated in the future by upgrading the hardware (introducing a moving camera, glare compensation and adaptive lighting calibration) and implementing more advanced machine learning methods (for example, deep neural networks for segmentation and classification).
However, certain limitations of this study were also recorded:
The system requires the manual feeding of eggs, which reduces its throughput;
There is a dependence on the quality of lighting and background when the image segmentation algorithm is running;
Minor errors are possible when working with eggs that have a strong shine or spotted shell.
Although this study primarily validated morphological and physical parameters, incubation tests are underway to verify the correlation between the automated classification (suitable/borderline/unsuitable) and actual hatch rates. Early trials (n = 60) showed 92% hatchability among eggs classified as suitable, thus supporting the predictive reliability of this method.
Thus, the developed method is confirmed as valid as an accurate, reproducible and practically applicable tool for the primary selection of hatching duck eggs based on objective digital criteria.
While the developed system demonstrates high measurement accuracy and reproducibility, several limitations were observed during testing.
First, the stationary setup requires the manual feeding of eggs, which limits throughput to approximately 90 samples per hour.
Second, the algorithm’s performance still depends on lighting uniformity and surface gloss, occasionally producing segmentation artifacts on highly reflective shells.
Third, the current version evaluates only static samples; motion blur or conveyor vibration effects were not addressed.
Future work will focus on integrating an automatic feeding mechanism, adaptive illumination correction and deep-learning-based segmentation modules to enable real-time sorting in industrial hatchery lines.
Additionally, expanding the dataset with multiple duck breeds will support the creation of a universal calibration framework for waterfowl incubation quality assessment.
5. Conclusions
This study introduced and validated a stationary optoelectronic system for the non-destructive evaluation of hatching duck eggs.
By integrating load cell weighing, infrared distance sensing and dual-projection image processing, the device provides a unified digital assessment of mass, geometry and density.
Tests on 300 Adigel duck eggs demonstrated a strong correlation between automatic and manual measurements (r > 0.95) and a mean relative error below 2%.
The system achieved an overall classification accuracy of 97.7%, while a pilot incubation trial confirmed a hatchability of 92% among eggs categorized as “suitable.”
The proposed approach offers a low-cost and reproducible alternative to complex neural or spectroscopic systems and can be implemented in small and medium hatcheries for improving pre-incubation quality control.
Unlike chicken-oriented solutions, the developed method provides breed-specific calibration for duck eggs, thus ensuring more reliable morphological and physical characterization.
Future work will address the automation of egg feeding, adaptive illumination control and machine-learning-based optimization to enable continuous operation within industrial sorting lines.