Abstract
Buckwheat and other grains have become influential in sustainable agriculture and food security owing to climate change. However, subpar storage conditions can result in the deterioration of the nutritional value and active components of buckwheat, making storage quality a significant research subject. This study examined common buckwheat (CB) and Tartary buckwheat (TB) stored at 4 °C, 30 °C, and 55 °C from 0 to 6 months to assess storage quality and its relationship to the preservation of active components. The results of agglomerative hierarchical clustering (AHC) and principal component analysis (PCA) showed that as storage temperature and time increased, both CB and TB exhibited the following differences: significant alterations in color due to an increase in browning index (B.I.), higher acidity from accelerated acid production at high temperatures, and a decrease in total phenolics, flavonoid content, and antioxidant capacity due to thermal degradation of functional components. In the storage quality assessment, no alteration in microstructure or degradation in components was detected after exposure to all times and temperatures, and the content of the primary bioactive compound, rutin, was CB (16.57–27.81 mg/100 g d.w.) and TB (707.70–787.58 mg/100 g d.w.), demonstrating buckwheat’s resistance to microbial contamination. Storage temperature significantly impacts buckwheat’s quality and bioactive components, making it an important element in establishing a sustainable food supply chain.
1. Introduction
Climate change has led to a global food crisis in recent years, prompting countries to focus on increasing food self-sufficiency. The United Nations’ 17 Sustainable Development Goals (SDGs) also emphasize the importance of food security and sustainable agriculture [1], as food crops provide nutrients and can introduce bioactive compounds to consumers, and their production and demand highlight their significance. Improper storage of food crops can lead to safety concerns. Improper storage conditions, such as temperature, light exposure, oxygen, and time, can often lead to issues with the quality, processing characteristics, shelf life, and safety of food crops. Research on the quality and changes in bioactive compounds of crops such as corn, quinoa, and soybeans during storage has shown that high-temperature storage can cause food quality deterioration, including change in appearance and color, protein denaturation, lipid oxidation, and bioactive compound degradation [2,3,4]. Additionally, high temperatures and humidity can also easily lead to microbiological hazards, losses in nutritional value and bioactive compounds, and a reduction in commercial value and food safety [5]. Therefore, evaluating appropriate storage conditions is an important research topic.
Buckwheat (Fagopyrum esculentum and F. tataricum) is known for its short growth period and easy cultivation, earning it the label of an “emergency crop.” The global area of harvested buckwheat in 2022 exceeded 2.236 million hectares, producing over 2.235 million metric tons [6]. The main cultivated varieties are common buckwheat (Fagopyrum esculentum Moench, CB) and Tartary buckwheat (Fagopyrum tataricum Gaertn, TB). Buckwheat is rich in starch (54.5%), proteins (12.3%), and lipids (3.8%), with higher levels of minerals like potassium, magnesium, and copper compared to other grains, providing high nutritional value [7,8]. Buckwheat (unhulled) contains high levels of phytochemicals (rutin and quercetin) and exhibits strong antioxidant and pest- and disease-resistant properties. It is one of the world’s important major cereal crops and has recently become an indicator crop for nutrition and health. In many Asian countries, the market competition is intense and the demand is substantial [9]. Therefore, the methods of storing buckwheat and preserving its quality are crucial research topics. Buckwheat is also rich in resistant starch, making it a low-glycemic-index (GI) food [10]. Additionally, buckwheat is gluten-free, making it suitable for consumption by individuals with celiac disease [11]. Buckwheat is rich in bioactive compounds, and is particularly abundant in antioxidant components, such as polyphenols and flavonoids, with TB scoring a higher content than CB [12]. Rutin is a major secondary metabolite in buckwheat [13]; it exhibits strong free radical scavenging ability, which improves plant tissue protection, and increases storage stability. Furthermore, in the human body, rutin can enhance capillary strength, and improve high blood sugar and abnormal blood lipids [14,15].
The applications and demand for buckwheat are rapidly increasing. It is widely cultivated for products like buckwheat noodles, hulled buckwheat groats, buckwheat flour, buckwheat tea, and bread [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19], gaining attention for its diverse applications in the food industry, thereby increasing market demand. Drying, storage, and shipping are among the critical factors for global grain circulation. In many Asian countries, room temperatures range from 26 to 32 °C. Due to buckwheat’s hard texture and its high content of rutin polyphenolic compounds, which provide effective resistance to pests and antimicrobial properties, it is often stored at room temperature in well-ventilated conditions. Additionally, some supply chains utilize storage at 4 to 12 °C (low temperature) to effectively maintain grain quality. During shipping and in summer container storage, grains can be exposed to high temperatures of up to 55 °C, which may increase enzyme activity, accelerate Maillard reactions, and lead to the degradation of polyphenolic compounds [20], thereby affecting both quality and safety. After harvest, grains with high moisture content can experience high respiration rates and heat release and are prone to fermentation and microbial proliferation [21]. Proper food storage is essential for maintaining food quality and safety, especially with grains, which have gained significant attention due to their diverse applications. Factors affecting storage quality include grain composition, moisture content, environmental temperature, humidity, and oxygen [22]. Improper storage conditions, such as high temperature and humidity, can lead to microbial growth, quality deterioration, and loss of bioactive components and antioxidant capacity [2,3,4].
Studies have explored the functional components and processing of buckwheat, including the changes in functional components and antioxidant capacity resulting from different processing methods and heat treatments [16,20,23]. However, there is relatively limited research on the storage quality and functional components of buckwheat. This study aims to compare the physical properties, secondary metabolites, and antioxidant capacity of common buckwheat (Taichung No. 5) and Tartary buckwheat (Taichung No. 2) stored at extremely high temperatures (55 °C) to simulate potential conditions during transportation, as well as at refrigerated temperatures (4 °C) and room temperature (30 °C) for 0 to 6 months. The results can provide buckwheat storage quality and reference in food processing in the future.
2. Materials and Methods
2.1. Materials and Chemicals
Taichung No. 5 (Fagopyrum esculentum Moench) and Taichung No. 2 (Fagopyrum ta-taricum Gaertn) were harvested in January 2023 (Central Taiwan Agricultural Cooperative, Taichung, Taiwan). Rutin, Trolox, 1,1-Diphenyl-2-picrylhydrazyl, gallic acid, 2,2-Azino-bis[3-ethylenzthiazoline-6-sulfonic acid], sterile count agar, Folin and Ciocalteu’s phenol reagent, and quercetin were purchased from Sigma-Aldrich Co. (Missouri, TX, USA). All reagents were LC grade. All solutions were prepared using deionized water with a resistivity of no < 18.2 MΩ cm−1.
2.2. Simulated Storage Test
The buckwheat was dried by a heat-pump dryer (MCGS, Bao Sheng Biotechnology Co., Tainan, Taiwan) for 24 h to maintain the water activity at 0.172–0.199, then stored at 4 °C, 30 °C, and 55 °C for 6 months.
2.3. Scanning Electron Microscope (SEM)
A dissecting stereomicroscope (Model SM Z 800, Nikon Co., Tokyo, Japan) and a scanning electron microscope (Model ABT-150S, Topcon Co., Tokyo, Japan) were used to capture the microstructure of the buckwheat samples.
2.4. Moisture and Water Activity Analysis
The buckwheat was dried by a heat-pump dryer for 55 °C and 24 h. Followed the method by Hsu et al. [24], the moisture (%) was calculated by Eq. 1. The water activity of buckwheat was measured by a water activity meter (Model Aqualab 3TE, Meter Group Inc., Washington, DC, USA).
where W0 is the original weight, and W1 is the weight of different storage temperature.
2.5. Color Analysis
Followed the method by Chiang and Chiang [25], the lightness value (L*), red/green coordinate (a*), and yellow/blue coordinate (b*) of all samples were measured by a color meter (Model ZE-2000, Nippon Denshoku Industries Co., Tokyo, Japan). The calculation of the color difference (ΔE) and browning index (BI) followed Equations (2)–(4).
2.6. Physical Quality Indices
pH, Titratable Acidity, and Total Soluble Solids of Buckwheat
Followed the method by Tsai et al. [26] and Hsu et al. [24], all samples were diluted with deionized water (1:10). The pH value was measured by a pH meter (Model SP-2300, Suntex Instruments Co., New Taipei, Taiwan). The total acidity was evaluated by titration with 0.1 N NaOH and expressed as citric acid. The total soluble solids were measured by a portable refractometer (Model MASTER-M, ATOGO Co., Tokyo, Japan).
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
Followed the method by Chiang and Chiang [25], all samples were analyzed by a Fourier-transform infrared spectrometer (FTIR) (Model Nicolet 6700, Thermo Fisher Scientific Co., Waltham, MA, USA) and MCT detector (Thermo Fisher Scientific Co., MA, USA). The scanning range was from 750 to 4000 cm−1. Spectra were collected, and the resolution was set at 2 cm−1/30 s.
2.8. Antioxidants and Its Capacities of Buckwheat
Followed the method by Lin et al. [27], total polyphenols and flavonoids were ex-pressed as gallic acid and quercetin equivalents (mg GAE/100g dry weight and mg QE/100 g dry weight), respectively. We measured the absorbance at 735 nm and 415 nm using an ELISA reader, respectively. DPPH and ABTS radical scavenging activity were expressed as Trolox equivalents (mg Trolox eq./100 g dry weight extracts). We measured the absorbance at 517 nm and 734 nm using an ELISA reader, respectively.
2.9. HPLC-DAD Analysis of Rutin
Followed the method by Chiang and Chiang [25], the high-performance liquid chromatography system with diode array detector (HPLC-DAD) is composed of an autosampler (Model PN5300, Postnova Co., Salt Lake City, UT, USA), a chromatographic pump (Model Chromaster 5110, Hitachi Co., Tokyo, Japan), a Mightysil RP-18GP column (250 mm, 4.6 mm i.d., 5.0 μm) (Kanto Co., Tokyo, Japan), and a diode array detector (Model L-2450, Hitachi Co., Tokyo, Japan). The mobile phase is a mixture of two solvents: A (0.1% H3PO4) and B (100% ACN). This mixture was analyzed for 38 min. The mobile phase flow rate was 1.00 mL/min at 25 °C, and the gradient was as follows: 0–5min, 5% B; 5–15 min, 37% B; 15–28 min, 40% B; 28–29 min, 95% B; 29–32 min, 50% B; 32–38 min, 5% B. These were injected into 10 μL of the samples. The DAD detection wavelength was set at 350 nm. Rutin was identified by comparing the retention time (min) of standards and quantified to dried weight (mg/g) by calibration curves from standards as well.
2.10. Microorganism Growth Tests
Following the method by Chen et al. [28], all samples were added to sterile physiological saline (SPS) and homogenized using a blender (7012G, Waring Co., Stamford, CT, USA). The appropriate dilutions were poured onto sterile count agar plates to determine the total plate count and poured onto 3MPetrifilm (YM) to determine the yeast and mold.
2.11. Statistical Analysis
All results are shown as mean ± standard deviation in the database (n = 3). Data were collected by the one-way analysis of variance (ANOVA) of SPSS version 12.0 software (IBM Co., Armonk, New York, NY, USA). Significance tests were determined by Duncan’s multiple range test (DMRT), and all comparisons were considered statistically significant if p < 0.05. Principal Component Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC) were performed using XLSTAT version 2024 software (Addinsoft Co., New York, NY, USA) for data analysis.
3. Results and Discussion
3.1. Microstructure
Buckwheat seeds consist of a husk and an endosperm. Due to the low moisture content after harvesting and drying, the seeds have a hard texture, with starch being the predominant component [7,8]. In this study, using a dissecting stereomicroscope and a scanning electron microscope, the microstructures of buckwheat can be observed. Buckwheat grains contain a high amount of starch, accounting for over 70% of their dry weight [29]. In this study, the aim was to investigate whether there would be any morphological changes in starch granules and other microstructures due to high-temperature storage during the storage period. Results from the stereomicroscope at 20× magnification (Figure 1) showed that the color of buckwheat tends to darken with increasing temperature and storage time, which is consistent with findings from other studies [3]. Increasing the temperature and extending the duration enhances enzyme activity and Maillard reactions, leading to changes in appearance and increased browning of the endosperm (Figure 1) [20]. Additionally, because buckwheat is a drought-resistant crop, its endosperm exhibits a highly dense and complex internal structure. From the scanning electron microscope at 1000× magnification, the buckwheat flour mainly consisted of starch granules, which appeared polygonal and smooth [29]. Additionally, the powder samples exhibited agglutinated starch granules. When observing buckwheat samples stored at different temperatures, both common buckwheat (CB) and Tartary buckwheat (TB) showed no significant changes between different groups (Figure 2).

Figure 1.
The influence of different storage temperatures on microstructure (20×) change of buckwheat. (A) Common buckwheat; (B) Tartary buckwheat.

Figure 2.
The influence of different storage temperatures on microstructure (20×) change of common buckwheat. CB and TB represent common buckwheat and Tartary buckwheat.
3.2. Quality
Moisture and temperature are important control factors for the storage period, quality, and safety of grains [30,31]. Moisture content is one of the critical factors affecting the biological activity of grains [32]. There were no significant differences in moisture content between the CB and TB samples as the storage temperature and time increased (CB: 7.23–7.25%; TB: 6.75–6.78%) (Table 1). When the moisture content of grains is below the critical level (14.5–15%), hydrolysis, respiration, and enzymatic activity, which affect quality, are less likely to occur [33]. Therefore, with appropriate packaging and storage, the low moisture content after harvesting and drying can effectively enhance the storage period and quality stability of the grains during distribution. With increased storage time, the water activity of both CB and TB samples showed an upward trend in all temperature groups, with the 55 °C group showing the highest increase (CB: 0.186–0.199; TB: 0.172–0.183) and the 30 °C group showing a slight increase (CB: 0.186–0.194; TB: 0.172–0.176). In comparison, the water activity at 4 °C showed no significant changes (CB: 0.186–0.187; TB: 0.172) (Table 1). The results indicate that high-temperature storage led to an increase in water activity, suggesting that the changes in quality due to high-temperature storage may be related to alterations in bound water and free water content. The soluble solid content of CB ranged from 0.98 to 1.02 °Brix, while TB had a soluble solid content ranging from 0.88 to 0.92 °Brix. Neither CB nor TB exhibited significant changes in soluble solid content with variations in storage temperature and time (Table 1).

Table 1.
Moisture content, water activity, and soluble solid content of buckwheat.
The color difference (ΔE) and browning index (B.I.) are important criteria for assessing browning and color changes in food products under high-temperature storage conditions [34]. Figure 3A indicates that the B.I. of CB and TB shows no significant drift at 4 °C and 30 °C over time, while an increase is observed at 55 °C (CB: 15.85–18.95; TB: 22.13–25.67). Figure 3B shows that with increasing storage time, the ΔE of CB and TB shows no significant trend at 4 °C, a slight increase at 30 °C (CB: 0.40–0.94; TB: 0.27–1.82), and a significant increase at 55 °C (CB: 1.16–3.81; TB: 1.86–4.10). As temperature and storage time increase, both CB and TB exhibit darker colors, with higher ΔE and increased B.I. Previous studies have found that increasing heat treatment temperature and time may lead to darker color and intensified browning in buckwheat [35]. In this study, it was observed that at 4 °C and 30 °C, due to enzyme activity and chemical reactions, there was slowly less occurrence of browning changes. The 55 °C condition primarily simulates the high-temperature effects experienced during shipping in container storage in summer in some Asian countries. Onigbinde and Akinyele also observed browning in maize under high-temperature storage conditions. This study aligns with these results [3].

Figure 3.
The influence of different storage temperatures on quality of buckwheat. (A) Browning index; (B) ΔE; (C) pH; (D) titratable acidity.
The pH and titratable acids of buckwheat during storage were observed. Figure 3C illustrates that the pH of both CB and TB showed no significant drift at 4 °C (CB: 6.69–6.70; TB: 6.71–6.72), slightly decreased at 30 °C (CB: 6.70–6.63; TB: 6.72–6.69), and significantly decreased at 55 °C (CB: 6.70–6.28; TB: 6.72–6.48) with increasing storage time. Figure 3D illustrates that the titratable acidity of CB and TB showed no significant trend at 4 °C (CB: 1.30–1.27%; TB: 0.95–0.97%), slightly increased at 30 °C (CB: 1.30–1.35%; TB: 0.96–1.20%), and significantly increased at 55 °C (CB: 1.30–1.53%; TB: 0.96–1.41%) with increasing storage time. As storage temperature and time increased, there was a decreasing trend in pH and an increasing trend in titratable acids. This could be due to the accelerated respiration of buckwheat during high-temperature storage, leading to increased organic acid production in the tricarboxylic acid cycle (TCA cycle) [36].
3.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
FTIR can be used to measure the bonding strength of functional groups, thereby allowing the detection of typical bond changes during storage [37]. In this study, the primary focus was to compare the functional group changes in buckwheat under extreme storage conditions (55 °C) for the long-term storage period (6 months). Figure 4 shows that both CB and TB exhibited peak variations in the range of 1540 to 1800 cm−1. Previous studies have utilized the range of 1540 to 1800 cm−1 as an indicator for identifying organic acids, such as lactic acid and acetic acid in starch [38]. Additionally, in Figure 4, the peaks at 2858 and 2927 cm−1 showed an increasing trend with longer high-temperature storage. The peak at 2858 cm−1 represents the symmetric stretching of -CH2 groups, and the peak at 2927 cm−1 represents the asymmetric stretching of -CH2 groups. Studies have indicated that absorption bands at 2858 and 2927 cm−1 correspond to the presence of carboxyl groups [39], which may be due to the increase in organic acid content due to high-temperature storage. This finding is consistent with the changes in titratable acidity observed in the study.
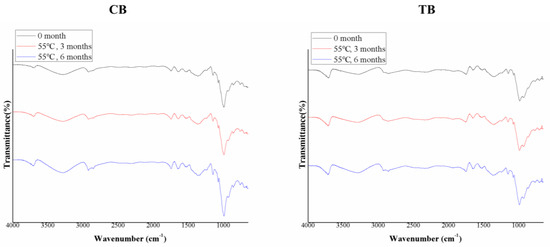
Figure 4.
FTIR spectra of the buckwheats. Common buckwheat (CB); Tartary buckwheat (TB).
3.4. Functional Compounds and Antioxidant Capacities
Polyphenols are secondary metabolites in plants, mainly produced in response to external biological or non-biological threats, rather than being essential for the plant’s survival. At 55 °C, high temperatures and oxidation readily promote the degradation of polyphenolic compounds during storage. Figure 5A shows that with increasing storage time, the total phenolic content in both CB and TB showed a significant decrease at 55 °C (CB: 117.19–96.41 mg GAE/100 g d.w.; TB: 143.92–129.80 mg GAE/100 g d.w.), while at 4 and 30 °C, there was no significant change over time. Figure 5B also shows that the flavonoid content of CB and TB significantly decreased with increasing storage time at 55 °C (CB: 191.21–160.04 mg QE/100 g d.w. TB: 437.89–405.29 mg QE/100 g d.w.), and a similar decreasing trend was observed at 30 °C (CB: 191.21–169.15 mg QE/100 g d.w.; TB: 437.89–415.83 mg QE/100 g d.w.). Previous studies have reported that high-temperature roasting leads to a decline in total polyphenols and flavonoids in buckwheat [20]. Additionally, Zieliński et al. found a decrease in total polyphenol content after buckwheat processing using extrusion at 120–160 °C [40]. Zhang et al. investigated the effects of heat treatment, high-pressure steam, and microwave heating on TB, and observed a decline in total polyphenols, flavonoids, and antioxidant capacity in buckwheat flour after heat processing [41]. This suggests that polyphenols may degrade due to high-temperature storage, which aligns with the results of related studies on the thermal processing of buckwheat. Buckwheat is rich in polyphenols and flavonoid compounds, including rutin and quercetin. However, these polyphenols and flavonoids are prone to degradation due to heat and time, which can lead to a reduction in antioxidant capacity [20].
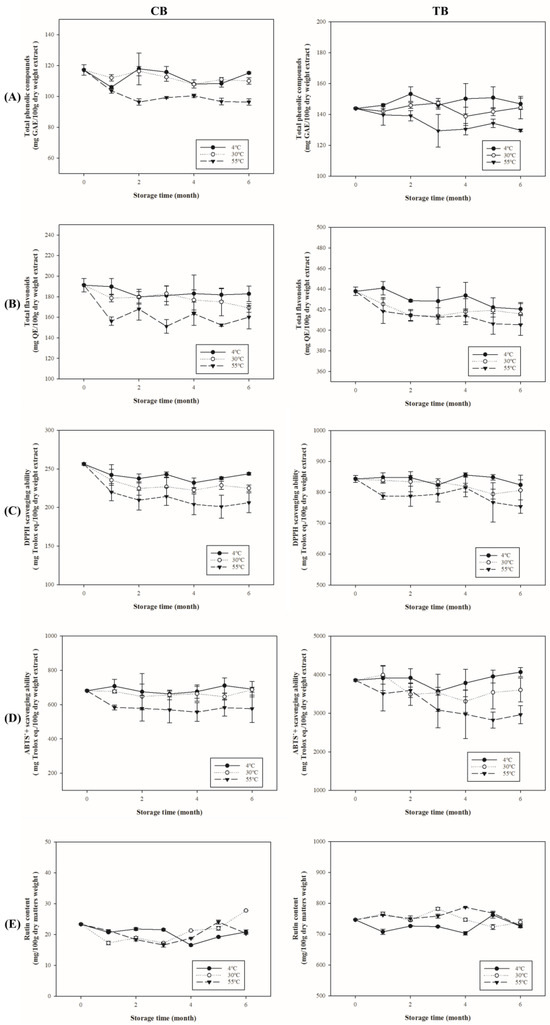
Figure 5.
The influence of different storage temperature on antioxidants and their capacities in buckwheats. (A) Total phenolic content; (B) total flavonoid content; (C) ABTS radical scavenging activity; (D) DPPH radical scavenging activity; (E) rutin.
Figure 5C show that with increasing storage time, the decrease in total polyphenol content led to a significant reduction in ABTS radical scavenging capacity for both CB and TB at 55 °C (CB: 681.04–576.23 mg Trolox eq./100 g d.w.; TB: 3858.24–2964.32 mg Trolox eq./100 g d.w.), while only a slight decrease was observed at 4 °C and 30 °C. As depicted in Figure 5D, with increasing storage time, the DPPH radical scavenging capacity also showed a decrease at 55 °C (CB: 256.39–206.20 mg Trolox eq./100 g d.w.; TB: 843.31–754.03 mg Trolox eq./100 g d.w.), while at 4 °C and 30 °C, there were no significant decreases observed. Several studies have indicated that total phenolic compounds and flavonoids are among the factors influencing antioxidant activity [42,43,44,45]. Phenolic compounds act as effective antioxidants due to the presence of hydroxyl groups on their aromatic rings, which can donate hydrogen and chelate metal ions [46]. The decrease in total phenolic and flavonoid content may lead to a reduction in antioxidant capacity. Additionally, such conditions can lead to fat oxidation, producing undesirable odors (rancid aroma). Oxidation products like malondialdehyde (MDA) interact with DNA and proteins in the human body, potentially causing mutagenic effects and contributing to atherosclerosis [47]. Our study measured MDA content (Figure S1), revealing a significant increase in MDA content at high temperatures (55 °C) over extended storage periods (p < 0.05). These results are consistent with previous research, which indicates that high-temperature storage may lead to fat oxidation and the formation of peroxidation products such as MDA [47]. Figure 5E indicates the rutin content of both CB (16.57–27.81 mg/100 g d.w.) and TB (707.70–787.58 mg/100 g d.w.) during storage periods. This study is consistent with the findings of [20], which reported that rutin is the most heat-resistant flavonoid in buckwheat. Rutin is relatively resistant to thermal degradation due to its heat-stable properties; therefore, its content did not show significant changes at the three storage temperatures (4 °C, 30 °C, 55 °C). However, a small amount of rutin was still hydrolyzed to quercetin by rutinase [48]. The study observed significant impacts on antioxidant activity, with a marked decrease in total polyphenols and total flavonoids, after 6 months of storage at 55 °C during storage testing. Consequently, buckwheat stored at lower temperatures exhibits better quality and storage stability.
3.5. The Influence of Total Plate Count/Yeast and Mold during Storage
The microbial composition in grains is highly diverse; however, bacteria and fungi have the greatest impact on storage quality [32]. Microbial growth, one cause of food spoilage, is related to moisture content and water activity. When the moisture content of grains reaches 15% to 18.5%, molds and yeasts begin to proliferate rapidly. When the moisture content exceeds 20%, bacterial growth becomes substantial [49]. In this study, the water content of buckwheat decreased after drying (CB: 7.24%; TB: 6.76%), leading to a decrease in water activity (CB: 0.186; CB: 0.172), which was not conducive to the growth of microorganisms. To ensure the presence of spores from spore-forming bacteria and mold that can survive extreme conditions during the packaging and storage of buckwheat, the total plate count, yeast, and mold are critical indicators of microbial growth. As shown in Table 2, the total plate count for CB and TB was the lowest when stored at 55 °C for 6 months, decreasing from 4.30 to 3.78 log CFU/g for CB and from 3.21 to 2.10 log CFU/g for TB. At 30 °C, the total plate count was the highest, increasing from 4.30 to 4.71 log CFU/g for CB and from 3.21 to 3.53 log CFU/g for TB. A temperature of 30 °C is optimal for the growth and proliferation of most mesophilic microorganisms commonly found in grains [50]. The trend of microbial growth at 4 °C was not significant. A similar trend can be observed in the study of yeast and mold (Table 2). Both CB and TB had < 1 log CFU/g of yeast and mold at 55 °C, and the growth of yeast and mold at 30 °C (CB: 2.10–2.78 log CFU/g; TB: 2.20–2.56 log CFU/g) was higher than that at 4 °C (CB: 2.10–2.36 log CFU/g; TB: 2.20–2.30 log CFU/g). Based on the yeast and mold count plate (3M Petrifilm (YM)), the results showed that buckwheat had predominantly mold colonies, with no yeast colonies present.

Table 2.
The influence of different storage temperatures on total bacterial count and yeast and mold of buckwheat.
3.6. Principal Component Analysis (PCA)
Chemometrics is the science of utilizing mathematical and statistical principles to process research data in food science, such as agglomerative hierarchical clustering (AHC) and principal component analysis (PCA). After performing AHC, the data can be further explored to understand the relationships between different clusters and experimental variables. PCA can reduce the dimensionality of experimental data to interpret the relationships between different experimental parameters and variances. In this study, the quality and functional compound results were subjected to AHC. CB was classified into three groups (C1, C2, and C3). C1 comprised samples from the unstored condition, 1- to 6-month storage at 4 and 30 °C, and 1-month storage at 55 °C. C2 included samples from 2 to 6 months of storage at 55 °C, while C3 consisted of samples stored for 6 months at 55 °C (Figure 6A). TB was classified into two groups (C1 and C2). C1 comprised samples from the unstored condition and all samples stored at 4 and 30 °C, while C2 included all samples stored at 55 °C (Figure 6B). The results indicated that the temperature effect was the main factor influencing the clustering, with the highest impact observed at high temperature (55 °C), and no significant differences observed among samples stored at 4 and 30 °C. After PCA, Figure 6C explained 74.19% of the overall data for CB (PC1 = 65.02%; PC2 = 9.17%), and Figure 6D explained 78.29% of the overall data for TB (PC1 = 68.63%; PC2 = 9.66%). A comparison of the AHC and PCA results shows that, in both CB and TB samples without storage or stored at lower temperatures, (1) L* values were higher (r = 0.973, −0.916), and B.I. (r = −0.978, 0.929) and ΔE (r = −0.986, 0.961) were lower, indicating that high-temperature storage caused buckwheat browning. (2) pH values were higher (r = 0.995, −0.950), and titratable acidity was lower (r = −0.608, 0.961), indicating changes in organic acid content due to high-temperature storage. (3) Total phenolic content (r = 0.883, −0.887), flavonoid content (r = 0.880, −0.861), ABTS radical scavenging capacity (r = 0.909, −0.916), and DPPH radical scavenging capacity (r = 0.894, −0.893) were higher, indicating the loss of buckwheat’s functionality due to high-temperature storage. (4) Moisture content (r = 0.043, 0.152), soluble solids (r = 0.095, 0.124), and rutin content (r = 0.036, 0.361) showed no significant differences (CB and TB showed opposite trends on the X-axis in the PCA, leading to the positive–negative differences in r-values). In conclusion, storage at temperatures above 55 °C caused the loss of quality and functionality in buckwheat during the storage process. The functional index component, rutin, was less susceptible to degradation during long-term storage, indicating its potential for development and application in the functional food market in the future. Buckwheat seeds are characterized by low moisture content, a hard texture, and a high rutin content. Due to these properties, quality degradation and microbial proliferation during storage are minimal. Buckwheat has the characteristics of good cereal crops. This study found that storage at 55 °C leads to noticeable browning of the seed and some degradation of rutin flavonoids. In contrast, storage at 4 °C better preserves the quality of the seeds, although the cost of storage and transportation must be considered.
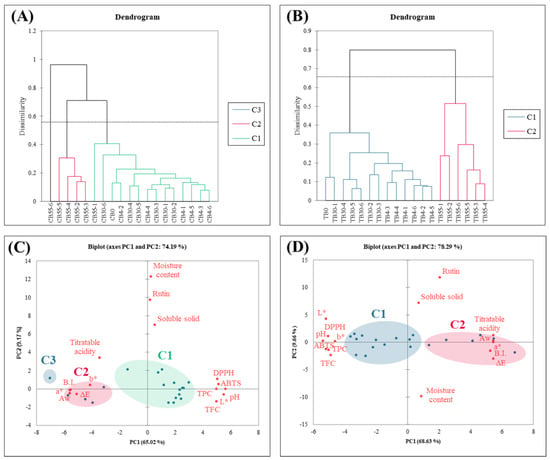
Figure 6.
Principal component analysis and agglomerative hierarchical clustering of buckwheat with different storage temperatures and time. (A) PCA of common buckwheat; (B) PCA of Tartary buckwheat; (C) AHC of common buckwheat; (D) AHC of Tartary buckwheat; the circles represent the clusters detected with AHC analysis; the solid fill type highlights the val-ues with PCA.
4. Conclusions
This study found that buckwheat exhibited better storage stability due to its rich content of rutin, which is known for its strong antioxidant properties and for resisting the influence of degradation with increasing storage temperature and time. Simulating potential conditions during transportation, these results show that storing buckwheat at lower temperatures mitigated quality degradation and inhibited microbial growth. Therefore, a cold chain (4 °C) is recommended to ensure the quality, functionality, and safety of buckwheat. Sustainable food supply chains are a crucial topic in today’s society. This study integrates food security with sustainable agriculture to maintain the stable quality and nutritional value of buckwheat under appropriate temperature management and storage conditions, effectively preventing food waste and disruptions or losses in the supply chain. These results provide valuable insights for the storage and processing of buckwheat and similar grains in the food industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriengineering6030178/s1, Figure S1: The influence of different storage temperatures on MDA content change of buckwheat.
Author Contributions
Conceptualization, Y.-L.C. and K.-M.Y.; Methodology, Y.-L.C. and K.-M.Y.; Formal analysis, Y.-L.C.; Writing—original draft preparation, Y.-L.C. and K.-M.Y.; Writing—review and editing, K.-M.Y. and X.-Y.S.; Visualization, J.-J.H. and Y.-A.M.; Data curation, K.-M.Y. and P.-Y.C.; Validation, J.-J.H. and Y.-A.M.; Investigation, K.-M.Y., X.-Y.S. and P.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Agriculture (MOA) under Project 112AS-4.2.2-FD-Z1.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The support of the FTIR and SEM measurements from the Instrument Center of National Chung Hsing University is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shen, Y.; Chen, Y.; Shi, X.; An, Y.; Yang, M.; Qi, Y. Assessing the role of global food commodity prices in achieving the 2030 agenda for SDGs. iScience 2024, 27, 108832. [Google Scholar] [CrossRef]
- Kibar, H.; Sönmez, F.; Temel, S. Effect of storage conditions on nutritional quality and color characteristics of quinoa varieties. J. Stored Prod. Res. 2021, 91, 101761. [Google Scholar] [CrossRef]
- Onigbinde, A.; Akinyele, I. Biochemical and nutritional changes in corn (Zea mays) during storage at three temperatures. J. Food Sci. 1988, 53, 117. [Google Scholar] [CrossRef]
- Pohndorf, R.S.; Meneghetti, V.L.; Paiva, F.F.; de Oliveira, M.; Elias, M.C. Kinetic evaluation of oxidative stability and physical degradation of soybean grains stored at different conditions. J. Food Process Preserv. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Cañizares, L.D.C.C.; Gaioso, C.A.; Timm, N.D.S.; Meza, S.L.R.; Ramos, A.H.; Oliveira, D.M.; Lutz, É.; Elias, M.C. Influence of broken kernels content on soybean quality during storage. Grain Oil Sci. Technol. 2024, 7, 105–112. [Google Scholar] [CrossRef]
- FAO. Food Security Indicators, Ethiopia. FAOSTAT Stat. Database. 2022. Available online: http://www.fao.org/faostat/en/#data/FS (accessed on 1 July 2024).
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.; Latif, M.; Randhawa, M. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- Kreft, I.; Golob, A.; Germ, M. A crop of high nutritional quality and health maintenance value: The importance of tartary buckwheat breeding. Agriculture 2023, 13, 1783. [Google Scholar] [CrossRef]
- Wijngaard, H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Christa, K.; Soral-Śmietana, M. Buckwheat grains and buckwheat products–nutritional and prophylactic value of their components–a review. Czech. J. Food Sci. 2008, 26, 153–162. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, G. Flavonoids as flower pigments:the formation of natural spectrum and its extension by genetic engineering. Plant Breed. 1991, 106, 1–26. [Google Scholar] [CrossRef]
- Fernandes, A.A.H.; Novelli, E.L.B.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimarães, J.F.C.; Junior, A.F. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Zhang, Q.H. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Sci. Nutr. 2001, 41, 451–464. [Google Scholar] [CrossRef]
- Klepacka, J.; Najda, A. Effect of commercial processing on polyphenols and antioxidant activity of buckwheat seeds. Int. J. Food Sci. Technol. 2021, 56, 661–670. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Kolasa, A.; Moscicki, L. Influence of buckwheat addition on physical properties, texture and sensory characteristics of extruded corn snacks. Pol. J. Food Nutr. Sci. 2013, 63, 239–244. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Technol. 2015, 44, 58–65. [Google Scholar] [CrossRef]
- Qin, P.; Wu, L.; Yao, Y.; Ren, G. Changes in phytochemical compositions, antioxidant and α-glucosidase inhibitory activities during the processing of tartary buckwheat tea. Food Res. Int. 2013, 50, 562–567. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, B.; Kaur, A.; Singh, N.; Kaur, M.; Kumari, S.; Yadav, M.P. Effect of infrared roasting on antioxidant activity, phenolic composition and Maillard reaction products of Tartary buckwheat varieties. Food Chem. 2019, 285, 240–251. [Google Scholar] [CrossRef]
- Harrington, J.F.; Kozlowski, T. Seed storage and longevity. Seed Biol. 1972, 3, 145–245. [Google Scholar] [CrossRef]
- Manandhar, A.; Milindi, P.; Shah, A. An overview of the post-harvest grain storage practices of smallholder farmers in developing countries. Agriculture 2018, 8, 57. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, C.; Yao, Y.; Xu, B. Alteration of phenolic profiles and antioxidant capacities of common buckwheat and Tartary buckwheat produced in China upon thermal processing. J. Sci. Food Agric. 2019, 99, 5565–5576. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.Y.; Yang, K.M.; Chiang, Y.C.; Lin, L.Y.; Chiang, P.Y. The browning properties, antioxidant activity, and α-glucosidase inhibitory improvement of aged oranges (Citrus sinensis). Foods 2024, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Chiang, P.Y. Accentuation of the browning characteristics and functional properties of aged tomatoes (Solanum Lycopersicon cv.). Food Chem. X 2024, 22, 101499. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Lin, L.Y.; Yang, K.M.; Chiang, Y.C.; Chen, M.H.; Chiang, P.Y. Effects of roasting sweet potato (Ipomoea batatas L. Lam.): Quality, volatile compound composition, and sensory evaluation. Foods 2021, 10, 2602. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) Essential oils: Volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mantilla, S.M.O.; Netzel, M.E.; Cozzolino, D.; Sivakumar, D.; Sultanbawa, Y. Physicochemical, antioxidant and microbial stability of Burdekin plum leathers. Int. J. Food Sci. Technol. 2024, 59, 2716–2726. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat starch: Structures, properties, and applications. Trends Food Sci. Technol. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Al-Yahya, S.A. Effect of storage conditions on germination in wheat. J. Agron. Crop Sci. 2001, 186, 273–279. [Google Scholar] [CrossRef]
- Moreno-Martínez, E.; Rivera, A.; Badillo, M.V. Effect of fungi and fungicides on the preservation of wheat seed stored with high and low moisture content. J. Stored Prod. Res. 1998, 34, 231–236. [Google Scholar] [CrossRef]
- Flor, O.; Palacios, H.; Suárez, F.; Salazar, K.; Reyes, L.; González, M.; Jiménez, K. New sensing technologies for grain moisture. Agriculture 2022, 12, 386. [Google Scholar] [CrossRef]
- Raudienė, E.; Rušinskas, D.; Balčiūnas, G.; Juodeikienė, G.; Gailius, D. Carbon dioxide respiration rates in wheat at various temperatures and moisture Contents. Mapan 2017, 32, 51–58. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Małgorzata, W.; Konrad, P.M.; Zieliński, H. Effect of roasting time of buckwheat groats on the formation of Maillard reaction products and antioxidant capacity. Food Chem. 2016, 196, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Ramstad, P.E.; Geddes, W.F. The Respiration and Storage Behavior of Soybeans; University of Minnesota Agricultural Experiment Station: Saint Paul, MN, USA, 1942; p. 156. [Google Scholar]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Donner, E.; Liu, Q. The effect of various extracting agents on the physicochemical and nutritional properties of pea starch. Starch-Stärke 2019, 71, 1900123. [Google Scholar] [CrossRef]
- Wu, M.L.; Nie, M.Q.; Wang, X.C.; Su, J.M.; Cao, W. Analysis of phenanthrene biodegradation by using FTIR, UV and GC–MS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1047–1050. [Google Scholar] [CrossRef]
- Zieliński, H.; Michalska, A.; Piskuła, M.K.; Kozłowska, H. Antioxidants in thermally treated buckwheat groats. Mol. Nutr. Food Res. 2006, 50, 824–832. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT-Food Sci. Technol. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Karamac, M.; Biskup, I.; Kulczyk, A. Fractionation of buckwheat seed phenolics and analysis of their antioxidant activity. Pol. J. Food Nutr. Sci. 2015, 65, 243–249. [Google Scholar] [CrossRef]
- Verardo, V.; Glicerina, V.; Cocci, E.; Frenich, A.G.; Romani, S.; Caboni, M.F. Determination of free and bound phenolic compounds and their antioxidant activity in buckwheat bread loaf, crust and crumb. LWT-Food Sci. 2018, 87, 217–224. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Carbiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K.; Otsuka, S.; Katsu, K. Breeding of buckwheat to reduce bitterness and rutin hydrolysis. Plants 2021, 10, 791. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Zhai, H.-C.; Huang, S.-X.; Cai, J.-P. A site-directed CO2 detection method for monitoring the spoilage of stored grains by insects and fungi in Chinese horizontal warehouses. J. Stored Prod. Res. 2014, 59, 146–151. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).