Abstract
Plant pathogens are an important agricultural problem, and early and rapid pathogen identification is critical for crop preservation. This work focuses on using fluorescence spectroscopy to characterize and compare healthy and fungal pathogen-infected wheat grains. The excitation–emission matrices of whole wheat grains were measured using a fluorescence spectrometer. The samples included healthy control samples and grains manually infected with Fusarium graminearum and Alternaria alternata fungi. The five distinct zones were identified by analyzing the location of the fluorescence peaks at each measurement. The zone centered at λem = 328/λex= 278 nm showed an increase in intensity for grains infected with both pathogens during all periods of the experiment. Another zone with the center λem = 480/λex = 400 nm is most interesting from the point of view of early diagnosis of pathogen development. A statistically significant increase of fluorescence for samples with F. graminearum is observed on day 1 after infection; for A. alternata, on day 2, and the fluorescence of both decreases to the control level on day 7. Moreover, shifts in the emission peaks from 444 nm to 452 nm were recorded as early as 2–3 h after infection. These results highlight fluorescence spectroscopy as a promising technique for the early diagnosis of fungal diseases in cereal crops.
1. Introduction
Rapid cheap monitoring of plant diseases in their early stages is an actual and important problem of food security and food safety: pathogens can destroy a crop and synthesize a wide range of toxins that are dangerous to animals and humans [1].
Different optical methods are widely used in the agriculture sector. Contrary to canonical usual laboratory methods like PCR, ELISA, etc., optical methods of analysis are non-invasive, rapid, simple, and have relatively low cost, enabling the performance of fast analyses in large scale. They most common are mainly vis–IR spectroscopy [2,3], Raman spectroscopy, Hyperspectral Imaging, Time-Resolved THz spectroscopy, and fluorescence-based techniques: more detailed in [4]. Examples of the application of optical methods for diagnosis of grain infection are IR spectroscopy in the MIR region [5] for wheat grain ergot disease and Raman spectroscopy for the detection of wheat grain fungal infections [6,7].
Fluorescence-based techniques are widely applied in the investigation of biological samples. Fluorescence is an excellent and important tool for plant and fungal tissues investigation. This technique combines fast data acquisition, high sensitivity and specificity, easy sample preparation, and relatively low cost. Plants contain many fluorescent compounds: proteins, porphyrins, chlorophylls, phenolic compounds, carotenoids, alkaloids, and others [8].
Chlorophyll are the most remarkable plant fluorophores, with its fluorescence and fluorescence induction kinetics actively used for assessing photosynthesis efficiency [9], plant health conditions, and diseases [10,11]. Some other fluorophores are also modified by external stress factors and can indicate disease-induced changes. Fluorescent molecules also hold great potential for studying fungal growth and development or monitoring fungal diseases and have been detected in and outside fungal cells and hyphae [12]. Fungi produce a large set of fluorescent metabolites, including proteins, coenzymes, vitamins, toxins, and pigments such as carotenoids, melanins, flavonoids, and quinones [13], as well as other secondary metabolites [14,15]. The fluorescence of cell wall components enables the observation of many plant tissues. Multispectral fluorescence imaging offers a rapid and efficient way of observing samples at the macroscopic level [16].
The most common pathogens are fungi, which can cause severe damage to crops, especially wheat. Fluorescence spectroscopy has been utilized for various analyses related to wheat grain including the analysis of grain flour [17] and the separation of wheat grains of different varieties [18]. Apple fruits and potato tubers affected by fungal infections were investigated by fluorescence spectroscopy [19,20], and also different seeds [21], onion bulbs [22], and Fusarium in wheat seeds [23] were tested for fusariosis. The majority of recent works on this theme are concentrated on the detection of fungal fluorescent metabolite aflatoxin; it is usual for maize [24,25,26,27,28,29], nuts (peanuts [30], pistachio [31], almonds [32]), and in a single found study for wheat [33]. Toxins and other fluorescent substances related to and important for wheat grain safety, such as Fusarium and another pathogenic fungus, cannot be detected with the same related simplicity. For their detection, the next methods are used; there are IR spectroscopy [34], RGB imaging [11,35,36], Hyperspectral Imaging [37,38,39,40,41,42], and less frequently vibrational spectroscopy methods [43].
Conventional fluorescence techniques that rely on measurements of emission or excitation spectra often prove insufficient for analyzing multicomponent systems with multiple undefined fluorophores. In such cases, the excitation–emission matrix (EEM) method can significantly enhance the analytical potential of the measurements. Existing known studies that utilized the EEM method focused on the monitoring of dissolved organic matter in water [44,45,46,47]. Handling the large amount of data generated by the EEM method requires specialized analysis techniques, such as chemometric analysis involving multivariate statistics and other approaches. The primary objectives of this data analysis are estimations of the method’s potential for classification and to identify and separate samples based on specified criteria. The most popular methods of data analysis in optical methods of plant pathogen detection are described in more detail in a specific review [4].
Thus, there is a lack of studies of wheat grain fungal infections, such as fusariosis and alternariosis, identified by fluorescence spectroscopy in general and by the EEM method in particular. The objective of this paper was to demonstrate the potential of fluorescence spectroscopy for the early detection of fungi on wheat grain at the presymptomatic stage of infection. This study focused on examining the autofluorescence of wheat seeds contaminated by two strains of filamentous fungi with different pigmentation. A detailed description and characterization of the spectral features of infection and a comparison between the influences of different fungal pathogens on wheat grains are provided. To achieve this, EEMs of whole grains, both healthy and contaminated by fungi, were collected.
2. Materials and Methods
2.1. Preparation of Fusarium graminearum and Alternaria alternata Cultures
The cultures F. graminearum and A. alternata were taken from the State collection of phytopathogenic microorganisms and plant varieties–identifiers of pathogenic strains of microorganisms at the All-Russian Research Institute of Phytopathology. Before measurement, cultures were stored for one week in plastic Petri dishes in a refrigerator at 3 °C.
2.2. Inoculation of Wheat Grains
Wheat grains (winter wheat variety Timiryazevka 150) were placed in glass Petri dishes and autoclaved in a pressure steam sterilizer (123 °C, 1.18 atm, 30 min) according to known microbiological recommendations [48] to ensure inactivation of microorganisms potentially residing in the grain to exclude their growth in parallel with the artificially inoculated fungi. Autoclaved wheat grains from the glass Petri dishes were then transferred to individual polystyrene sterile tissue culture dishes with a diameter of 40 mm. The number of dishes corresponded to the number of experimental days (days 0, 1, 2, 4, and 7, i.e., 5 dishes for each sample: wet control, Fusarium-infected, Alternaria-infected; dry control was examined only on days 0 and 7), totaling 17 dishes for one experiment. Thus, contacts with environmental factors, possible changes in humidity and contamination of samples during measurements were excluded. The dishes and all other labware were kept covered at all times. Flushes were prepared from flasks containing cultures of F. graminearum and A. alternata. For this purpose, 10 mL of sterilized distilled water was introduced into each flask using a Pasteur pipette, and the flasks were gently stirred for 5 min to saturate the water with spores of the infecting culture. The flushes were then poured into clean plastic containers. Using Pasteur pipettes, the flushes were transferred into Petri dishes, with 1 mL in each dish. Control samples (referred to as “wet”) were also wetted with 1 mL of sterilized distilled water. After adding the flushes, the Petri dishes were sealed with Parafilm tape and packed in white paper and a sealed bag. All the dishes were then placed in a thermostat at 28 °C for 7 days.
The first measurement was taken 2–3 h after infection (day 0). Further, measurements were taken on days 1, 2, 4, and 7. Three experiments were performed over a period of six weeks. In each experiment, the infection of wheat with fungi was performed in the same way.
Photographs in Figure 1 depict control wet wheat seeds and wet wheat infected with fungi after 1, 2, 4, and 7 days of incubation in the second experiment. Visual inspection revealed the presence of fungal mycelium on the surface, notably for F. graminearum on day 4 and for A. alternata on day 2 (see Figure 1). In the first and third experiments, the presence of A. alternata mycelium was visible only on day 4 (results not shown). These data indicate that additional methods for the early diagnosis of wheat seeds are needed, as conventional imaging methods are inconclusive during the first days of infection.


Figure 1.
Wet control wheat and wheat infected with F. graminearum and A. alternata on different days after infection in experiment No. 2.
2.3. Measurement of Fluorescence of Control and Infected Grains and F. graminearum and A. alternata Cultures
The fluorescence of the control grains and those corrupted by F. graminearum or A. alternata was studied using a Jasco FP-8300 spectrofluorometer (JASCO Applied Sciences, Victoria, BC, Canada). The grain measurements were conducted in a special cell for solid and powder samples holding approximately 15 grains (see photo in the “Supplementary Materials”). All parameters (see “Supplementary Materials”) were the same for all measurements and were selected such that the intensity peaks for wet grains corresponded to approximately ~20% of the instrument’s range. In addition, the width of the “excitation bandwidth” was maximized to minimize the influence of the grain arrangement geometry in the cuvette. Each sample was measured five to nine times (2–3 experiments with 2–3 independent measurements each), so the EEMs show the mean for 5–9 measurements. The measurements were performed at room temperature (~22 °C).
Fluorescence measurements of F. graminearum or A. alternata fungal cultures grown on agar in Petri dishes were also conducted using glass slides in the spectrofluorometer (see photo in the “Supplementary Materials”). The slides and agar did not contribute to the fluorescence of the fungal culture (see photo in the “Supplementary Materials”).
2.4. Principal Component Analysis
The measured fluorescence spectra were not subjected to any processing. The only thing that was done to them is the artificial peak λem = 500/λex = 450 nm was cut out.
To use principal component analysis (PCA), the emission–excitation matrices were transformed into a single column. The Nipals (Nonlinear Iterative Partial Least Squares) algorithm [49] was then used with the following parameters: number of principal components—4, maximum number of iterations—104, data scaling—‘noscale’, convergence accuracy—10−10. To represent the loading matrices, the inverse was performed from the column to obtain the emission–excitation matrix for loading values.
2.5. Finding the Peaks of the Emission–Excitation Matrix
The algorithm for finding local maxima is as follows. The EEM matrix was divided into squares with a side of 2 nm. A local maximum was considered if the maximum intensity value of points in a square exceeded the maximum values of points in neighboring squares and exceeded a threshold value (usually 30% of the maximum EEM value). This algorithm found the same maximum values as the software of the Jasco FP-8300 spectrofluorometer.
3. Results
The figure shows the typical EMM of the fungi F. graminearum and A. alternata grown in Petri dishes (Figure 2). The fluorescence peak of amino acids in the emission region of λem = 334–332 nm and excitation region of λex = 277–279 nm is well observed. We also observed peaks in area λem = 422–433/λex = 338–343 nm and λem = 451–463/λex = 360–364 nm. The obtained peaks are not related to agar fluorescence (see “Supplementary Materials”). The peak λem = 451–463/λex = 360–364 nm can be associated with many biologically important substances (see peak 2 in Table 1). It is interesting that the maximum of the amino acid peak of A. alternata (λem = 334/λex = 279 nm) is shifted to the long-wave region compared with the peak of F. graminearum (λem = 332/λex = 277 nm). This shift is also observed for seed samples infected with these fungi (Figure 3). This discrepancy is likely attributable to the differing protein and, consequently, amino acid compositions of the fungi.
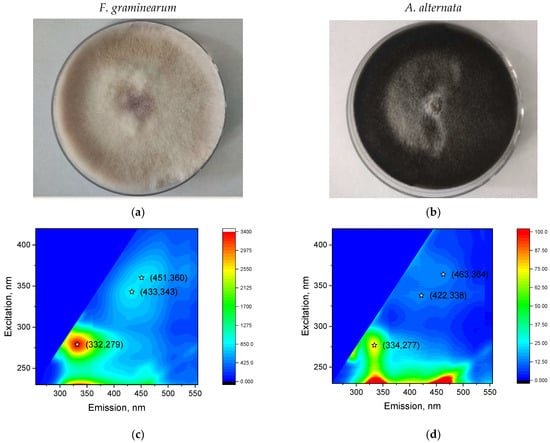
Figure 2.
Cultures of F. graminearum (a) and A. alternata (b) in plastic Petri dishes (diameter 9 cm). Typical EEMs of fungal cultures F. graminearum (c) and A. alternata (d). The spectrofluorometer settings were the same for all measurements (see “Supplementary Materials”). Asterisks on EEMs denote peaks indicating emission and excitation wavelengths.
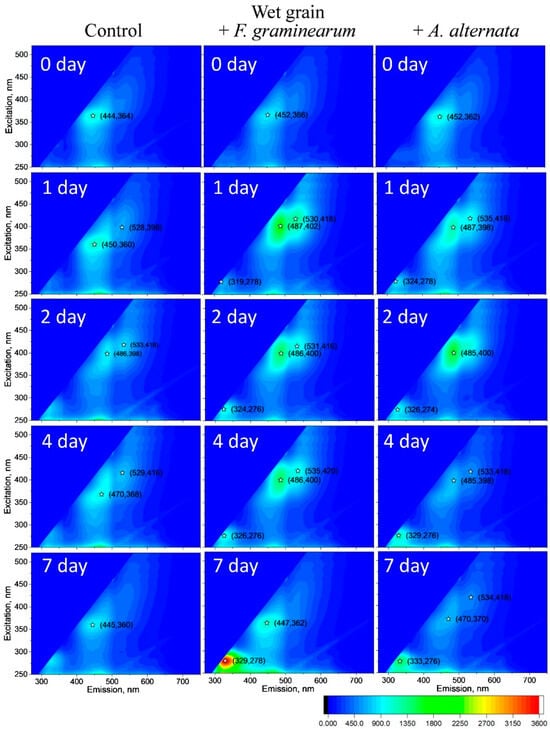
Figure 3.
EEMs depict wet wheat and wheat infected with F. graminearum and A. alternata on days 0, 1, 2, 4, and 7 following infection. EEMs are averaged over three experiments, each comprising 2–3 independent measurements. Consistency was maintained across all measurements using identical spectrofluorometric settings. Asterisks on EEMs denote peaks indicating emission and excitation wavelengths.
Figure 3 displays averaged (over three experiments or 5–9 independent samples) EEMs of wet wheat (control) and wheat infected with F. graminearum and A. alternata on days 0, 1, 2, 4, and 7 after inoculation. The EMMs reveal discernible differences in spectra as early as the first hours and days after infection. In wheat infected with A. alternata and F. graminearum, the peak maximum after 2–3 h (0 day) shifts from the λem = 444/λex = 364 nm area to the λem = 452/λex = 362–366 nm area and to the λem = 487/λex = 398–402 after 1 day. This shift is likely attributable to chitin in cell walls and metabolites of fungi resulting from their growth on wheat [8]. Intriguingly, fluorescence from the fungal cultures of F. graminearum and A. alternata did not exhibit peaks in the λem = 486/λex = 400 region (Figure 2). The closest peaks were observed at λem = 451/λex = 360 nm for F. graminearum and λem = 463/λex = 364 nm for A. alternata (Figure 3).
On the first day after inoculation with A. alternata, a peak emerges associated with the absorption and fluorescence of amino acids (Phe, Tyr, and Trp) at λem = 324–328/λex = 276–278 nm [50,51]. In the following days of incubation, the intensity of this peak increases. Notably, for F. graminearum, the amino acid peak (λem = 324/λex = 276 nm) appears on the second day and increases sharply in subsequent days (Figure 3).
The peak in the region λem = 485–483/λex = 400–402 nm behaves interestingly. For F. graminearum, there is an increase in this peak for day 1 and then a decrease in intensity. For A. alternata, in this region, the peak has a maximum on the second day, and on the following days, the peak also decreases. This indicates a decrease in active growth of fungi after hyphae emergence on day 4–7 of the experiment.
A similar pattern was observed for the control wet wheat grains. There, the position of the main peak also changes. There is a shift from λem = 440/λex = 360 nm from the start of the experiment to λem = 486/λex = 398 nm on day 2 (Figure 3). The peak then reverts back to λem = 445/λex = 360 nm on day 7. This may indicate that some fungi may also grow on the control wheat in a small amount, but this is not the case. Characteristically, no such changes were recorded for dry wheat grain. The EMMs did not change during the whole experiment (Figure 4). It should be noted that the spectra for dry wheat grains were much higher in intensity than the spectra of wet grains. This is due to the quenching of emission of fluorescence by water, especially in the visible and not ultraviolet region.
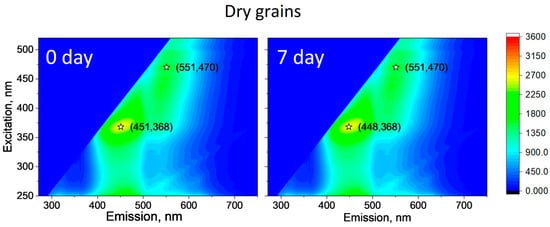
Figure 4.
EEMs depict dry wheat on days 0 and 7. EMMs are averaged over three experiments, each comprising 2–3 independent measurements. Consistency was maintained across all measurements using identical spectrofluorometer settings. Asterisks on EEMs denote peaks indicating emission and excitation wavelengths.
3.1. Principal Component Analysis
Various chemometric methods are often used to analyze spectral data. The most popular of them is principal component analysis (PCA). In fact, the method allows reducing multivariate spectral data by dividing them and selecting the parts of the spectrum where the maximum variability of the data is observed. In our case, we also applied PCA (Figure 5). Figure 5 shows the data separately by day from 0 (few hours), 1, 2, 4, and 7 days after infection. On the first and last days, in addition to wet control and infected samples, PCA results for dry wheat are shown. It can be seen that PC1 and, to a lesser extent, PC2 of the dry wheat measurements differ from the wet samples both on day 0 and day 7 (Figure 5a,i). Wet control and infected samples at day 0 did not differ (Figure 5a,b). However, after 1 day, infected samples, especially for F. graminearum, were well separated from control samples (Figure 5d). The separation is maximized by the PC3 component. On the second day, both F. graminearum- and A. alternata-infected samples were well separated by PC3 and PC4 (Figure 5f). However, on days 4 and 7, separation by PC3 and PC4 became impossible. But by day 7, samples with F. graminearum became well separated by PC2 (Figure 5i).
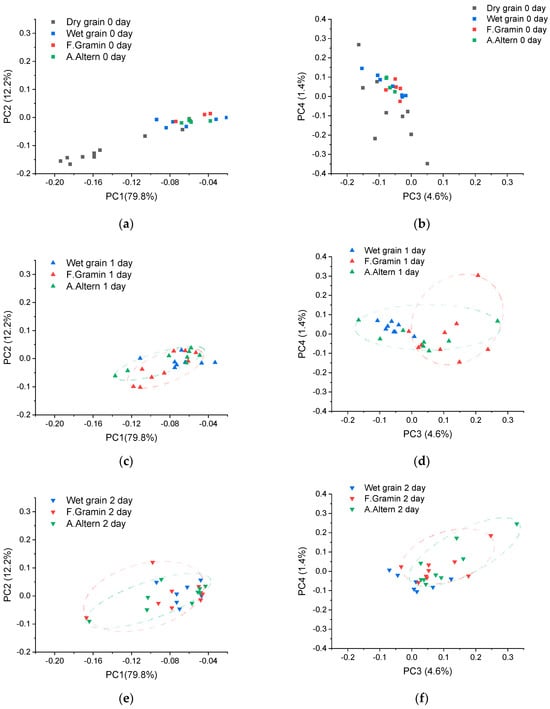
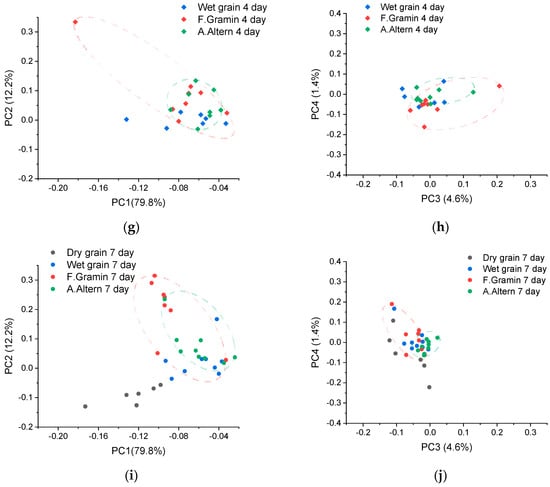
Figure 5.
Results of PCA for dry (day 0 and 7) and wet wheat and for wheat infected with F. graminearum and A. alternata at 0 (few hours, (a,b)), 1 (c,d), 2 (e,f), 4 (g,h), and 7 (i,j) days after infection. Results for PC1 and PC2 are shown on the left and for PC3 and PC4 on the right. The areas of control (blue), F. graminearum (red), and A. alternata (green) infected samples are marked with ellipses. Ellipses were constructed for the purpose of visualisation so that all points of the corresponding group were inside them.
The loading matrices for different excitation and emission wavelengths for the different components PC1 (a), PC2 (b), PC3 (c), PC4 (d) are shown in Figure 6. The loading matrices show which regions of the spectra contribute most to PC1–PC4. The loading matrix of PC1 (79% of total data variance) is generally similar to the spectrum of dry wheat (Figure 4) and most likely this component is responsible for moisture. Indeed, the maximum differences between dry and wet samples were in PC1 (Figure 6a). The loading matrices for PC2 (12.2% of the total variance) have a predominant amino acid peak, so it is not surprising to see good separation between the control and F. graminearum samples at day 7 of observation (Figure 6b). The amino acid peak for samples infected by F. graminearum is clearly visible on the fluorescence spectra (Figure 3, day 7). The loading matrix for PC3 (4.6% of the total variance) has peaks in the λem = 487/λex = 412 nm (positive) and λem = 430/λex = 364 nm (negative). The area near these peaks appears to be the main area for early detection of fungal growth. In the following sections, we will elaborate on the possibility of early detection of fungi in this area. In PC4 (1.7% of total variance), the positive region actually repeats the entire PC3 zone (the region between λem = 400–520/λex = 350–430 nm), while the negative region repeats part of PC1 and PC2 in the region (λem = 520–620/λex = 430–520 nm).
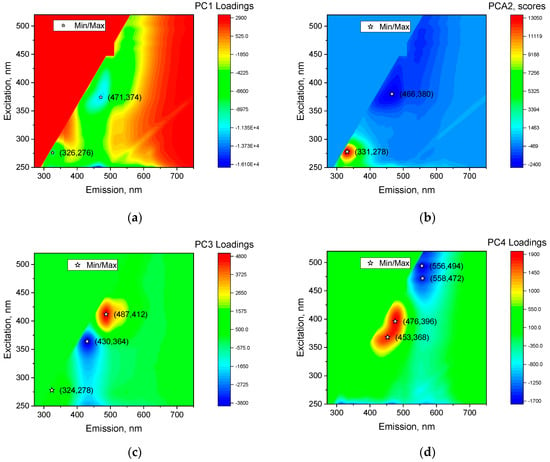
Figure 6.
The loading matrices for different excitation and emission wavelengths for different components, PC1 (a), PC2 (b), PC3 (c), and PC4 (d). Asterisks on EEMs denote peaks indicating emission and excitation wavelengths.
Although PCA performed on a small number of samples separates the data quite well, we decided to analyze and describe additional peaks in different regions of the spectrum.
3.2. Analysis of Peaks
Because the peaks in the spectra (Figure 3) varied in both intensity and location, we decided to analyze these parameters separately. Figure 7a shows the locations of the peaks along the emission and excitation axes for each dimension of our study. Peaks were found using a software program; the following parameters were set in the program to reduce the number of peaks: a minimum distance of 10 nm for excitation and emission to the next peak and a minimum peak intensity of 500 a.u. (arbitrary units). These parameters were the same for all spectra. Figure 7b–f show the locations of the peaks separately by days.
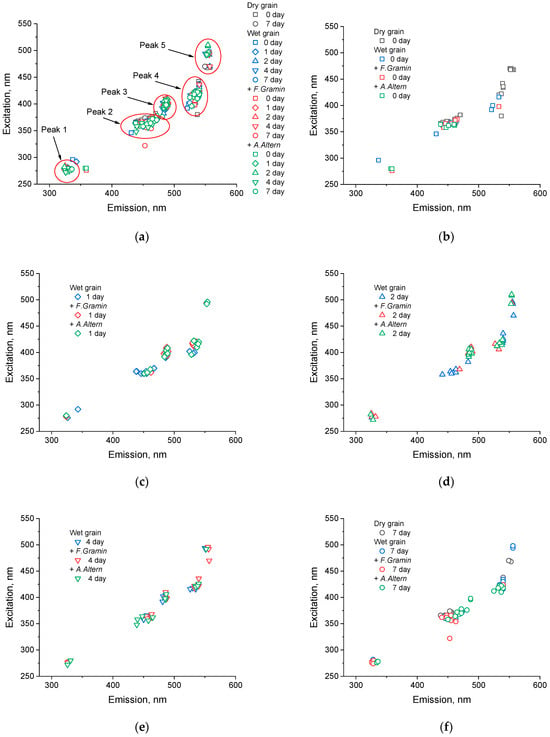
Figure 7.
The peaks of each measurement of EEMs depict wet wheat and wheat infected with F. graminearum and A. alternata for all days (a), on day 0 (b), day 1 (c), day 2 (d), day 4 (e), and day 7 (f) following infection. Consistency was maintained across all measurements with identical spectrofluorometer settings.
Figure 7a shows that the locations of the peaks are concentrated at several points on the spectrum. It was decided to number them from one to five. Table 1 presents the literature data on substances that have peaks in areas close to those found by us. We realize the conditionality of this division because many substances have peaks in different zones. For example, lignin is a class of polymers of different length and consists of different monomeric structures. Its fluorescence peaks have been found in several emission/excitation regions [52]. In addition, the fluorescence peak of a substance depends on its interaction with other substances and can vary greatly, which is intensified in the case of living plant or fungal cells. The fluorescence of many compounds is affected by their conformational states and diffusion processes, and driven by molecular environments, pH, types of bonds, etc., for instance, for hydroxycinnamic acids [53]. The fluorescence intensity and emission spectra of NADPH and NADH can be changed upon its binding to proteins [54].
Substances whose peaks fall into several zones in the table are highlighted in grey (Table 1). For the peaks found, graphs of intensity changes on different days were plotted, and statistical analysis of intensity differences between control and fungus-infected samples was performed (Figure 8).
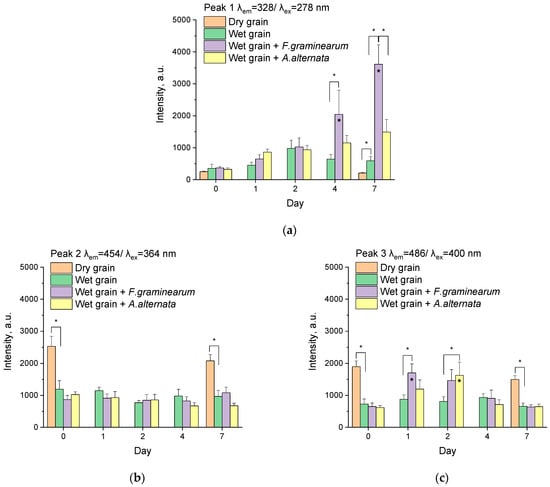
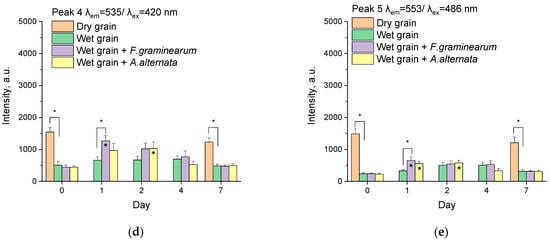
Figure 8.
The fluorescence intensities of the peaks on different days after infection with phytopathogen for dry wheat grains, wet wheat grains (control), and wheat grains infected by A. alternata and F. graminearum. The peaks are located in λem = 328/λex = 278 nm (a), λem = 454/λex = 364 nm (b), λem = 486/λex = 400 nm (c), λem = 535/λex = 420 nm (d), and λem = 553/λex = 486 nm (e). Intensities of the peaks are averages of 5–9 independent measurements in 3 experiments. Standard errors of mean are set as an error. The spectrofluorometer settings were the same for all measurements. * (above column)—the difference between this group and wet grain in same day at this time (p < 0.05, Two-way ANOVA with Tukey’s Test). * (inside column)—the difference between this group and the group in the same category on day 0 (p < 0.05, Two-way ANOVA with Tukey’s Test).
3.2.1. Peak 1
As can be seen from the figure (Figure 7a), peak 1 has an average location λem = 328/λex = 278 nm. This is the only peak for which the substances reliably identified are proteins and amino acids (Table 1). Nearby peaks (e.g., λem = 360/λex = 280 nm), which were observed in the first days of the experiments (Figure 7b,c), in some experiments may belong to lignins (Table 1, [52]). Peak 1 appears on the samples of the first and subsequent days and is mainly formed by fungus-infected samples (Figure 7c–f). Analysis of intensity changes at λem = 328/λex = 278 nm showed that there is a sharp increase in intensity on the first day in samples infected with A. alternata, and by day 4–7 in samples with F. graminearum (Figure 8a), with the increase in the latter being statistically significant (p < 0.05, Two-way ANOVA with Tukey’s Test). The intensity of uninfected dry samples does not change during the week of the experiment, whereas in the control wet samples, there is an increase up to day 2 and a slight drop, which may indicate a possible infection of the control samples. F. graminearum samples at day 7 also were significantly different from A. alternata samples (p < 0.05, Two-way ANOVA with Tukey’s Test).
3.2.2. Peak 2
Peak 2 with the center λem = 455/λex = 364 nm is, in our opinion, related to the fluorescence of wheat grains (Figure 7a). This peak is seen on all days of the study, with only the control wet wheat visible for samples on day 2 (Figure 7d). The nearby peak 3, which has a higher intensity for infected samples, makes it difficult to fix peak 2. The intensity of samples at λem = 455/λex = 364 nm is almost unchanged on different experimental days (Figure 8b). An insignificant trend of decreasing intensity for A. alternata is observed. This decrease is probably because this phytopathogen develops inside the grain and uses plant substances fluorescing in this range during its life activity.
The fluorescence peak with the center in the region λem = 455/λex = 364 nm may be due to many compounds found in plant and fungi cells, such as important molecules for the organization of life activity: NADH [8,55,56]; terpenoids [57,58]; components of plant immunity against phytopathogenic fungi and viruses: phenolic [8,59,60,61,62], chlorogenic, and folic acids [8,63,64], flavonoids [57,62,65], alkaloids; components of cell membranes: chitin, suberin, sporopollenin; and products of vital activity (lipofuscin). As already noted, phytopathogens also have peaks in this area (Figure 2). In addition to the usual biotic fluorophores, mycotoxins may contribute to fluorescence in this region.
3.2.3. Peak 3
The peak with the center λem = 486/λex = 400 nm was the most important for the early detection of phytopathogenic fungi. While during the first hours after infection (0 day), the peak was undetectable (Figure 7b), after one day, phytopathogen-infected seeds gave a stable peak in this region (Figure 7c). Interestingly, this peak persisted on days 2 and 4 after infection (Figure 7d,e), but on day 7, the peak remained only in two samples infected with A. alternata (Figure 7f). The fluorescence intensities at λem = 486/λex = 400 nm for a week after infection are shown in Figure 8c. Fluorescence intensities increase statistically significantly on the first day after infection for F. graminearum and on the second for A. alternata (p < 0.05, Two-way ANOVA with Tukey’s Test). Fluorescence intensity then decreases by day 7 and is visually unchanged from the control (wet wheat grains, Figure 8c). The investigated peak does not seem to be related to the hyphae of the phytopathogens, because at 1–2 days after infection, the hyphae were not clearly visible (traces of hyphae are visible only for A. alternata on the second day, Figure 1). In addition, the fluorescence of fungal cultures with hyphae did not show any peaks in the region λem = 486/λex = 400 nm (Figure 2). Possible sources of fluorescence could be the already mentioned sporopollenin, cutin, suberin, lignin, or terpenoids as well as metabolites (phenolics, quinones, Table 1).
3.2.4. Peak 4
Peak 4 has a broad excitation band and a rather narrow emission band with the center λem = 536/λex = 420 nm. A characteristic feature of this peak is the practical absence of peaks of contaminated samples at the beginning of the experiment (day 0, Figure 7b). The pattern of changes in intensities on different days of the experiment (Figure 8d) did not differ practically from peak 3 (Figure 8c). Although the changes compared to the control were less pronounced, for F. graminearum one day after infection, statistically significant differences from the control were obtained (p < 0.05, Two-way ANOVA with Tukey’s Test, Figure 8d).
In addition to the already discussed substances (terpenoids, sporopollenin), phospholipids and flavins can contribute to the fluorescence intensity in this range (Table 1). Also, various pigments, such as melanin, which is more typical for Alternaria phytopathogens, and carotenoids—more typical for Fusarium—have peaks in this range of excitation/emission.
3.2.5. Peak 5
On the EEMs, peak 5 can be divided into two peaks, one lower with excitation at ~470 nm and the other above with excitation at ~495 nm (Figure 7a). The lower peak is mainly associated with the fluorescence of dry grain samples; the upper, with wet infected and control samples. Characteristically, at the beginning of the experiment and on day 7, the peaks for infected samples in this area are not registered. The fluorescence intensities of the upper and lower peaks did not differ practically; therefore, the data for these peaks were combined into a single peak 5 with center λem = 553/λex = 486 nm (Figure 7a). The changes in fluorescence intensities (Figure 8e) actually replicate the data for peak 4 (Figure 8d). As for the fluorescent substances, in addition to the already mentioned substances, tannins possess fluorescence peaks close to the investigated region (Table 1).

Table 1.
Fluorescence peaks of important biological substances in plant cells and phytopathogens. Substances are divided into five peaks depending on the emission/excitation range. Some substances marked in grey contribute to several peaks.
Table 1.
Fluorescence peaks of important biological substances in plant cells and phytopathogens. Substances are divided into five peaks depending on the emission/excitation range. Some substances marked in grey contribute to several peaks.
| Number and Center of the Peaks (λex/λem, nm) | Substances | Maximum of λex/λem for Substances, nm | References |
|---|---|---|---|
| 1 (328/278) | Amino acids, proteins | 282–363/255–295 | [8,66] |
| Lignin | 360/280 | [52] | |
| 2 (455/364) | Phenolic acids (hydroxycinnamic), ferulic acids, coumaric acids | 410–460/340–380 | [8,59,60,61,62] |
| Pteridine compounds (folic acid, neopterin etc.) | 373–458/331–361 | [8,63,64] | |
| Chitin | 450–458/254, 305, 370–373 | [8] | |
| 413/335 | [67,68,69] | ||
| Coumarin | 455/280 and 340 | [8] | |
| Chlorogenic acid | 430–440/320–330 | [70] | |
| NADPH, NADH | 440–480/330–380 | [8,55,56] | |
| Cellulose | 410–460/330–340 | [8,66] | |
| Lignin | 419–458/282, 331–345 | [62,66] | |
| Suberin | 450–460/355 | [62] | |
| Lipofuscin | 460–670/345–490 | [71,72,73] | |
| Flavonoids | 440–610/365 | [57,62,65] | |
| Alkaloids | 410–600/360–380 | [57,58,74] | |
| Sporopollein | 400–650/300–550 | [57,58,75] | |
| Terpenoids | 400–725/250–395 | [57,58] | |
| Mycotoxins (deoxynivalenol, nivalenol, zearalenone, and alternariol) | 398–465/295–393 | [76,77,78,79,80,81] | |
| Metabolites (quinones) | 430–480/270–400 | [82,83] | |
| 3 (486/400) | Sporopollenin, cutin, suberin, lignin | 400–650/300–550 | [57,58,75,84] |
| Lipofuscin | 430–670/340–490 | [72,73] | |
| Terpenoids | 400–725/250–395 | [57,58] | |
| Metabolites (quinones) | 430–480/270–400 | [82,83] | |
| 4 (536/420) | Flavins (riboflavin, FAD, FMN) | 540/360–445 | [8,56,85] |
| Carotenoids | 520–560/400–500 | [57,65] | |
| Sporopollenin | 400–650/300–550 | [57,58,75] | |
| Melanin | 543–548/469–471 | [86] | |
| Phospholipids | 540,560/436 | [87] | |
| Lipofuscin | 430–670/340–490 | [72,73] | |
| Terpenoids | 400–725/250–395 | [57,58] | |
| 5 (553/486) | Some lignin and/or cuticle compounds | 590 and 540/488 | [53] |
| Flavonoids | 540–550/488 | [52] | |
| Lignin | 530–560/488 | [52] | |
| Tannins | 500–650/488 | [52] | |
| Sporopollenin | 400–650/300–550 | [57,58,75] | |
| Melanin | 543–548/469–471 | [86] | |
| Phospholipids | 540,560/436 | [87] | |
| Lipofuscin | 430–670/340–490 | [72,73] |
4. Discussion
In this work, we obtained striking differences in EMMs (or as they are often called, 3D fluorescence spectra) in phytopathogen-infected F. graminearum and A. alternata and control wheat grains. These differences are visible in the early stages of infection, 1–2 days after infection, when no visual differences from the control are observed (Figure 1). Using localization of peaks in all measurements, the five excitation/emission zones of the most interesting from the point of view of phytopathogen fluorescence were identified and analyzed. Also, PCA processing was applied to reduce the data and isolate the most important regions in the spectral data. The peaks of single measurements formed five zones with rather sharp boundaries with centers in Peak 1–Peak 5 (Table 1). Based on the localizations of these zones and their fluorescence intensities on different days after infection, we analyzed possible components that may be crucial for early detection of phytopathogens using fluorescence.
In [7], analysis of near infrared spectra of infected by F. graminearum seeds shows that there are increased protein, fat, and cellulose and decreased water and starch, and microscopy demonstrated the presence of fungus mycelium on grain surfaces. Accordingly, in the present work, the appearance of an aromatic amino acids peak and a peak around emissions at 480 nm and excitation at 400 nm (Peak 3) was observed on fungi-inoculated grains spectra.
There are differences in intensities of this peak between two types of pathogenic fungi. A possible explanation is the difference in host–pathogen interactions. Fusarium occupied the grain surface actively at the beginning of infection process, whereas Alternaria invaded the inner part of grain. That is why the amino acid peak for Fusarium is more pronounced than it is for Alternaria. Already, on the 7th day, there is noticeable softening of grains affected by Alternaria, while Fusarium produces a dry rot.
Peaks in field emission 420–570 nm and excitation 340–520 nm are a complex of fluorophores. The wheat grain comprises several tissues: embryo, endosperm, and outer layers. Autofluorescence of cereal grains is associated with various compounds, including pigments, mainly carotenoids. Fluorescence of the whole grain is limited by its outer shell and in most cases is due to phenolic compounds [88] of pericarp cell walls [89,90]. They have protective functions, including against fungal diseases [91]. It is a well-known fact that the fluorescent component of plant cell walls is ferulic acid [92]. Phenolic acids in grains exist in both free and bound forms with a heterogeneous distribution in the grain. They are concentrated in the outer layers, with the lowest content in the endosperm. The major phenolic acids in cereal grains are ferulic and p-coumaric acids, which are mainly present in insoluble fractions associated with the cell wall components [93]. Phenolic compounds are also represented by lignin and other hydroxycinnamic acids [53].
The following grain fluorophores should also be mentioned. Xanthophylls are located in the embryo, grain coat, and starchy endosperm; flavones are located in the embryo and seed coat [94,95]; lignin cross-links are in the cell walls of wheat grain outer layers [96]. It should be noted that the fluorescence of main compounds overlaps significantly in the spectra.
The statistically significant increase in the λem = 486/λex = 400 nm peak after the first day for F. graminearum and after two days for A. alternata may be due to both changes in the fluorophores of the grain itself and fluorophores formed in the phytopathogens. The fluorescence of lignin is quenched due to the presence of covalent linkages to hemicelluloses. Fungal or bacterial degradation of grains led to the disruption of such bonds, thus increasing the brightness of lignin fluorescence [52]. The peak shift can be due to both the appearance of new and the destruction of old fluorophores, and their modification. It is most likely that, due to the prevailing process of destruction of the grain shell, a decrease in the fluorescence of the original fluorophores and an increase in the intensity of compounds associated with the vital activity of the fungus will be observed.
Fungal infection may initiate a shift of peak caused by a variety of fungal pigments [97] (melanins [12], carotenoids), metabolites (lipofuscin, ergosterol, NADPH, NADH, pyridoxine) [12,82], and toxins [98]. Fungi and their spores themselves have emission peaks in the region of 400–500 nm when excited in the range of 250–350 nm [99,100,101]. This fluorescence can be ascribed to the reduced form of the NADPH, which is known to emit fluorescence in the blue region with a maximum ~440–465 nm [102]. In [103], fluorophores emitting between 490 and 540 nm under 370–410 nm excitation were proposed to be flavoproteins and considered as a marker of fungal metabolic activity. Flavins have emission maximums above 500 nm, 520 nm for free riboflavin, FMN, and FAD [104]. Flavins, bound to proteins, change their fluorescence; their emission maximum can vary in the range of 500–530 nm depending on the redox state and the local environment [105]. Protein bonding of NADPH also affects its fluorescence [101].
A different pigment composition for Fusarium and Alternaria was described in a few papers [97,106]. Pigment melanin is more typical for A. alternata [107]; carotenoids is more typical for F. graminearum [108,109]. The major Fusarium carotenoid is neurosporaxanthin, a carboxylic xanthophyll [110].
Substances that can create a unique fluorescent footprint for phytopathogens may be mycotoxins [111]. For F. graminearum, the predominant mycotoxins detected in samples inoculated with the strains were deoxynivalenol (DON), nivalenol, and zearalenone [112,113]. For zearalenone, the fluorescence peak lies in the λem = 465/λex = 330 nm region [76]. There are many conflicting data for DON. Fluorescence peaks have been detected over a wide range of emission/excitation wavelengths: λem = 339/λex = 295 nm [77], λem = 403/λex = 325 nm [78], and λem = 459/λex = 393 nm [79]. In the case of A. alternata, the major toxin alternariol also has different peaks depending on pH and buffer, λem = 398–440/λex = 340–355 nm [80,81].
The literature data on mycotoxin fluorescence generally agree with the peaks measured for the fungal cultures, λem = 433/λex = 343 nm and λem = 451/λex = 360 nm for F. graminearum and λem = 459/λex = 393 nm and λem = 459/λex = 393 nm for A. alternata (Figure 2). However, this region overlaps with the intrinsic fluorescence of wheat grains (λem = 454/λex = 364 nm, Peak 2), and the main detectable peak at infection (λem = 486/λex = 400 nm, Peak 3) is shifted to the longer wavelength region. This peak, in our opinion, may be due to fungal metabolites, among which may be phenolic and quinones compounds. The shift in the fluorescence spectra of metabolites of various fungi from the λem = 300–350 nm region in normal growth medium to the 430–480 nm region in the presence of humate in the medium was previously reported in [82]. The absorption peak of quinones also lies in this region [83]. The shift of emission peaks to the long-wavelength region was also observed in other works. Thus, for maize kernels infected with fungal infection, shifts to the long-wave region of the emission spectrum in the range of 450–500 nm at 365 nm excitation were detected [114].
The observed uneven changes in spectral features over time may also be associated with the peculiarities of the life cycle of the fungus, which represent an alternation of stages, which correspond to changes of fungal metabolic activity, in combination with grain degradation processes. According to [115], the fluorescence of phytopathogens can either increase or decrease during growth. When mycelium was young, at the stage of germination of conidia or ascospores, fluorescence was minimal. Mature, highly coiled, and fragmented mycelium fluoresced brightly. After aging and melanization, mycelial cells became non-fluorescent again, which the authors explained by oxidation of phenolic compounds. In our case, we see a drop in fluorescence to the control level by days 4–7 of the experiment in the long wavelength region of excitation and emission. At the same time, fluorescence in the amino acid region apparently associated with fungi hyphae continued to grow.
The EEM method is, of course, inconvenient for practical application, but experiments carried out with its instruments will help in the development of inexpensive simple devices for rapid scanning of grain for suspected infestation by the pathogens under investigation, F. graminearum and A. alternata. In our study, the wavelengths characterizing the presymptomatic presence of fungus on grain were determined as λem = 486/λex = 400 nm in combination with λem = 328/λex = 278 nm. We can recommend this approach using fluorescence for grain monitoring and pre-selection of potentially infected grain from the post-harvest stage. More reliable and validated methods such as molecular biological, PCR, or ELISA are suggested to verify the presence of infection.
We also envisage the use of complementary optical spectroscopic non-invasive methods such as Raman and IR spectroscopy. These techniques allow for more precise identification of specific chemical compounds. The use of such combinations of methods is already being realized in ongoing research in our laboratory.
5. Conclusions
This study has demonstrated that fluorescence spectroscopy can detect wheat infection by F. graminearum and A. alternata fungi at an early stage (1–2 days after inoculation), even before visible fungal mycelium appears. By analyzing the distribution of fluorescence peaks, a zone with a center (λem = 480/λex = 400 nm) was identified in which fluorescence peaks appear in phytopathogen-infected samples after one day, and the fluorescence intensity is about two times higher than in control samples. Moreover, shifts in the emission peaks at 8 nm to the long-wavelength region were recorded as early as 2–3 h after infection.
Differences in the character of phytopathogen growth for F. graminearum and A. alternata in different spectral ranges were revealed. In the λem = 480/λex = 400 nm range and farther regions, fluorescence maxima for F. graminearum are observed 1 day after infection, and for A. alternata, on the 2nd day, and by the 7th day, fluorescence decreases to the control level. The amino acid peak (λem = 328/λex = 278 nm) showed a sharp (about twofold) increase for A. alternata on the first day, while for Fusarium, this peak grew strongly after 4–7 days of the experiment.
Since the range centered on λem = 480/λex = 400 nm showed the greatest differences between the spectra of infected and uninfected samples, possible fluorophores for early detection were suggested from the literature, among which the most likely are metabolites (phenolic and quinones compounds) of fungi. These results highlight fluorescence spectroscopy as a promising technique for the early diagnosis of fungal diseases in cereal crops. The different positions of fluorescence peaks, differences in peak intensity, as well as different dynamics of fluorescence changes on different days can be used for the identification of phytopathogens.
Fluorescence spectroscopy does not require any sample preparation and processing, but the considered EEM method is rather complicated to implement and process and expensive for performing routine tasks. This study has the limitation of laboratory experiments and should be continued in practice to field experiments and for a limited number of wavelengths within conventional fluorescence spectroscopy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriengineering6030179/s1. Table S1: Settings of the fluorimeter Jasco FP-8300 for measuring control and infected grain; Table S2: Settings of the fluorimeter Jasco FP-8300 for measuring fungal hyphae; Figure S1: Fluorescence measurements for pure cultures of F. graminearum, A. alternata and agar were measured on cover glass; Figure S2: EEMs of the cover glass used to measure the cultures in Figure 1; Figure S3: EEMs of agar on the cover glass in Figure 1; Figure S4: Cuvette for solid and powder samples with wheat grains. It holds approximately 15 grains; List of abbreviations.
Author Contributions
Conceptualization, S.V.G. and R.M.S.; methodology, O.K.P. and T.A.M.; validation, N.A.S. and O.K.P.; formal analysis, R.M.S.; investigation, T.A.M., V.M.A., and O.K.P.; resources, V.M.A. and N.A.S.; data curation, R.M.S., T.A.M., and V.M.A.; writing—original draft preparation, R.M.S., T.A.M., and O.K.P.; writing—review and editing, N.A.S. and S.V.G.; visualization, R.M.S. and T.A.M.; supervision, S.V.G.; project administration, R.M.S.; funding acquisition, S.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Science and Higher Education of the Russian Federation (075-15-2022-315) for the organization and development of a World-class research center “Photonics”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.e.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Ogly, G. Modern physical methods and technologies in agriculture. Phys. Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Kremneva, O.Y.; Danilov, R.Y.; Sereda, I.; Tutubalina, O.; Pachkin, A.; Zimin, M. Spectral characteristics of winter wheat varieties depending on the development degree of Pyrenophora tritici-repentis. Precis. Agric. 2023, 24, 830–852. [Google Scholar] [CrossRef]
- Graeff, S.; Link, J.; Claupein, W. Identification of powdery mildew (Erysiphe graminis sp. tritici) and take-all disease (Gaeumannomyces graminis sp. tritici) in wheat (Triticum aestivum L.) by means of leaf reflectance measurements. Open Life Sci. 2006, 1, 275–288. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Matveeva, T.A.; Sarimov, R.M.; Simakin, A.V.; Stepanova, E.V.; Moskovskiy, M.N.; Dorokhov, A.S.; Izmailov, A.Y. Optical methods for the detection of plant pathogens and diseases. Agriengineering 2023, 5, 1789–1812. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Borisov, E.; Povolotskiy, A.; Borzenko, S.; Gulyaev, A.; Gerasimenko, S.; Dorochov, A.; Khamuev, V.; Moskovskiy, M. Investigation of Spectroscopic Peculiarities of Ergot-Infected Winter Wheat Grains. Foods 2023, 12, 3426. [Google Scholar] [CrossRef]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and identification of fungal infections in intact wheat and sorghum grain using a hand-held Raman spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef] [PubMed]
- Moskovskiy, M.N.; Sibirev, A.V.; Gulyaev, A.A.; Gerasimenko, S.A.; Borzenko, S.I.; Godyaeva, M.M.; Noy, O.V.; Nagaev, E.I.; Matveeva, T.A.; Sarimov, R.M. Raman spectroscopy enables non-invasive identification of mycotoxins p. Fusarium of winter wheat seeds. Photonics 2021, 8, 587. [Google Scholar] [CrossRef]
- Pan, Y.-L. Detection and characterization of biological and other organic-carbon aerosol particles in atmosphere using fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current approaches to light conversion for controlled environment agricultural applications: A review. Horticulturae 2022, 8, 885. [Google Scholar] [CrossRef]
- Zhou, C.; Mao, J.; Zhao, H.; Rao, Z.; Zhang, B. Monitoring and predicting Fusarium wilt disease in cucumbers based on quantitative analysis of kinetic imaging of chlorophyll fluorescence. Appl. Opt. 2020, 59, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, M.; Mouhu, K.; Hautsalo, J.; Jacobson, D.; Jalli, M.; Himanen, K. Image-based time series analysis to establish differential disease progression for two Fusarium head blight pathogens in oat spikelets with variable resistance. Front. Plant Sci. 2023, 14, 1126717. [Google Scholar] [CrossRef] [PubMed]
- Knaus, H.; Blab, G.A.; van Veluw, G.J.; Gerritsen, H.C.; Wösten, H.A. Label-free fluorescence microscopy in fungi. Fungal Biol. Rev. 2013, 27, 60–66. [Google Scholar] [CrossRef]
- Christiansen, J.V.; Isbrandt, T.; Petersen, C.; Sondergaard, T.E.; Nielsen, M.R.; Pedersen, T.B.; Sørensen, J.L.; Larsen, T.O.; Frisvad, J.C. Fungal quinones: Diversity, producers, and applications of quinones from Aspergillus, Penicillium, Talaromyces, Fusarium, and Arthrinium. Appl. Microbiol. Biotechnol. 2021, 105, 8157–8193. [Google Scholar] [CrossRef]
- Jha, Y. Differential fungal metabolite accumulation in response to abiotic and biotic stresses. In Fungal Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 457–467. [Google Scholar]
- Fedoseeva, E.; Patsaeva, S.; Stom, D.; Terekhova, V. Excitation-dependent fluorescence helps to indicate fungal contamination of aquatic environments and to differentiate filamentous fungi. Photonics 2022, 9, 692. [Google Scholar] [CrossRef]
- Corcel, M.; Devaux, M.-F.; Guillon, F.; Barron, C. Comparison of UV and visible autofluorescence of wheat grain tissues in macroscopic images of cross-sections and particles. Comput. Electron. Agric. 2016, 127, 281–288. [Google Scholar] [CrossRef]
- Zeković, I.; Lenhardt, L.; Dramićanin, T.; Dramićanin, M.D. Classification of intact cereal flours by front-face synchronous fluorescence spectroscopy. Food Anal. Methods 2012, 5, 1205–1213. [Google Scholar] [CrossRef]
- Ram, M.; Seitz, L.M.; Dowell, F.E. Natural fluorescence of red and white wheat kernels. Cereal Chem. 2004, 81, 244–248. [Google Scholar] [CrossRef]
- Matveyeva, T.A.; Sarimov, R.M.; Simakin, A.V.; Astashev, M.E.; Burmistrov, D.E.; Lednev, V.N.; Sdvizhenskii, P.A.; Grishin, M.Y.; Pershin, S.M.; Chilingaryan, N.O. Using fluorescence spectroscopy to detect rot in fruit and vegetable crops. Appl. Sci. 2022, 12, 3391. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Lednev, V.N.; Sibirev, A.V.; Gudkov, S.V. The use of fluorescence spectra for the detection of scab and rot in fruit and vegetable crops. Front. Phys. 2021, 8, 640887. [Google Scholar] [CrossRef]
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G. Development of an environmentally friendly technology for the treatment of aqueous solutions with high-purity plasma for the cultivation of cotton, wheat and strawberries. ChemEngineering 2022, 6, 91. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. Comparing visual and image analysis techniques to quantify fusarium basal rot severity in mature onion bulbs. Horticulturae 2021, 7, 156. [Google Scholar] [CrossRef]
- Bashilov, A.M.; Efremenkov, I.Y.; Belyakov, M.V.; Lavrov, A.V.; Gulyaev, A.A.; Gerasimenko, S.A.; Borzenko, S.I.; Boyko, A.A. Determination of Main spectral and luminescent characteristics of winter wheat seeds infected with pathogenic microflora. Photonics 2021, 8, 494. [Google Scholar] [CrossRef]
- Bartolić, D.; Mutavdžić, D.; Carstensen, J.M.; Stanković, S.; Nikolić, M.; Krstović, S.; Radotić, K. Fluorescence spectroscopy and multispectral imaging for fingerprinting of aflatoxin-B1 contaminated (Zea mays L.) seeds: A preliminary study. Sci. Rep. 2022, 12, 4849. [Google Scholar] [CrossRef] [PubMed]
- Chavez, R.A.; Opit, G.; Opoku, B.; Stasiewicz, M.J. Spectral kernel sorting based on high-risk visual features associated with mycotoxin contamination reduces aflatoxin and fumonisin contamination in maize from Ghana. Food Control 2023, 151, 109788. [Google Scholar] [CrossRef]
- Qu, M.; Tian, S.; Yu, H.; Liu, D.; Zhang, C.; He, Y.; Cheng, F. Single-kernel classification of deoxynivalenol and zearalenone contaminated maize based on visible light imaging under ultraviolet light excitation combined with polarized light imaging. Food Control 2023, 144, 109354. [Google Scholar] [CrossRef]
- Rathna Priya, T.; Manickavasagan, A. Characterising corn grain using infrared imaging and spectroscopic techniques: A review. J. Food Meas. Charact. 2021, 15, 3234–3249. [Google Scholar] [CrossRef]
- Smeesters, L.; Kuntzel, T.; Thienpont, H.; Guilbert, L. Handheld fluorescence spectrometer enabling sensitive aflatoxin detection in maize. Toxins 2023, 15, 361. [Google Scholar] [CrossRef]
- Yao, H.; Zhu, F.; Kincaid, R.; Hruska, Z.; Rajasekaran, K. A Low-Cost, Portable Device for Detecting and Sorting Aflatoxin-Contaminated Maize Kernels. Toxins 2023, 15, 197. [Google Scholar] [CrossRef]
- Gu, S.; Chen, W.; Wang, Z.; Wang, J. Rapid determination of potential aflatoxigenic fungi contamination on peanut kernels during storage by data fusion of HS-GC-IMS and fluorescence spectroscopy. Postharvest Biol. Technol. 2021, 171, 111361. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H. Design and development of an on-line fluorescence spectroscopy system for detection of aflatoxin in pistachio nuts. Postharvest Biol. Technol. 2020, 159, 111016. [Google Scholar] [CrossRef]
- Bertani, F.; Businaro, L.; Gambacorta, L.; Mencattini, A.; Brenda, D.; Di Giuseppe, D.; De Ninno, A.; Solfrizzo, M.; Martinelli, E.; Gerardino, A. Optical detection of aflatoxins B in grained almonds using fluorescence spectroscopy and machine learning algorithms. Food Control 2020, 112, 107073. [Google Scholar] [CrossRef]
- Turksoy, S.; Kabak, B. Determination of aflatoxins and ochratoxin A in wheat from different regions of Turkey by HPLC with fluorescence detection. Acta Aliment. 2020, 49, 118–124. [Google Scholar] [CrossRef]
- Almoujahed, M.B.; Rangarajan, A.K.; Whetton, R.L.; Vincke, D.; Eylenbosch, D.; Vermeulen, P.; Mouazen, A.M. Non-destructive detection of fusarium head blight in wheat kernels and flour using visible near-infrared and mid-infrared spectroscopy. Chemom. Intell. Lab. Syst. 2024, 245, 105050. [Google Scholar] [CrossRef]
- Francesconi, S.; Harfouche, A.; Maesano, M.; Balestra, G.M. UAV-based thermal, RGB imaging and gene expression analysis allowed detection of Fusarium head blight and gave new insights into the physiological responses to the disease in durum wheat. Front. Plant Sci. 2021, 12, 628575. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Jin, N.; Gu, C.; Chen, Y.; Huang, Y. Evaluation of efficacy of fungicides for control of wheat fusarium head blight based on digital imaging. IEEE Access 2020, 8, 109876–109890. [Google Scholar] [CrossRef]
- Liang, K.; Huang, J.; He, R.; Wang, Q.; Chai, Y.; Shen, M. Comparison of Vis-NIR and SWIR hyperspectral imaging for the non-destructive detection of DON levels in Fusarium head blight wheat kernels and wheat flour. Infrared Phys. Technol. 2020, 106, 103281. [Google Scholar] [CrossRef]
- Nadimi, M.; Brown, J.; Morrison, J.; Paliwal, J. Examination of wheat kernels for the presence of Fusarium damage and mycotoxins using near-infrared hyperspectral imaging. Meas. Food 2021, 4, 100011. [Google Scholar] [CrossRef]
- Rangarajan, A.K.; Whetton, R.L.; Mouazen, A.M. Detection of fusarium head blight in wheat using hyperspectral data and deep learning. Expert Syst. Appl. 2022, 208, 118240. [Google Scholar] [CrossRef]
- Rieker, M.E.; Lutz, M.A.; El-Hasan, A.; Thomas, S.; Voegele, R.T. Hyperspectral Imaging and Selected Biological Control Agents for the Management of Fusarium Head Blight in Spring Wheat. Plants 2023, 12, 3534. [Google Scholar] [CrossRef]
- Vincke, D.; Eylenbosch, D.; Jacquemin, G.; Chandelier, A.; Pierna, J.A.F.; Stevens, F.; Baeten, V.; Mercatoris, B.; Vermeulen, P. Near infrared hyperspectral imaging method to assess Fusarium Head Blight infection on winter wheat ears. Microchem. J. 2023, 191, 108812. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Lin, F.; Yin, X.; Gu, C.; Qiao, H. Development and evaluation of a new spectral disease index to detect wheat fusarium head blight using hyperspectral imaging. Sensors 2020, 20, 2260. [Google Scholar] [CrossRef]
- Jia, B.; Wang, W.; Ni, X.; Chu, X.; Yoon, S.; Lawrence, K. Detection of mycotoxins and toxigenic fungi in cereal grains using vibrational spectroscopic techniques: A review. World Mycotoxin J. 2020, 13, 163–178. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Goffin, A.; Mailler, R.; Varrault, G.; Vulliet, E.; Morlay, C.; Nauleau, F.; Guérin, S.; Rocher, V. Fluorescence excitation/emission matrices as a tool to monitor the removal of organic micropollutants from wastewater effluents by adsorption onto activated carbon. Water Res. 2021, 190, 116749. [Google Scholar] [CrossRef]
- Jutaporn, P.; Armstrong, M.; Coronell, O. Assessment of C-DBP and N-DBP formation potential and its reduction by MIEX® DOC and MIEX® GOLD resins using fluorescence spectroscopy and parallel factor analysis. Water Res. 2020, 172, 115460. [Google Scholar] [CrossRef]
- Omanović, D.; Marcinek, S.; Santinelli, C. TreatEEM—A software tool for the interpretation of fluorescence excitation-emission matrices (EEMs) of dissolved organic matter in natural waters. Water 2023, 15, 2214. [Google Scholar] [CrossRef]
- Shi, W.; Zhuang, W.-E.; Hur, J.; Yang, L. Monitoring dissolved organic matter in wastewater and drinking water treatments using spectroscopic analysis and ultra-high resolution mass spectrometry. Water Res. 2021, 188, 116406. [Google Scholar] [CrossRef]
- Pandey, S.C.; Pande, V.; Sati, D.; Samant, M. Advanced Microbial Techniques in Agriculture, Environment, and Health Management; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Brereton, R.G. Introduction to multivariate calibration in analytical chemistry. Analyst 2000, 125, 2125–2154. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Jameson, D.M. Introduction to Fluorescence; Taylor & Francis: Abingdon, UK, 2014. [Google Scholar]
- Donaldson, L. Autofluorescence in plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Chateigner-Boutin, A.-L.; Guillon, F.; Devaux, M.-F.; Abdollahi, H.; Duponchel, L. Multi-excitation hyperspectral autofluorescence imaging for the exploration of biological samples. Anal. Chim. Acta 2019, 1062, 47–59. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Fernández-Marín, B.; Duke, S.O.; Hernández, A.; López-Arbeloa, F.; Becerril, J.M. Autofluorescence: Biological functions and technical applications. Plant Sci. 2015, 236, 136–145. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Jacobson, B.C.; Muller, M.G.; Sheets, E.E.; Badizadegan, K.; Carr-Locke, D.L.; Crum, C.P.; Boone, C.W.; Dasari, R.R.; Van Dam, J. NAD (P) H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res. 2002, 62, 682–687. [Google Scholar]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy for monitoring metabolism in animal cells and tissues. In Histochemistry of Single Molecules: Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 15–43. [Google Scholar]
- Roshchina, V.V. Autofluorescence of plant secreting cells as a biosensor and bioindicator reaction. J. Fluoresc. 2003, 13, 403–420. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Yashin, V.A.; Kononov, A.V. Autofluorescence of developing plant vegetative microspores studied by confocal microscopy and microspectrofluorimetry. J. Fluoresc. 2004, 14, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, R.; O’brien, T.; Lee, J. Studies on the aleurone layer I. Oonventional and fluorescence microscopy of the cell wall with emphasis on phenol-carbohydrate complexes in wheat. Aust. J. Biol. Sci. 1972, 25, 23–34. [Google Scholar] [CrossRef]
- Saadi, A.; Lempereur, I.; Sharonov, S.; Autran, J.; Manfait, M. Spatial distribution of phenolic materials in durum wheat grain as probed by confocal fluorescence spectral imaging. J. Cereal Sci. 1998, 28, 107–114. [Google Scholar] [CrossRef]
- Beaugrand, J.; Crônier, D.; Thiebeau, P.; Schreiber, L.; Debeire, P.; Chabbert, B. Structure, chemical composition, and xylanase degradation of external layers isolated from developing wheat grain. J. Agric. Food Chem. 2004, 52, 7108–7117. [Google Scholar] [CrossRef]
- Donaldson, L.; Williams, N. Imaging and spectroscopy of natural fluorophores in pine needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef]
- Kopczynski, K.; Kwasny, M.; Mierczyk, Z.; Zawadzki, Z. Laser Induced Fluorescence System for Detection of Biological Agents: European Project FABIOLA; SPIE: Bellingham, WA, USA, 2005; Volume 5954. [Google Scholar]
- Dalterio, R.; Nelson, W.; Britt, D.; Sperry, J.; Tanguay, J.; Suib, S. The steady-state and decay characteristics of primary fluorescence from live bacteria. Appl. Spectrosc. 1987, 41, 234–241. [Google Scholar] [CrossRef]
- Roshchina, V.; Mel’nikova, E. Pollen chemosensitivity to ozone and peroxides. Russ. J. Plant Physiol. 2001, 48, 74–83. [Google Scholar] [CrossRef]
- Pöhlker, C.; Huffman, J.A.; Pöschl, U. Autofluorescence of atmospheric bioaerosols–fluorescent biomolecules and potential interferences. Atmos. Meas. Tech. Discuss. 2011, 4, 5857–5933. [Google Scholar] [CrossRef]
- Bonfante-Fasolo, P.; Faccio, A.; Perotto, S.; Schubert, A. Correlation between chitin distribution and cell wall morphology in the mycorrhizal fungus Glomus versiforme. Mycol. Res. 1990, 94, 157–165. [Google Scholar] [CrossRef]
- Vierheiling, H.; Böckenhoff, A.; Knoblauch, M.; Juge, C.; Van Bel, A.J.; Grundler, F.; Piche, Y.; Wyss, U. In vivo observations of the arbuscular mycorrhizal fungus Glomus mosseae in roots by confocal laser scanning microscopy. Mycol. Res. 1999, 103, 311–314. [Google Scholar] [CrossRef]
- Dreyer, B.; Morte, A.; Pérez-Gilabert, M.; Honrubia, M. Autofluorescence detection of arbuscular mycorrhizal fungal structures in palm roots: An underestimated experimental method. Mycol. Res. 2006, 110, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Cartelat, A.; Álvarez-Fernández, A.; Moya, I.; Cerovic, Z.G. Time-resolved spectral studies of blue− green fluorescence of artichoke (Cynara cardunculus L. var. scolymus) leaves: Identification of chlorogenic acid as one of the major fluorophores and age-mediated changes. J. Agric. Food Chem. 2005, 53, 9668–9678. [Google Scholar] [CrossRef] [PubMed]
- Billinton, N.; Knight, A.W. Seeing the Wood through the Trees: A Review of Techniques for Distinguishing Green Fluorescent Protein from Endogenous Autofluorescence. Anal. Biochem. 2001, 291, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Eldred, G.E.; Miller, G.V.; Stark, W.S.; Feeney-Burns, L. Lipofuscin: Resolution of discrepant fluorescence data. Science 1982, 216, 757–759. [Google Scholar] [CrossRef]
- Andersson, H.; Baechi, T.; Hoechl, M.; Richter, C. Autofluorescence of living cells. J. Microsc. 1998, 191, 1–7. [Google Scholar] [CrossRef]
- Roshchina, V.a.V. Fluorescing World of Plant Secreting Cells; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Roshchina, V.; Mel’nikova, E.; Kovaleva, L. Changes in fluorescence during development of the male gametophyte. Russ. J. Plant Physiol. 1997, 44, 36–44. [Google Scholar]
- Faisal, Z.; Fliszár-Nyúl, E.; Dellafiora, L.; Galaverna, G.; Dall’Asta, C.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interaction of zearalenone-14-sulfate with cyclodextrins and the removal of the modified mycotoxin from aqueous solution by beta-cyclodextrin bead polymer. J. Mol. Liq. 2020, 310, 113236. [Google Scholar] [CrossRef]
- Scafuri, B.; Varriale, A.; Facchiano, A.; D’Auria, S.; Raggi, M.E.; Marabotti, A. Binding of mycotoxins to proteins involved in neuronal plasticity: A combined in silico/wet investigation. Sci. Rep. 2017, 7, 15156. [Google Scholar] [CrossRef]
- Duan, N.; Li, C.; Song, M.; Ren, K.; Wang, Z.; Wu, S. Deoxynivalenol fluorescence aptasensor based on AuCu bimetallic nanoclusters and MoS2. Microchim. Acta 2022, 189, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, X.; Zhou, Y.; Fu, F. Deoxynivalenol Detection beyond the Limit in Wheat Flour Based on the Fluorescence Hyperspectral Imaging Technique. Foods 2024, 13, 897. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interactions of mycotoxin alternariol with cyclodextrins and its removal from aqueous solution by beta-cyclodextrin bead polymer. Biomolecules 2019, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Effects of microenvironmental changes on the fluorescence signal of alternariol: Magnesium induces strong enhancement in the fluorescence of the mycotoxin. Int. J. Mol. Sci. 2021, 22, 8692. [Google Scholar] [CrossRef]
- Khundzhua, D.; Patsaeva, S.; Terekhova, V.; Yuzhakov, V. Spectral characterization of fungal metabolites in aqueous medium with humus substances. J. Spectrosc. 2013, 2013, 538608. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence Spectroscopy Reveals Ubiquitous Presence of Oxidized and Reduced Quinones in Dissolved Organic Matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Berg, R.H.; Beachy, R.N. Fluorescent protein applications in plants. Methods Cell Biol. 2008, 85, 153–177. [Google Scholar]
- Chance, B.; Schoener, B.; Oshino, R.; Itshak, F.; Nakase, Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J. Biol. Chem. 1979, 254, 4764–4771. [Google Scholar] [CrossRef]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz–Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Investig. Ophthalmol. Vis. Sci. 2001, 42, 241–246. [Google Scholar]
- Ramanujam, N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000, 2, 89–117. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Symons, S.; Dexter, J. Aleurone and pericarp fluorescence as estimators of mill stream refinement for various Canadian wheat classes. J. Cereal Sci. 1996, 23, 73–83. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H. Imaging of the blue, green, and red fluorescence emission of plants: An overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Ndolo, V.; Beta, T. Types and Distribution of Phenolic Compounds in Grains. In Cereal Grain-Based Functional Foods: Carbohydrate and Phytochemical Components, Beta, T., Camire, M.E., Eds.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar] [CrossRef]
- Galland, M.; Boutet-Mercey, S.; Lounifi, I.; Godin, B.; Balzergue, S.; Grandjean, O.; Morin, H.; Perreau, F.; Debeaujon, I.; Rajjou, L. Compartmentation and dynamics of flavone metabolism in dry and germinated rice seeds. Plant Cell Physiol. 2014, 55, 1646–1659. [Google Scholar] [CrossRef]
- Zandomeneghi, M. Fluorescence of cereal flours. J. Agric. Food Chem. 1999, 47, 878–882. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.-L.; Lapierre, C.; Alvarado, C.; Yoshinaga, A.; Barron, C.; Bouchet, B.; Bakan, B.; Saulnier, L.; Devaux, M.-F.; Girousse, C.; et al. Ferulate and lignin cross-links increase in cell walls of wheat grain outer layers during late development. Plant Sci. 2018, 276, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Pettersson, H.; Olsen, M.; Börjesson, T. Real-time PCR detection of Fusarium species in Swedish oats and correlation to T-2 and HT-2 toxin content. World Mycotoxin J. 2010, 3, 77–88. [Google Scholar] [CrossRef]
- Pan, Y.-L.; Hill, S.C.; Pinnick, R.G.; House, J.M.; Flagan, R.C.; Chang, R.K. Dual-excitation-wavelength fluorescence spectra and elastic scattering for differentiation of single airborne pollen and fungal particles. Atmos. Environ. 2011, 45, 1555–1563. [Google Scholar] [CrossRef]
- Raimondi, V.; Palombi, L.; Cecchi, G.; Lognoli, D.; Trambusti, M.; Gomoiu, I. Remote detection of laser-induced autofluorescence on pure cultures of fungal and bacterial strains and their analysis with multivariate techniques. Opt. Commun. 2007, 273, 219–225. [Google Scholar] [CrossRef]
- Raimondi, V.; Agati, G.; Cecchi, G.; Gomoiu, I.; Lognoli, D.; Palombi, L. In vivo real-time recording of UV-induced changes in the autofluorescence of a melanin-containing fungus using a micro-spectrofluorimeter and a low-cost webcam. Opt. Express 2009, 17, 22735–22746. [Google Scholar] [CrossRef]
- König, K.; Berns, M.W.; Tromberg, B.J. Time-resolved and steady-state fluorescence measurements of β-nicotinamide adenine dinucleotide-alcohol dehydrogenase complex during UVA exposure. J. Photochem. Photobiol. B Biol. 1997, 37, 91–95. [Google Scholar] [CrossRef]
- Ganzlin, M.; Marose, S.; Lu, X.; Hitzmann, B.; Scheper, T.; Rinas, U. In situ multi-wavelength fluorescence spectroscopy as effective tool to simultaneously monitor spore germination, metabolic activity and quantitative protein production in recombinant Aspergillus niger fed-batch cultures. J. Biotechnol. 2007, 132, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Marose, S.; Lindemann, C.; Scheper, T. Two-dimensional fluorescence spectroscopy: A new tool for on-line bioprocess monitoring. Biotechnol. Prog. 1998, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.P.; Ouedraogo, D.; Orozco-Gonzalez, Y.; Gadda, G.; Gozem, S. Alternative strategy for spectral tuning of flavin-binding fluorescent proteins. J. Phys. Chem. B 2023, 127, 1301–1311. [Google Scholar] [CrossRef]
- Sandra, R.P.-S.; Gabriela, S.S.; Jazmina, C.R.A.; Helen, F.L.; Rita, C.R.G. Production of Melanin Pigment by Fungi and Its Biotechnological Applications. In Melanin; Miroslav, B., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Gao, J.; Wenderoth, M.; Doppler, M.; Schuhmacher, R.; Marko, D.; Fischer, R. Fungal melanin biosynthesis pathway as source for fungal toxins. MBio 2022, 13, e00219–e00222. [Google Scholar] [CrossRef] [PubMed]
- Cambaza, E. Comprehensive description of Fusarium graminearum pigments and related compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef]
- Ryabova, N.; Tupolskikh, T.; Serdyuk, V.; Gordeeva, N. Analysis of infection with fungi of the genus Fusarium seed and vegetative organs of crops. E3S Web Conf. 2021, 273, 01019. [Google Scholar] [CrossRef]
- Ávalos Cordero, F.J.; Pardo Medina, J.; Parra Rivero, O.; Ruger Herreros, M.M.; Rodríguez Ortiz, L.R.; Hornero Méndez, D. Carotenoid Biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A. Key global actions for mycotoxin management in wheat and other small grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Janaviciene, S.; Venslovas, E.; Kadziene, G.; Matelioniene, N.; Berzina, Z.; Bartkevics, V.; Suproniene, S. Diversity of mycotoxins produced by Fusarium strains infecting weeds. Toxins 2023, 15, 420. [Google Scholar] [CrossRef]
- Alisaac, E.; Mahlein, A.-K. Fusarium head blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Detecting maize inoculated with toxigenic and atoxigenic fungal strains with fluorescence hyperspectral imagery. Biosyst. Eng. 2013, 115, 125–135. [Google Scholar] [CrossRef]
- Wu, C.H.; Warren, H.L. Natural Autofluorescence in Fungi, and its Correlation With Viability. Mycologia 1984, 76, 1049–1058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).