Advancing Leaf Nutritional Characterization of Blueberry Varieties Adapted to Warm Climates Enhanced by Proximal Sensing
Abstract
1. Introduction
2. Materials and Methods
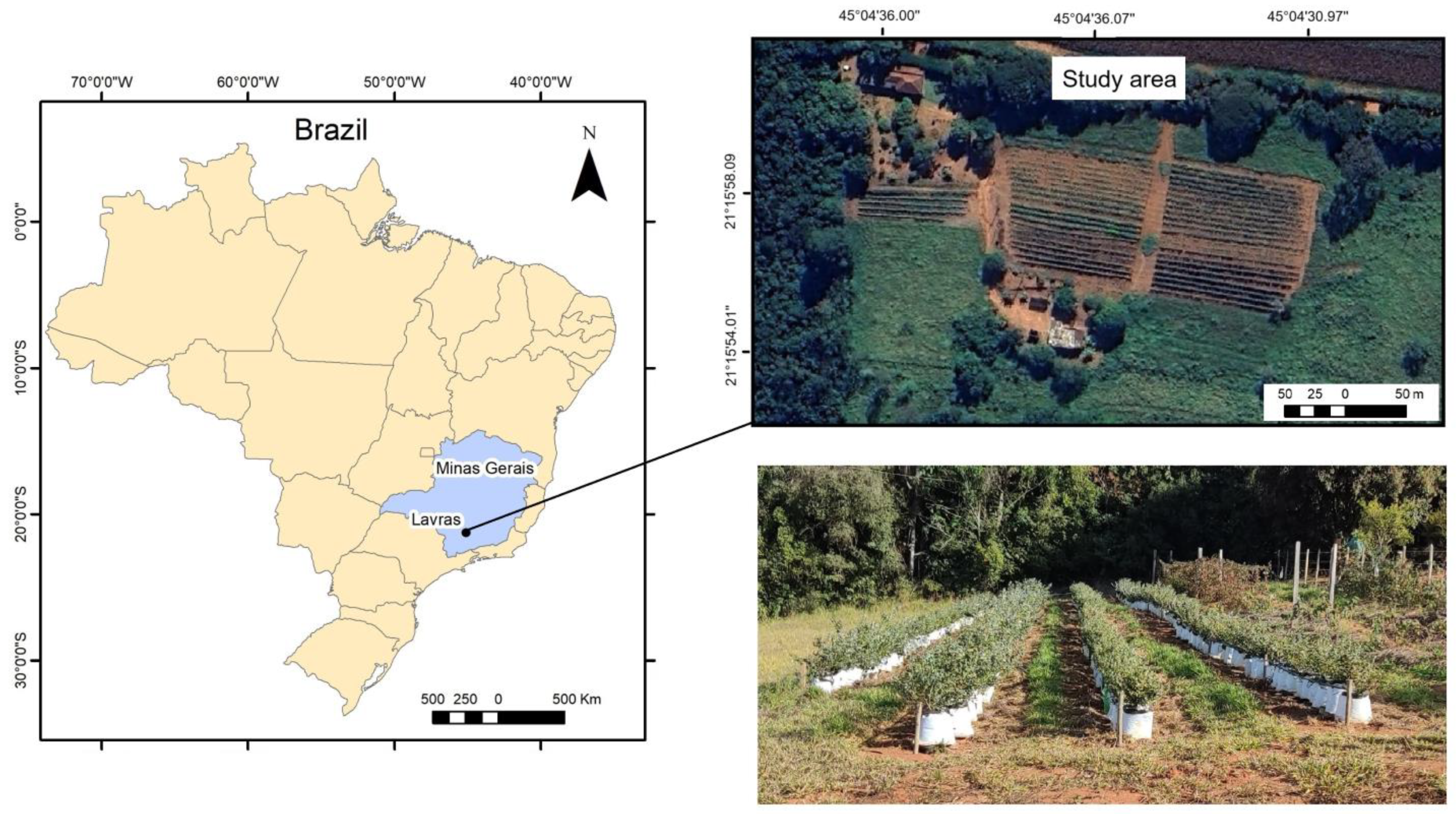
2.1. Study Area and Sampling
2.2. Laboratory Analyses
2.3. Statistical Analyses
3. Results and Discussion
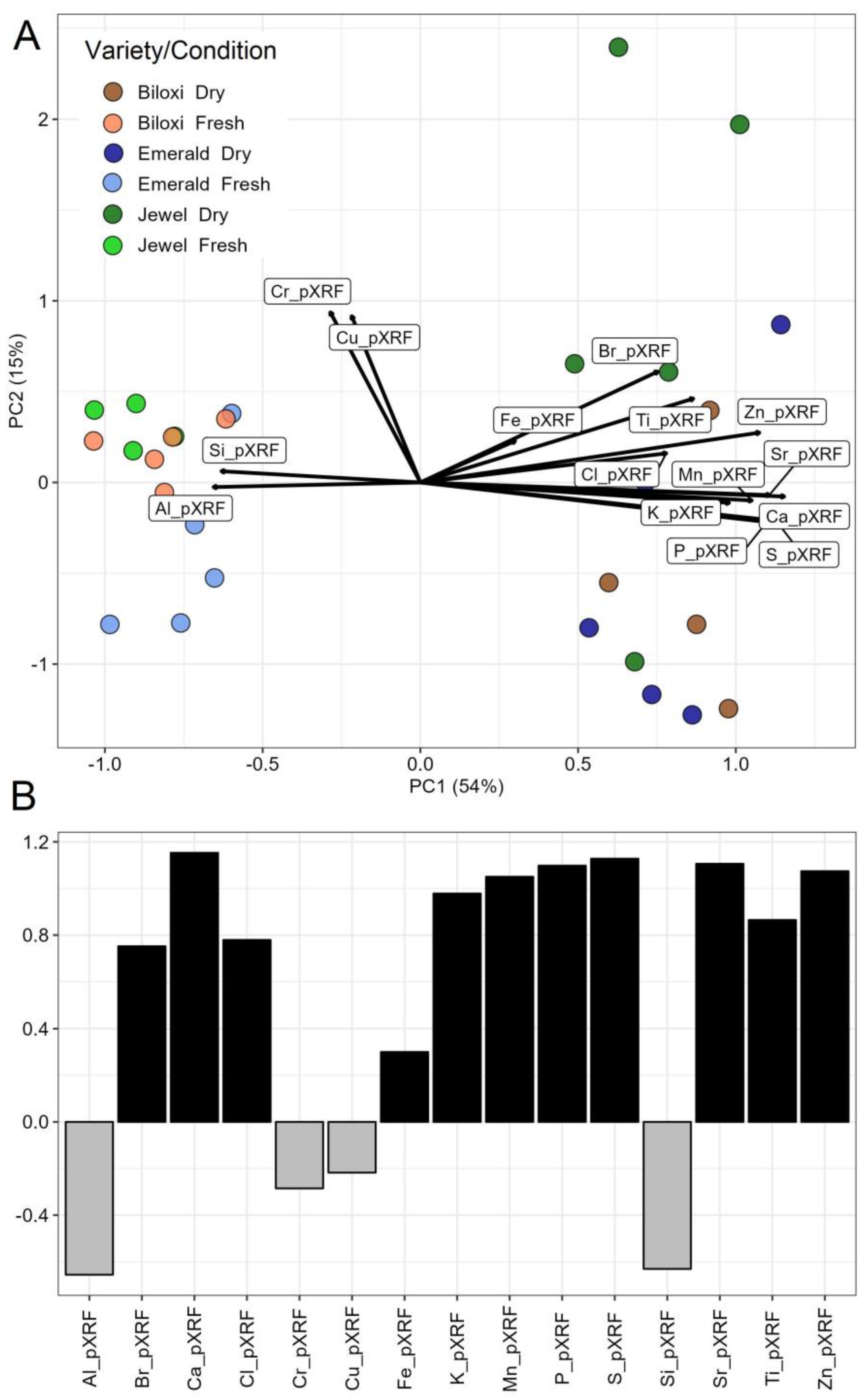
3.1. Characterization of Nutritional Differences of Blueberry Varieties
3.2. Effects of Moisture on pXRF Results
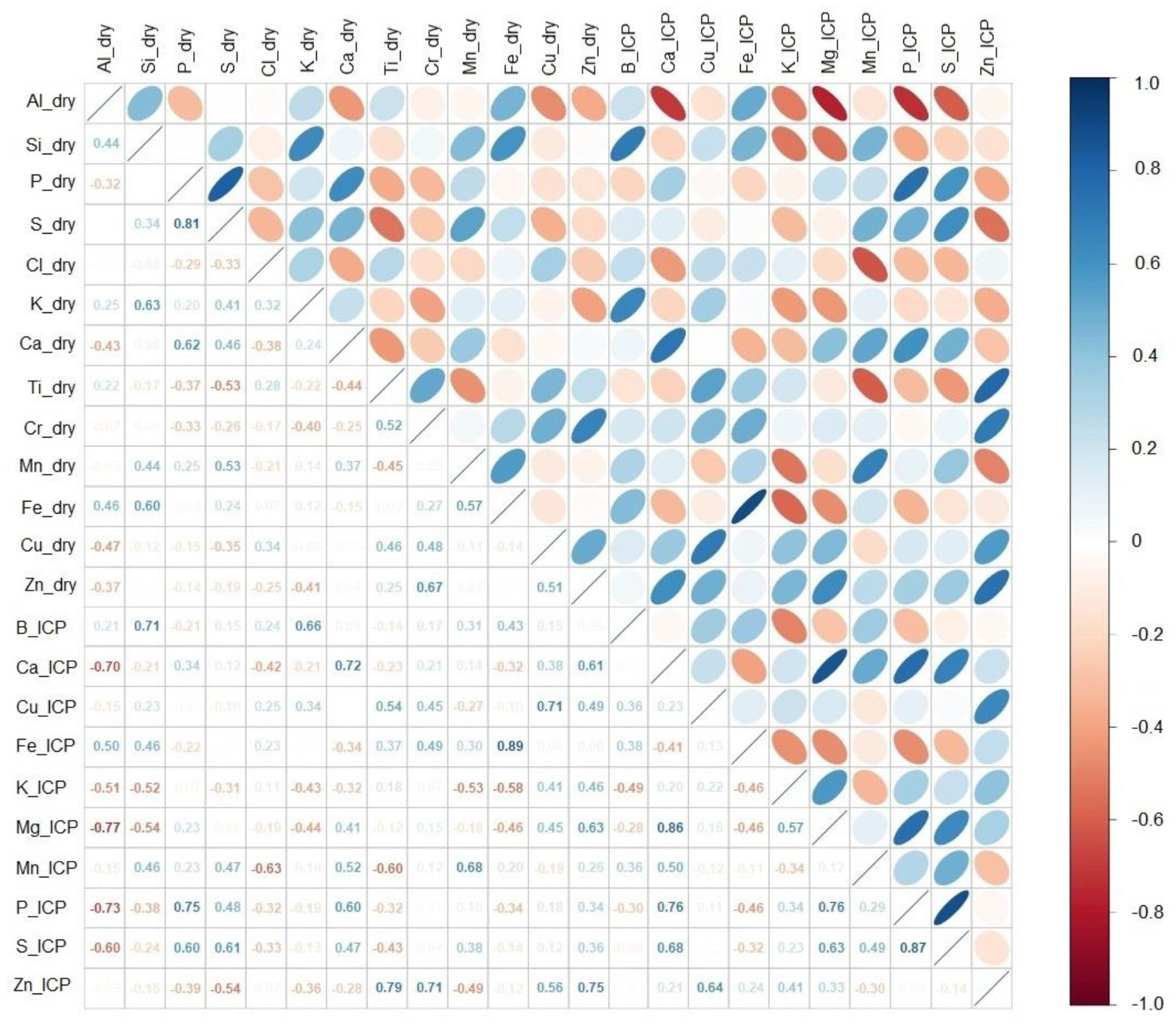
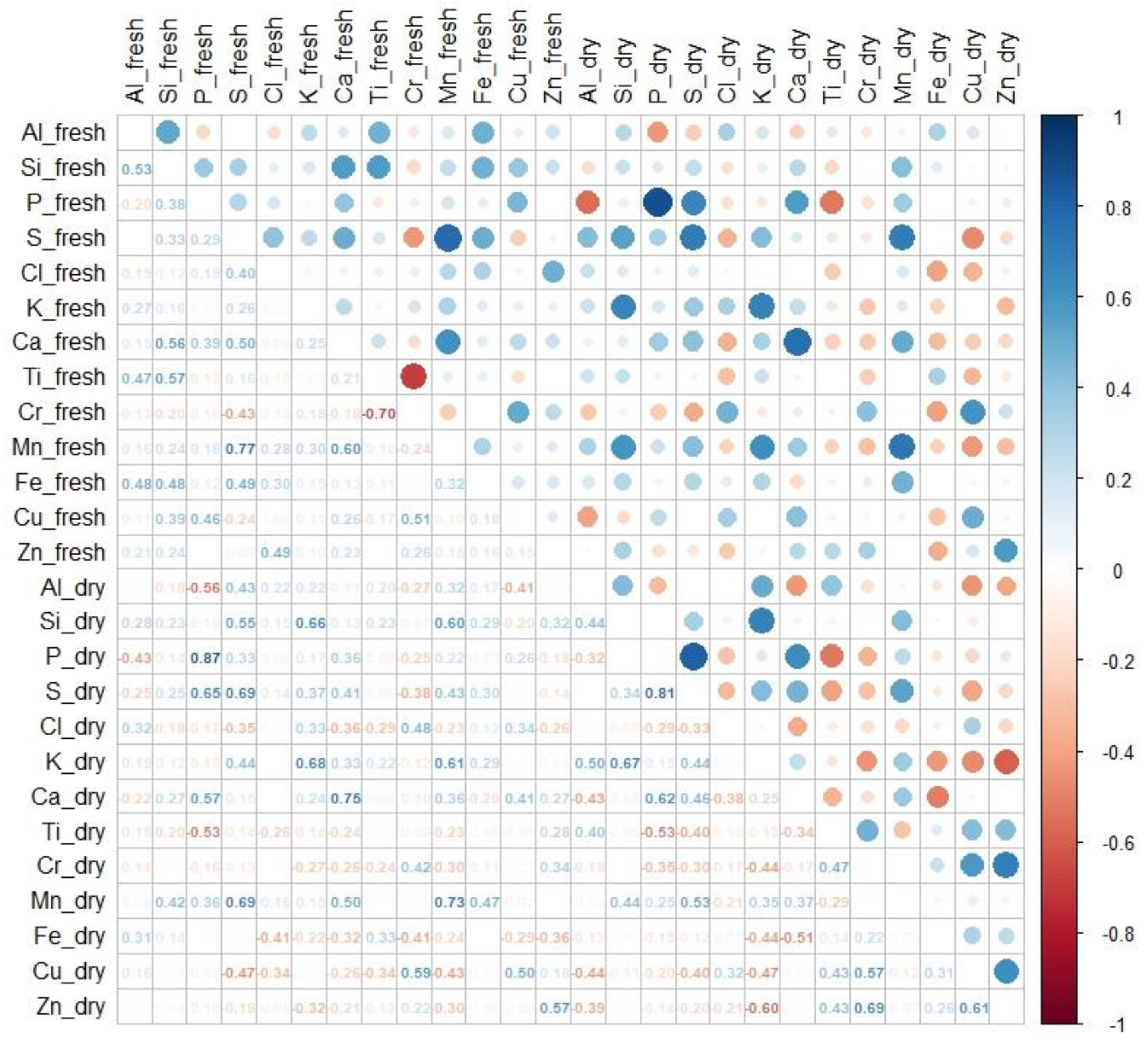
3.3. Correlations between pXRF and ICP Results
3.4. Prediction of Blueberry Varieties Based on the Elemental Contents of Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, E.K.; Lee, S.Y.; Ghanem, N.; Vazquez, A.R.; Johnson, S.A. Protective effects of blueberries on vascular function: A narrative review of preclinical and clinical evidence. Nutr. Res. 2023, 120, 20–57. [Google Scholar] [CrossRef]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Yuan, B.Z.; Sun, J. Bibliometric analysis of blueberry (Vaccinium corymbosum L.) research publications based on Web of Science. Food Sci. Technol. 2022, 42, e96321. [Google Scholar] [CrossRef]
- Doyle, J.W.; Nambeesan, S.U.; Malladi, A. Physiology of nitrogen and calcium nutrition in blueberry (Vaccinium sp.). Agronomy 2021, 11, 765. [Google Scholar] [CrossRef]
- Martini, D.; Marino, M.; Venturi, S.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del Bo, C. Blueberries and their bioactives in the modulation of oxidative stress, inflammation and cardio/vascular function markers: A systematic review of human intervention studies. J. Nutr. Biochem. 2023, 111, 109154. [Google Scholar] [CrossRef]
- Carpenedo, S.; Raseira, M.d.C.B.; Franzon, R.C. Importância e Perspectivas para a Cultura do Mirtilo no Brasil; Documentos 526; Embrapa Clima Temperado: Pelotas, Brazil, 2022. [Google Scholar]
- Williamson, J.G.; Lyrene, P.M. Blueberry Varieties for Florida. Edis 1969, 2004, 1–9. [Google Scholar] [CrossRef]
- Antunes, L.E.C.; Baccan, R. Cultivares de Mirtilos para Produção em Vasos, 1st ed.; Embrapa: Pelotas, Brazil, 2023. [Google Scholar]
- Medina, R.B.; Cantuarias-Avilés, T.E.; Angolini, S.F.; Silva, S.R.d. Performance of ‘emerald’ and ‘jewel’ blueberry cultivars under no-chill incidence. Pesqui. Agropecuária Trop. 2018, 48, 147–152. [Google Scholar] [CrossRef]
- Lima, F.N.d.; Miranda, G.S.; Yamanishi, O.K.; Pires, M.d.C.; Pereira, E.D.S.A.R. Ecophysiology of the Southern Highbush blueberry cv. Biloxi in response to nitrogen fertigation. Comun. Sci. 2020, 11, e3245. [Google Scholar] [CrossRef]
- Ratnaparkhe, M.B. Blueberry. In Fruits and Nuts. Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 2017–2018. [Google Scholar]
- Rufato, A.D.R.; Antunes, L.E.C. Técnicas de Produção de Framboesa e Mirtilo; Embrapa Clima Temperado: Brasília, Brazil, 2016; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/158076/1/Tecnicas-de-Producao-de-Framboesa-e-Mirtilo-Incluido.pdf (accessed on 27 June 2024).
- Cortés-Rojas, M.E.; Mesa-Torres, P.A.; Grijalba-Rativa, C.M.; Pérez-Trujillo, M.M. Rendimiento y calidad de frutos de los cultivares de arándano Biloxi y Sharpblue en Guasca, Colombia. Agron. Colomb. 2016, 34, 33–41. [Google Scholar] [CrossRef]
- Medina, R.B.; Tezotto-Uliana, J.V.; Santoro, M.B.; Silva, S.R.d. Postharvest quality of “Emerald” blueberry cultivated in a subtropical region. Pesqui. Agropecu. Bras. 2022, 57, e02683. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Hirzel, J. Can the Firmness, Weight, and Size of Blueberry Fruit Be Enhanced through the Application of Low Amounts of Calcium to the Soil? Plants 2024, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, H.; Quaggio, J.A.; Mattos, D., Jr.; Boaretto, R.M.; van Raij, B. Recomendações de Adubação e Calagem para o Estado de São Paulo (Boletim Técnico 100); IAC: Campinas, Brazil, 2022. [Google Scholar]
- Ishfaq, M.; Kiran, A.; Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Borges, C.S.; Weindorf, D.C.; Carvalho, G.S.; Guilherme, L.R.G.; Takayama, T.; Curi, N.; Lima, G.J.E.O.; Ribeiro, B.T. Foliar elemental analysis of Brazilian crops via portable X-ray fluorescence spectrometry. Sensors 2020, 20, 2509–2525. [Google Scholar] [CrossRef]
- Weindorf, D.C.; Bakr, N.; Zhu, Y. Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Adv. Agron. 2014, 128, 1–45. [Google Scholar] [CrossRef]
- Andrade, R.; Silva, S.H.G.; Benedet, L.; Araújo, E.F.d.; Carneiro, M.A.C.; Curi, N. A Proximal Sensor-Based Approach for Clean, Fast, and Accurate Assessment of the Eucalyptus spp. Nutritional Status and Differentiation of Clones. Plants 2023, 12, 561. [Google Scholar] [CrossRef]
- McGladdery, C.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Paulette, L.; Podar, D.; Pearson, D.; Kusi, N.Y.O.; Duda, B. Elemental assessment of vegetation via portable X-ray fluorescence (PXRF) spectrometry. J. Environ. Manag. 2018, 210, 210–225. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Li, W.; Zhang, C.; Wan, M.; Huang, B.; Zhao, Y. Correction of in-situ portable X-ray fluorescence (PXRF) data of soil heavy metal for enhancing spatial prediction. Environ. Pollut. 2019, 254, 112993. [Google Scholar] [CrossRef]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for environmental assessment of soils: Not just a point and shoot method. Environ. Int. 2020, 134, 105250. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.d.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, Brazil, 1997. [Google Scholar]
- Miller, R. Nitric-Perchloric Acid Wet Digestion in an Open Vessel. In Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1998; pp. 57–61. [Google Scholar]
- R Development Core Team. R: A Language and Environmental for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package “corrplot”. R Development Core Team. 2017, Volume 18. Available online: https://cran.r-project.org/web/packages/corrplot/corrplot.pdf (accessed on 12 May 2024).
- Ferreira, D.F. Sisvar: A guide for its bootstrap procedures in multiple comparisons. Ciência Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package “factoextra”: Extract and visualize the results of multivariate data analyses. R Development Core Team. 2022. Available online: https://cran.r-project.org/web/packages/factoextra/factoextra.pdf (accessed on 12 May 2024).
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Carlier, E.; Marina Zapata, L. Mineral composition of blueberries (Vaccinium corymbosum) cultivated in the northeast region of Argentina. Rev. Iberoam. Tecnol. Postcosecha 2021, 22, 71–85. [Google Scholar]
- Totad, M.G.; Sharma, R.R.; Sethi, S.; Joshi, A.; Verma, M.K.; Sharma, V.K.; Singh, S.; Dhiman, M.R.; Jaiswal, S. Genotypic variability in nutritional and functional attributes of blueberry varieties grown in northern-western Himalayas. J. Food Sci. Technol. 2020, 57, 2251–2258. [Google Scholar] [CrossRef]
- Rivaneira, M.F. Concentración de nutrientes en hojas de diferente estado de desarrollo en arándano. RIA. Rev. Investig. Agropecu. 2012, 38, 247–250. [Google Scholar]
- Arrington, M.; DeVetter, L.W. Foliar applications of calcium and boron do not increase fruit set or yield in northern highbush blueberry (Vaccinium corymbosum). HortScience 2017, 52, 1259–1264. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A. Effect of foliar fertilization of microelements on highbush blueberry (Vaccinium corumbosum L.) nutrient status and yield components in cutover peatlands. Agron. Res. 2019, 17, 133–143. [Google Scholar]
- Clark, J.R.; Creech, D.; Austin, M.E.; Ferree, M.E.B.; Lyrene, P.; Mainland, M.; Makus, D.; Neuwndorff, L.; Patten, K.; Spiers, J.M. Foliar elemental analysis of southern highbush, rabbiteye, and highbush blueberries in the southern United States. Horttechnology 1994, 4, 351–354. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P.; Kafkas, N.E.; Yesil, B.; Cieśliński, S. Foliar application of some macronutrients and micronutrients improves yield and fruit quality of highbush blueberry (Vaccinium corymbosum L.). Horticulturae 2022, 8, 664. [Google Scholar] [CrossRef]
- Sahraoui, H.; Hachicha, M. Effect of soil moisture on trace elements concentrations. J. Fundam. Appl. Sci. 2017, 9, 468–484. [Google Scholar] [CrossRef]
- Ribeiro, B.T.; Weindorf, D.C.; Borges, C.S.; Guilherme, L.R.G.; Curi, N. Foliar analysis via portable X-ray fluorescence spectrometry: Experimental considerations. Spectrochim. Acta Part. B At. Spectrosc. 2021, 186, 106320. [Google Scholar] [CrossRef]
- Stockmann, U.; Cattle, S.R.; Minasny, B.; McBratney, A.B. Utilizing portable X-ray fluorescence spectrometry for in-field investigation of pedogenesis. Catena 2016, 139, 220–231. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulphur in crop production—Invited paper. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.V.d.; Lima, G.J.d.O.; Guilherme, L.R.G.; Carneiro, M.A.C.; Ribeiro, B.T. Towards direct and eco-friendly analysis of plants using portable X-ray fluorescence spectrometry: A methodological approach. Chemosphere 2023, 339, 665583. [Google Scholar] [CrossRef] [PubMed]
- Towett, E.K.; Shepherd, K.D.; Drake, B.L. Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: Quantification under different analytical parameters. X-ray Spectrom. 2016, 45, 117–124. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, Q.; Weindorf, D.C.; Yang, B.; Yuan, Z.; Yang, J. Determination of trace element concentrations in organic materials of, J. “intermediate-thickness” via portable X-ray fluorescence spectrometry. J. Anal. Spectrom. 2022, 37, 2461–2469. [Google Scholar] [CrossRef]
- Costa Junior, G.T.; Nunes, L.C.; Gomes, M.H.F.; Almeida, E.d.; Carvalho, H.W.P.d. Direct determination of mineral nutrients in soybean leaves under vivo conditions by portable X-ray fluorescence spectroscopy. X-ray Spectrom. 2020, 49, 274–283. [Google Scholar] [CrossRef]
- Antonangelo, J.; Zhang, H. Soil and plant nutrient analysis with a portable XRF probe using a single calibration. Agronomy 2021, 11, 2118. [Google Scholar] [CrossRef]
- Otaka, A.; Hokura, A.; Nakai, I. Determination of trace elements in soybean by X-ray fluorescence analysis and its application to identification of their production areas. Food Chem. 2014, 147, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, F.M.G.; Carvalho, G.S.; Hein, P.R.G.; Napoli, A.; Wojcieszak, R.; Guilherme, L.R.G. Artificial Neural Networks to Distinguish Charcoal from Eucalyptus and Native Forests Based on Their Mineral Components. Energy Fuels 2020, 34, 9599–9608. [Google Scholar] [CrossRef]


| CRM | Al | Ca | Cl | Cu | Fe | K | Mn | P | S | Si | Ti | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -------------------------------------------------------------%-------------------------------------------------------------------- | ||||||||||||
| CS-P | – | 87 | – | – | – | 87 | 102 | 81 | 82 | – | – | 100 |
| 1573a | 136 | 73 | 72 | 137 | 138 | 72 | 121 | 56 | 60 | – | – | 197 |
| 1547 | 506 | 80 | 190 | 198 | 181 | 85 | 135 | 78 | 64 | – | – | 147 |
| Variety | Statistics | B | Ca | Cu | Fe | K | Mg | Mn | P | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| -------------------------------------------------------------------mg kg−1---------------------------------------------------- | |||||||||||
| Emerald | Mean | 269.92 | 5725.50 | 3.27 | 3343.39 | 8296.18 | 1572.52 | 534.08 | 1862.20 | 2993.91 | 17.94 |
| SD | 58 | 1012 | 1 | 6946 | 2870 | 344 | 74 | 399 | 470 | 4 | |
| Jewel | Mean | 263.60 | 6787.21 | 5.34 | 2123.23 | 10,278.41 | 2109.30 | 426.94 | 1775.18 | 2564.49 | 28.24 |
| SD | 54 | 2644 | 3 | 3194 | 808 | 499 | 141 | 452 | 673 | 12 | |
| Biloxi | Mean | 255.75 | 7627.59 | 5.14 | 119.88 | 9050.80 | 2108.72 | 480.19 | 2491.53 | 3441.49 | 16.13 |
| SD | 80 | 1429 | 3 | 30 | 1793 | 508 | 46 | 443 | 652 | 5 | |
| Variety | Cond. | Stats | Al | Si | P | S | Cl | K | Ca | Ti | Cr | Mn | Fe | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ------------------------------------------------------------mg kg−1------------------------------------------------------- | |||||||||||||||
| Emerald | Fresh | Mean | 695 aA | 2874 aA | 137 bB | 143 bA | 116 aA | 1839 b | 1140 bA | 0 bA | 3 aB | 287 bA | 150 bA | 9 aB | 13 bA |
| SD | 434.8 | 1239.0 | 7.9 | 48.8 | 112.2 | 632.7 | 254.7 | 0.3 | 0.8 | 102.5 | 30.7 | 1.0 | 1.0 | ||
| Dry | Mean | 522 aA | 2023 aA | 799 aB | 1356 aA | 164 aA | 8418 aA | 6925 aB | 3 aA | 3 aA | 663 aA | 278 aA | 7 bB | 24 aA | |
| SD | 227.3 | 441.1 | 50.0 | 84.5 | 54.3 | 1636.0 | 607.3 | 1.0 | 1.2 | 194.7 | 75.8 | 1.1 | 2.2 | ||
| Jewel | Fresh | Mean | 799 aA | 2176 aA | 119 bB | 11 bC | 26 bA | 1623 b | 973 bA | 0 bA | 4 aA | 195 bA | 126 bA | 10 aA | 14 bA |
| SD | 418.2 | 877.4 | 18.5 | 20.1 | 41.7 | 331.4 | 128.6 | 0.0 | 0.3 | 47.6 | 36.3 | 1.0 | 2.0 | ||
| Dry | Mean | 320 bB | 1721 aA | 620 aB | 856 aB | 270 aA | 7449 aA | 6879 aB | 4 aA | 4 aA | 495 aA | 258 aA | 10 aA | 26 aA | |
| SD | 140.8 | 246.6 | 128.1 | 251.4 | 125.6 | 1456.1 | 1063.9 | 1.8 | 1.6 | 105.2 | 66.6 | 2.6 | 4.1 | ||
| Biloxi | Fresh | Mean | 586 aA | 3301 aA | 171 bA | 74 bB | 62 bA | 2478 b | 1218 bA | 0 bA | 4 aA | 229 bA | 134 bA | 10 aA | 12 bA |
| SD | 311.5 | 536.2 | 26.1 | 17.4 | 137.7 | 2133.3 | 85.5 | 0.0 | 0.3 | 32.7 | 23.0 | 0.4 | 1.5 | ||
| Dry | Mean | 149 bB | 1697 bA | 1000 aA | 1385 aA | 243 aA | 7918 aA | 7962 aA | 1 aB | 3 aA | 640 aA | 249 aA | 10 aA | 23 aA | |
| SD | 160.9 | 488.9 | 204.0 | 197.2 | 88.5 | 1805.0 | 443.5 | 1.0 | 1.1 | 97.0 | 49.6 | 2.0 | 3.0 | ||
| Variety | Condition | Ca | Cu | Fe | K | Mn | P | S | Zn |
|---|---|---|---|---|---|---|---|---|---|
| -------------------------------------------------------mg kg−1----------------------------------------------------- | |||||||||
| Emerald | ICP | 5726 | 3 | 3343 | 8296 | 534 | 1862 | 2994 | 18 |
| Dry | 6925 | 7 | 278 | 8418 | 663 | 799 | 1356 | 24 | |
| Fresh | 1140 | 9 | 150 | 1839 | 287 | 137 | 143 | 13 | |
| Jewel | ICP | 6787 | 5 | 2123 | 10,278 | 427 | 1775 | 2564 | 28 |
| Dry | 6879 | 10 | 258 | 7449 | 495 | 620 | 856 | 26 | |
| Fresh | 973 | 10 | 126 | 1623 | 195 | 119 | 11 | 14 | |
| Biloxi | ICP | 7628 | 5 | 120 | 9051 | 480 | 2492 | 3441 | 16 |
| Dry | 7962 | 10 | 249 | 7918 | 640 | 1000 | 1385 | 23 | |
| Fresh | 1218 | 10 | 134 | 2478 | 229 | 171 | 74 | 12 | |
| Variety | Emerald | Jewel | Biloxi | General | ||||
|---|---|---|---|---|---|---|---|---|
| Dry | Fresh | Dry | Fresh | Dry | Fresh | Dry | Fresh | |
| Ca | 0.34 | 0.22 | 0.87 | 0.89 | 0.44 | 0.67 | 0.72 | 0.41 |
| Cu | 0.66 | −0.38 | 0.88 | 0.33 | 0.29 | −0.33 | 0.71 | 0.22 |
| Fe | −0.34 | 0.57 | 0.69 | −0.27 | 0.87 | 0.60 | 0.71 | 0.22 |
| K | −0.89 | −0.96 | −0.82 | −0.21 | −0.56 | −0.44 | −0.73 | −0.43 |
| Mn | 0.73 | 0.65 | 0.88 | 0.81 | 0.15 | −0.44 | 0.68 | 0.61 |
| P | 0.85 | 0.27 | 0.86 | 0.86 | 0.48 | 0.90 | 0.75 | 0.84 |
| S | 0.16 | −0.45 | 0.88 | 0.76 | −0.03 | 0.12 | 0.61 | 0.24 |
| Zn | 0.84 | −0.39 | 0.66 | −0.01 | 0.92 | 0.82 | 0.75 | 0.29 |
| Element | Variety | Condition | Equation * | R2 |
|---|---|---|---|---|
| Ca Ca | Jewel | Dry | CaICP = 2.1622x − 8087 | 0.76 |
| Jewel | Fresh | CaICP = 18.284x − 11,012 | 0.79 | |
| Cu | Jewel | Dry | CuICP = 1.065x − 5.741 | 0.78 |
| Fe | Biloxi | Dry | FeICP = 0.534x − 12.967 | 0.76 |
| K | Emerald | Dry | KICP = −1.5603x + 21,431 | 0.79 |
| K | Emerald | Fresh | KICP = −4.3644x + 16,320 | 0.93 |
| K | Jewel | Dry | KICP = −0.4552x + 13,669 | 0.67 |
| Mn | Jewel | Dry | MnICP = 1.1794x − 156.93 | 0.78 |
| Mn | Jewel | Fresh | MnICP = 2.3995x − 39.814 | 0.66 |
| P | Emerald | Dry | PICP = 6.803x − 3576.1 | 0.73 |
| P | Jewel | Dry | PICP = 3.0482x − 113.36 | 0.75 |
| P | Jewel | Fresh | PICP = 20.877x − 709.14 | 0.73 |
| P | Biloxi | Fresh | PICP = 15.226x − 111.56 | 0.80 |
| P | General | Fresh | PICP = 15.371x − 147.12 | 0.71 |
| S | Jewel | Dry | SICP = 2.367x + 539.29 | 0.78 |
| Zn | Emerald | Dry | ZnICP = 1.4748x − 17.258 | 0.70 |
| Zn | Biloxi | Dry | ZnICP = 1.4984x − 18.634 | 0.85 |
| Zn | Biloxi | Fresh | ZnICP = 2.6286x − 16.255 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.H.G.; Berardo, M.C.; Rosado, L.R.; Andrade, R.; Teixeira, A.F.S.; Duarte, M.H.; Bócoli, F.A.; Carneiro, M.A.C.; Curi, N. Advancing Leaf Nutritional Characterization of Blueberry Varieties Adapted to Warm Climates Enhanced by Proximal Sensing. AgriEngineering 2024, 6, 3187-3202. https://doi.org/10.3390/agriengineering6030182
Silva SHG, Berardo MC, Rosado LR, Andrade R, Teixeira AFS, Duarte MH, Bócoli FA, Carneiro MAC, Curi N. Advancing Leaf Nutritional Characterization of Blueberry Varieties Adapted to Warm Climates Enhanced by Proximal Sensing. AgriEngineering. 2024; 6(3):3187-3202. https://doi.org/10.3390/agriengineering6030182
Chicago/Turabian StyleSilva, Sérgio H. G., Marcelo C. Berardo, Lucas R. Rosado, Renata Andrade, Anita F. S. Teixeira, Mariene H. Duarte, Fernanda A. Bócoli, Marco A. C. Carneiro, and Nilton Curi. 2024. "Advancing Leaf Nutritional Characterization of Blueberry Varieties Adapted to Warm Climates Enhanced by Proximal Sensing" AgriEngineering 6, no. 3: 3187-3202. https://doi.org/10.3390/agriengineering6030182
APA StyleSilva, S. H. G., Berardo, M. C., Rosado, L. R., Andrade, R., Teixeira, A. F. S., Duarte, M. H., Bócoli, F. A., Carneiro, M. A. C., & Curi, N. (2024). Advancing Leaf Nutritional Characterization of Blueberry Varieties Adapted to Warm Climates Enhanced by Proximal Sensing. AgriEngineering, 6(3), 3187-3202. https://doi.org/10.3390/agriengineering6030182






