Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Materials
2.2. Determination of Physicochemical Parameters
2.3. Colorimetry Analysis
2.4. CPE Procedure
2.5. Conventional Extraction Procedure
2.6. Polyphenol Recovery
2.7. Quantification of Total Polyphenol Content
2.8. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.9. Evaluation of Antiradical Activity (DPPH Assay)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of Peach Cultivars
3.2. Optimization of the Extraction Procedure
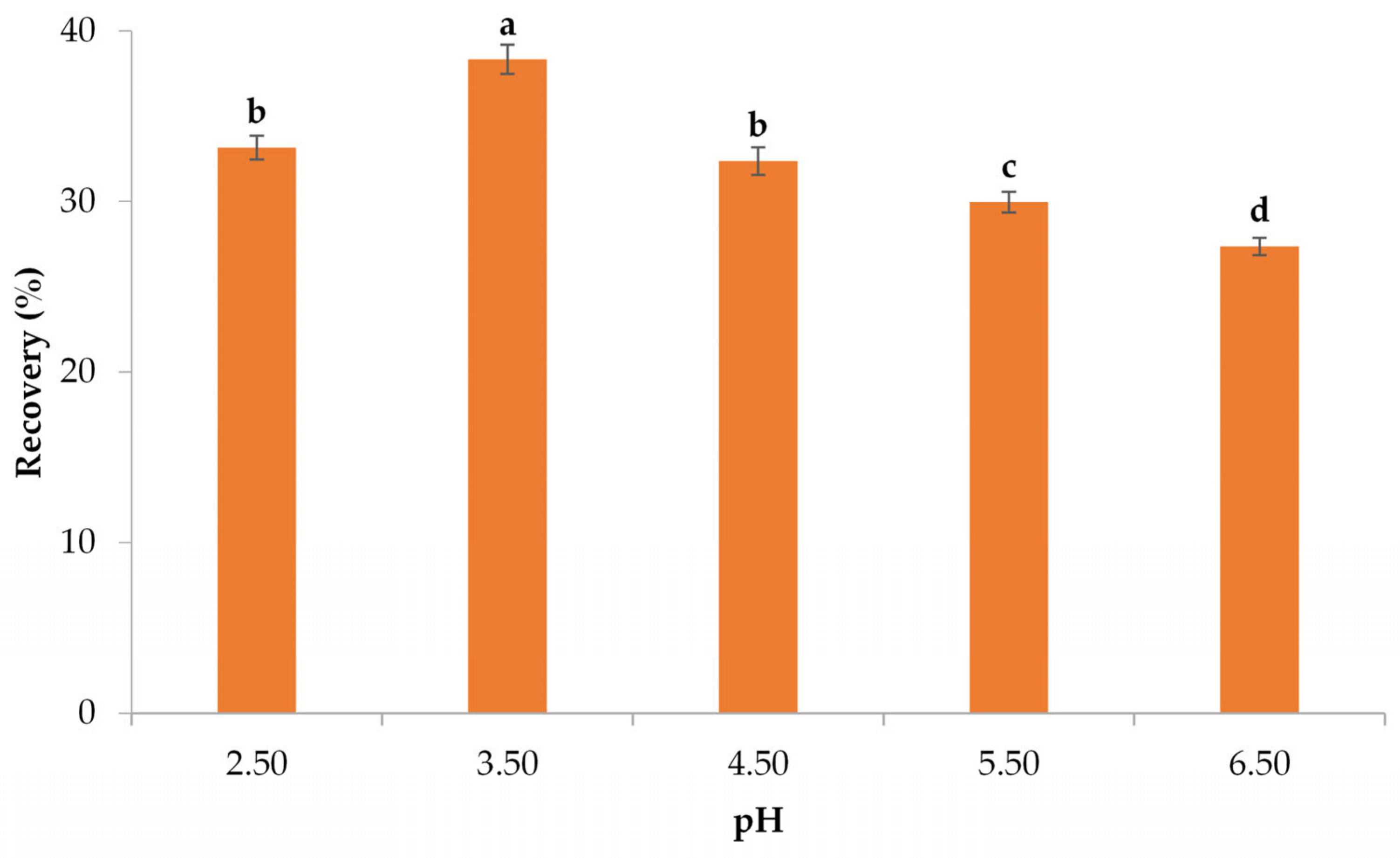
3.2.1. Impact of pH
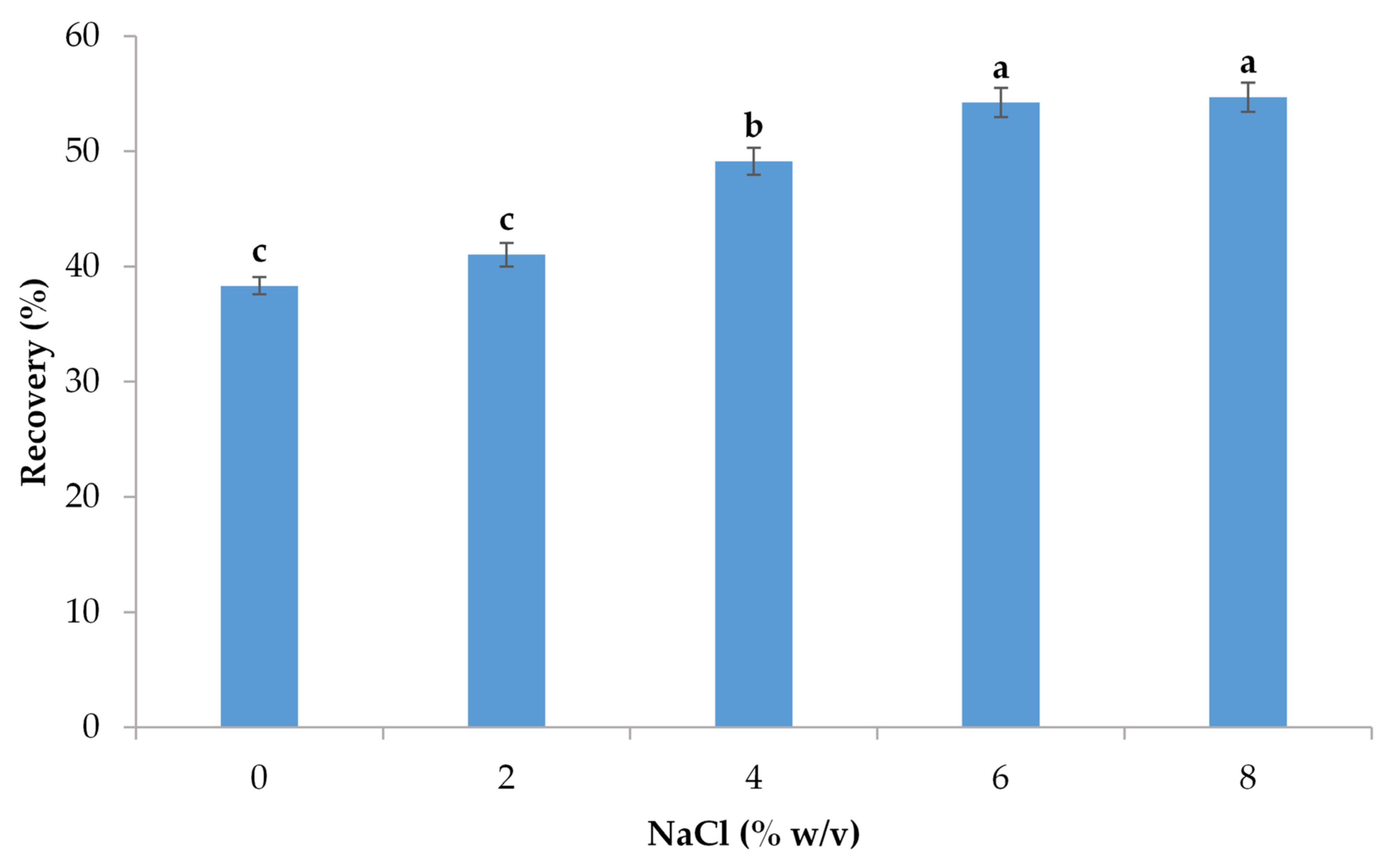
3.2.2. Impact of Ionic Strength
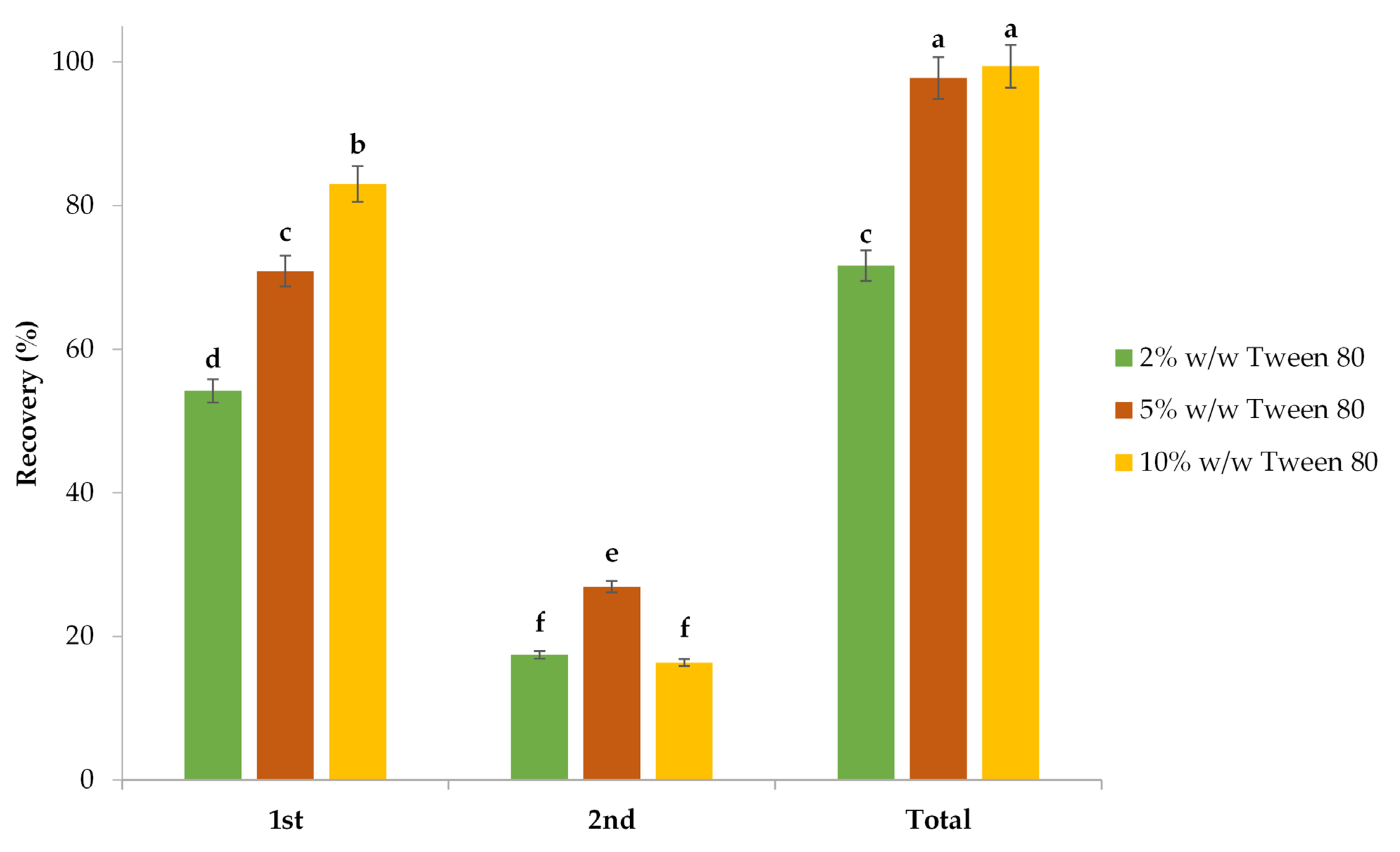
3.2.3. Impact of Surfactant Concentration and Extraction Frequency
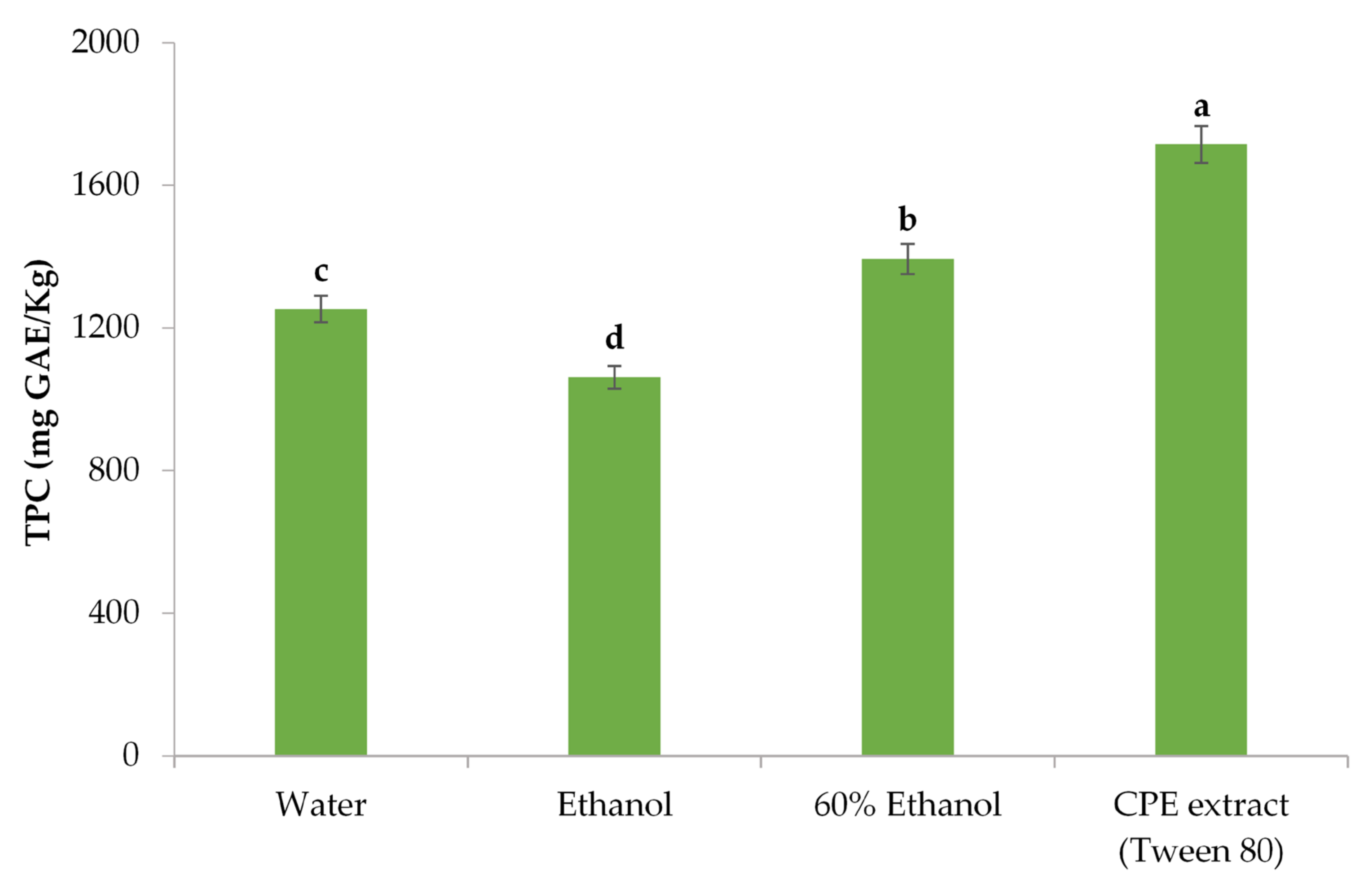
3.2.4. Impact of the Extraction Solvent
3.3. Antioxidant Activity of Extracted Polyphenols
3.4. Principal Component Analysis (PCA)
3.5. Multivariate Correlation Analysis (MCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gedük, A.Ş.; Atsız, S. LC-MS/MS Phenolic Composition of Peach (Prunus persica (L.) Batsch) Extracts and an Evaluation of Their Antidiabetic, Antioxidant and Antibacterial Activities. S. Afr. J. Bot. 2022, 147, 636–645. [Google Scholar] [CrossRef]
- Rudke, C.R.M.; Zielinski, A.A.F.; Ferreira, S.R.S. From Biorefinery to Food Product Design: Peach (Prunus persica) By-Products Deserve Attention. Food Bioprocess Technol. 2023, 16, 1197–1215. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L. Genetic Resources, Breeding Programs in China, and Gene Mining of Peach: A Review. Hortic. Plant J. 2020, 6, 205–215. [Google Scholar] [CrossRef]
- Mannino, G.; Ricciardi, M.; Gatti, N.; Serio, G.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. Changes in the Phytochemical Profile and Antioxidant Properties of Prunus persica Fruits after the Application of a Commercial Biostimulant Based on Seaweed and Yeast Extract. Int. J. Mol. Sci. 2022, 23, 15911. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Minas, I.; Cirilli, M.; Torres, R.; Bassi, D.; Costa, G. Peach for the Future: A Specialty Crop Revisited. Sci. Hortic. 2022, 305, 111390. [Google Scholar] [CrossRef]
- Anthony, B.M.; Minas, I.S. Redefining the Impact of Preharvest Factors on Peach Fruit Quality Development and Metabolism: A Review. Sci. Hortic. 2022, 297, 110919. [Google Scholar] [CrossRef]
- Veerappan, K.; Natarajan, S.; Chung, H.; Park, J. Molecular Insights of Fruit Quality Traits in Peaches, Prunus persica. Plants 2021, 10, 2191. [Google Scholar] [CrossRef]
- Dabbou, S.; Maatallah, S.; Castagna, A.; Guizani, M.; Sghaeir, W.; Hajlaoui, H.; Ranieri, A. Carotenoids, Phenolic Profile, Mineral Content and Antioxidant Properties in Flesh and Peel of Prunus persica Fruits during Two Maturation Stages. Plant Foods Hum. Nutr. 2017, 72, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and Health Benefits. Food Rev. Int. 2022, 38, 1703–1734. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive Evaluation of Late Season Peach Varieties (Prunus persica L.): Fruit Nutritional Quality and Phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Principal Component Analysis (PCA) of Physicochemical Compounds’ Content in Different Cultivars of Peach Fruits, Including Qualification and Quantification of Sugars and Organic Acids by HPLC. Eur. Food Res. Technol. 2019, 245, 929–938. [Google Scholar] [CrossRef]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and Carotenoid Composition of Canned Peaches (Prunus Persica) and Apricots (Prunus Armeniaca) as Affected by Variety and Peeling. Food Res. Int. 2013, 54, 448–455. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and Comparison of Vitamin C, Phenolic Compounds, Antioxidant Properties and Metal Chelating Activity of Pulp and Peel from Selected Peach Cultivars. LWT—Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative Study of Early- and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro- Nutrient Profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef]
- Jung, K.-M.; Kim, S.-Y.; Lee, G.-W.; Kim, I.-D.; Park, Y.-S.; Dhungana, S.K.; Kim, J.-H.; Shin, D.-H. Quality Characteristics and Antioxidant Activity of Unripe Peach (Prunus persica L. Batsch) Extracts with Distilled Water Coupled with Ultrasonication and Prethanol-A. Int. J. Fruit Sci. 2020, 20, S111–S122. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Song, G.; Xie, M.; Zhang, H. Melatonin Enhances the Waterlogging Tolerance of Prunus persica by Modulating Antioxidant Metabolism and Anaerobic Respiration. J. Plant Growth Regul. 2021, 40, 2178–2190. [Google Scholar] [CrossRef]
- Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods 2022, 11, 2944. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Shukla, R.; Shukla, A. A Review on Peach (Prunus persica): An Asset of Medicinal Phytochemicals. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2186–2200. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Synthesis of Silver Chloride Nanoparticles Using Prunus persica L. Outer Peel Extract and Investigation of Antibacterial, Anticandidal, Antioxidant Potential. Green Chem. Lett. Rev. 2016, 9, 132–142. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; He, L.; Cheng, J.; Li, J.; Tao, W.; Mao, G.; Zhang, H.; Linhardt, R.J.; Ye, X.; et al. Structural Characterization and Anti-Proliferative Activities of Partially Degraded Polysaccharides from Peach Gum. Carbohydr. Polym. 2019, 203, 193–202. [Google Scholar] [CrossRef]
- Chojnacka, K.; Lewandowska, U. Chemopreventive Effects of Polyphenol-Rich Extracts against Cancer Invasiveness and Metastasis by Inhibition of Type IV Collagenases Expression and Activity. J. Funct. Foods 2018, 46, 295–311. [Google Scholar] [CrossRef]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Costa, G.; Andreotti, C.; Paolini, M. Comparison between in Toto Peach (Prunus persica L. Batsch) Supplementation and Its Polyphenolic Extract on Rat Liver Xenobiotic Metabolizing Enzymes. Food Chem. Toxicol. 2016, 97, 385–394. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Nalbantsoy, A.; Yalcin, H.T.; Yesil-Celiktas, O. Cytotoxic, Antimicrobial and Nitric Oxide Inhibitory Activities of Supercritical Carbon Dioxide Extracted Prunus persica Leaves. Mol. Biol. Rep. 2020, 47, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Peach (Prunus persica)—Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas: Nutritional and Health Benefits; Hussain, S.Z., Naseer, B., Qadri, T., Fatima, T., Bhat, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 207–217. ISBN 978-3-030-75502-7. [Google Scholar]
- Kim, H.-R.; Kim, I.-D.; Dhungana, S.K.; Kim, M.-O.; Shin, D.-H. Comparative Assessment of Physicochemical Properties of Unripe Peach (Prunus persica) and Japanese Apricot (Prunus mume). Asian Pac. J. Trop. Biomed. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Zhou, M.; Wu, X. Antioxidant Profile of Thinned Young and Ripe Fruits of Chinese Peach and Nectarine Varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Peng, X.; Xu, H.; Yuan, X.; Leng, L.; Meng, Y. Mixed Reverse Micellar Extraction and Effect of Surfactant Chain Length on Extraction Efficiency. Sep. Purif. Technol. 2016, 160, 117–122. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Fan, H.; Zhu, D.; Wu, X.; Huang, X.; Tang, J. Separation and Purification of Alkaloids from Sophora Flavescens Ait. by Focused Microwave-Assisted Aqueous Two-Phase Extraction Coupled with Reversed Micellar Extraction. Ind. Crops Prod. 2016, 86, 231–238. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Freire, M.G.; Coutinho, J.A.P. Aqueous Two-Phase Systems: Towards Novel and More Disruptive Applications. Fluid Phase Equilibria 2020, 505, 112341. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-Based Processes for Wastewater Nutrient Recovery: Technology, Challenges, and Future Direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef]
- Chen, Y.; Du, K.; Li, J.; Bai, Y.; An, M.; Tan, Z.; Chang, Y. A Green and Efficient Method for the Preconcentration and Determination of Gallic Acid, Bergenin, Quercitrin, and Embelin from Ardisia Japonica Using Nononic Surfactant Genapol X-080 as the Extraction Solvent. Int. J. Anal. Chem. 2018, 2018, 1707853. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, M.R.; Al-Garawi, Z.S.; Thani, M.Z. Promising Technique, Cloud Point Extraction: Technology & Applications. J. Phys. Conf. Ser. 2021, 1853, 012064. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef]
- Santana, C.M.; Ferrera, Z.S.; Rodríguez, J.J.S. Use of Non-Ionic Surfactant Solutions for the Extraction and Preconcentration of Phenolic Compounds in Water Prior to Their HPLC-UV Detection. Analyst 2002, 127, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, K.; Katsoyannos, E.; Chatzilazarou, A. Antioxidant Activity and Thermal Stability of Oleuropein and Related Phenolic Compounds of Olive Leaf Extract after Separation and Concentration by Salting-Out-Assisted Cloud Point Extraction. Antioxidants 2014, 3, 229–244. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus Limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, M.; Li, J.; Su, Z.; Cai, Z.; Shen, Z.; Ma, R.; Yan, J.; Yu, M. Carotenoid Profiling of Yellow-Flesh Peach Fruit. Foods 2022, 11, 1669. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in Peach Fruit: A Breeding Perspective. Hortic. Res. 2016, 3, 15067. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the Fruit Quality and Phytochemical Compounds in Peach and Nectarine Cultivars. Plants 2023, 12, 1618. [Google Scholar] [CrossRef]
- Chatzilazarou, A. Application of Cloud Point Extraction with the Aid of Genapol X-080 in the Pre-Concentration of Lycopene and Total Carotenoids from Red Fleshed Orange. Ernähr.—Wiss. Prax. 2011, 35, 5–13. [Google Scholar]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodríguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Melamine Sponge Decorated with Copper Sheets as a Material with Outstanding Properties for Microextraction of Sulfonamides Prior to Their Determination by High-Performance Liquid Chromatography. J. Chromatogr. A 2018, 1554, 28–36. [Google Scholar] [CrossRef]
- Santalad, A.; Burakham, R.; Srijaranai, S.; Srijaranai, S.; Deming, R.L. Role of Different Salts on Cloud-Point Extraction of Isoprocarb and Promecarb Insecticides Followed by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2012, 50, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Mortada, W.I.; Hassanien, M.M.; El-Asmy, A.A. Cloud Point Extraction of Some Precious Metals Using Triton X-114 and a Thioamide Derivative with a Salting-out Effect. Egypt. J. Basic Appl. Sci. 2014, 1, 184–191. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) Based Microemulsions: Preclinical Drug Delivery, Toxicity and Antimicrobial Applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306–322. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef]
- Di Vaio, C.; Graziani, G.; Marra, L.; Cascone, A.; Ritieni, A. Antioxidant Capacities, Carotenoids and Polyphenols Evaluation of Fresh and Refrigerated Peach and Nectarine Cultivars from Italy. Eur. Food Res. Technol. 2008, 227, 1225–1231. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in Total Phenolics, Antioxidant Activity and Metabolic Characteristics in Peach Fruits at Different Stages of Ripening. LWT 2023, 178, 114586. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Changes in Extractable and Non-Extractable Polyphenols and Their Antioxidant Properties during Fruit On-Tree Ripening in Five Peach Cultivars. Hortic. Plant J. 2019, 5, 137–144. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
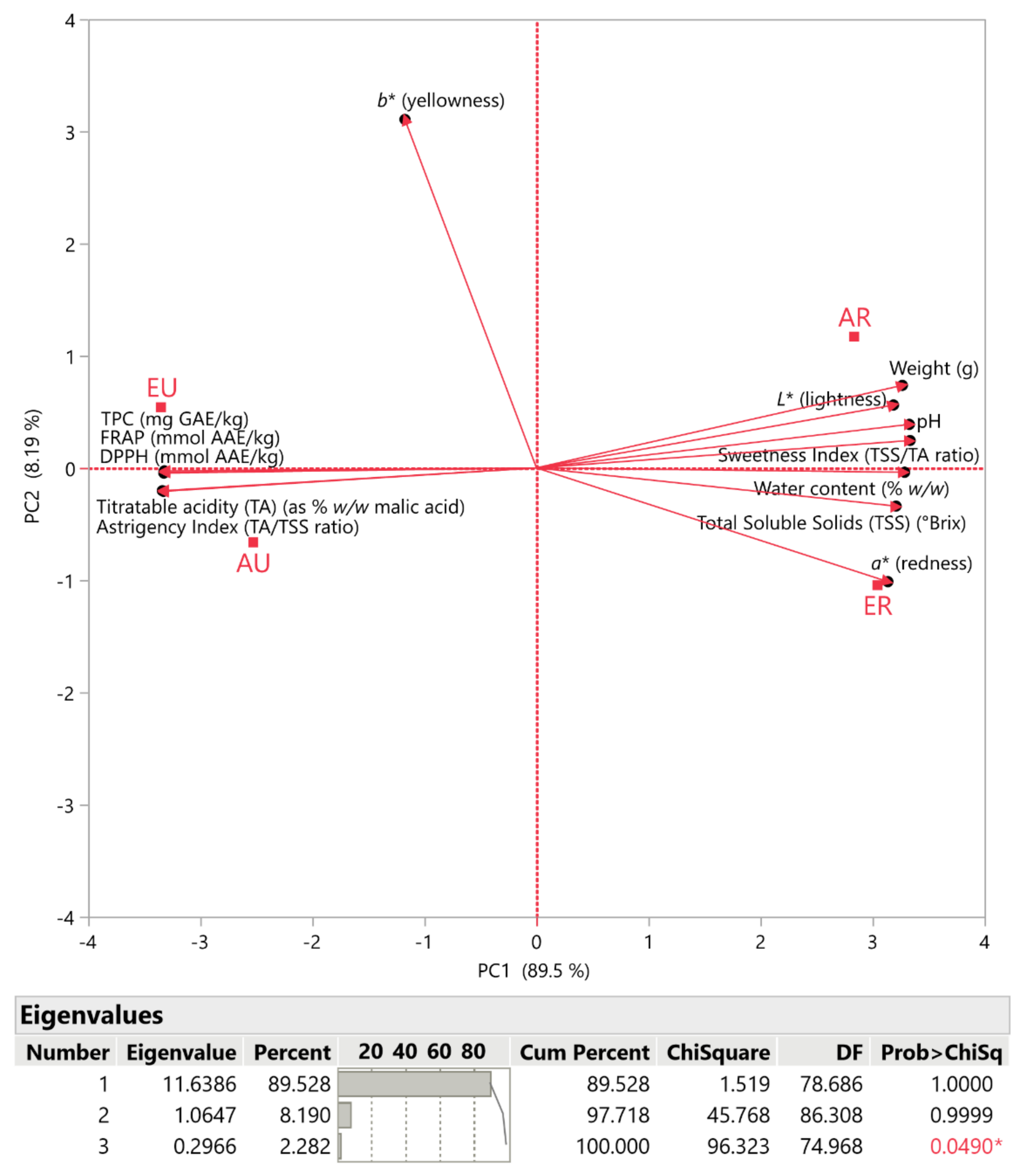
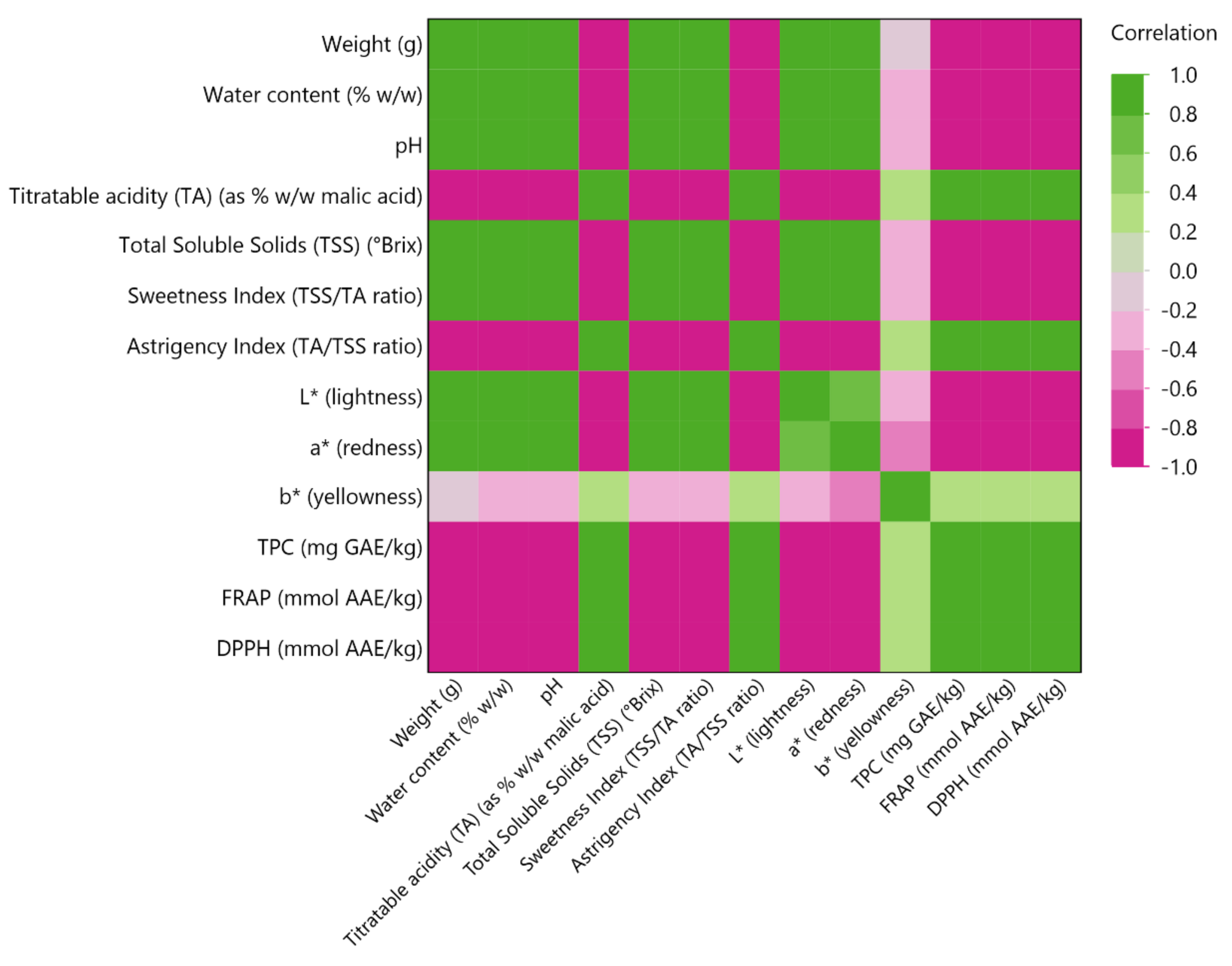
| Parameters | Samples | |||
|---|---|---|---|---|
| AU | AR | EU | ER | |
| Weight (g) | 121.62 ± 2.43 d | 389.33 ± 7.78 a | 138.31 ± 3.45 c | 340.35 ± 8.85 b |
| Water content (% w/w) | 75.82 ± 2.12 b | 88.36 ± 2.47 a | 77.12 ± 2.08 b | 90.77 ± 2.72 a |
| pH | 3.70 ± 0.02 c | 4.27 ± 0.01 a | 3.65 ± 0.02 d | 4.20 ± 0.01 b |
| Titratable acidity (TA) (as % w/w malic acid) | 0.71 ± 0.06 a | 0.28 ± 0.03 b | 0.81 ± 0.07 a | 0.33 ± 0.03 b |
| Total Soluble Solids (TSS) (°Brix) | 6.12 ± 0.18 c | 10.76 ± 0.32 b | 6.83 ± 0.21 c | 12.61 ± 0.38 a |
| Sweetness Index (TSS/TA ratio) | 8.65 ± 0.48 b | 38.64 ± 3.01 a | 8.46 ± 0.47 b | 38.35 ± 2.34 a |
| Astrigency Index (TA/TSS ratio) | 0.12 ± 0.01 a | 0.03 ± 0 b | 0.12 ± 0.01 a | 0.03 ± 0 b |
| L* (lightness) | 62.1 ± 0.1 c | 72.3 ± 0.2 a | 58.4 ± 0.1 d | 68.5 ± 0.2 b |
| a* (redness) | −10.6 ± 0.1 c | 21.1 ± 0.1 b | −14.6 ± 0.1 d | 30.2 ± 0.1 a |
| b* (yellowness) | 40.1 ± 0.1 c | 60.5 ± 0.1 b | 65.2 ± 0.1 a | 38.3 ± 0.1 d |
| Samples | TPC (mg GAE/kg) | FRAP (mmol AAE/kg) | DPPH (mmol AAE/kg) |
|---|---|---|---|
| AU | 1465.32 ± 42.49 b | 7.33 ± 0.16 b | 5.12 ± 0.15 b |
| AR | 696.74 ± 20.21 c | 3.47 ± 0.08 c | 2.33 ± 0.07 c |
| EU | 1714.53 ± 49.72 a | 8.57 ± 0.19 a | 6.08 ± 0.18 a |
| ER | 753.21 ± 21.84 c | 3.76 ± 0.08 c | 2.54 ± 0.08 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering 2023, 5, 2139-2154. https://doi.org/10.3390/agriengineering5040131
Giovanoudis I, Athanasiadis V, Chatzimitakos T, Kalompatsios D, Mantiniotou M, Bozinou E, Gortzi O, Nanos GD, Lalas SI. Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering. 2023; 5(4):2139-2154. https://doi.org/10.3390/agriengineering5040131
Chicago/Turabian StyleGiovanoudis, Ioannis, Vassilis Athanasiadis, Theodoros Chatzimitakos, Dimitrios Kalompatsios, Martha Mantiniotou, Eleni Bozinou, Olga Gortzi, George D. Nanos, and Stavros I. Lalas. 2023. "Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method" AgriEngineering 5, no. 4: 2139-2154. https://doi.org/10.3390/agriengineering5040131
APA StyleGiovanoudis, I., Athanasiadis, V., Chatzimitakos, T., Kalompatsios, D., Mantiniotou, M., Bozinou, E., Gortzi, O., Nanos, G. D., & Lalas, S. I. (2023). Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering, 5(4), 2139-2154. https://doi.org/10.3390/agriengineering5040131













