Forage Properties of Fresh and Composted Cotton Gin Byproducts as Feed Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Locations and Composting
2.2. Composting Process and Compositional Analyses
2.3. Statistical Analyses
3. Results
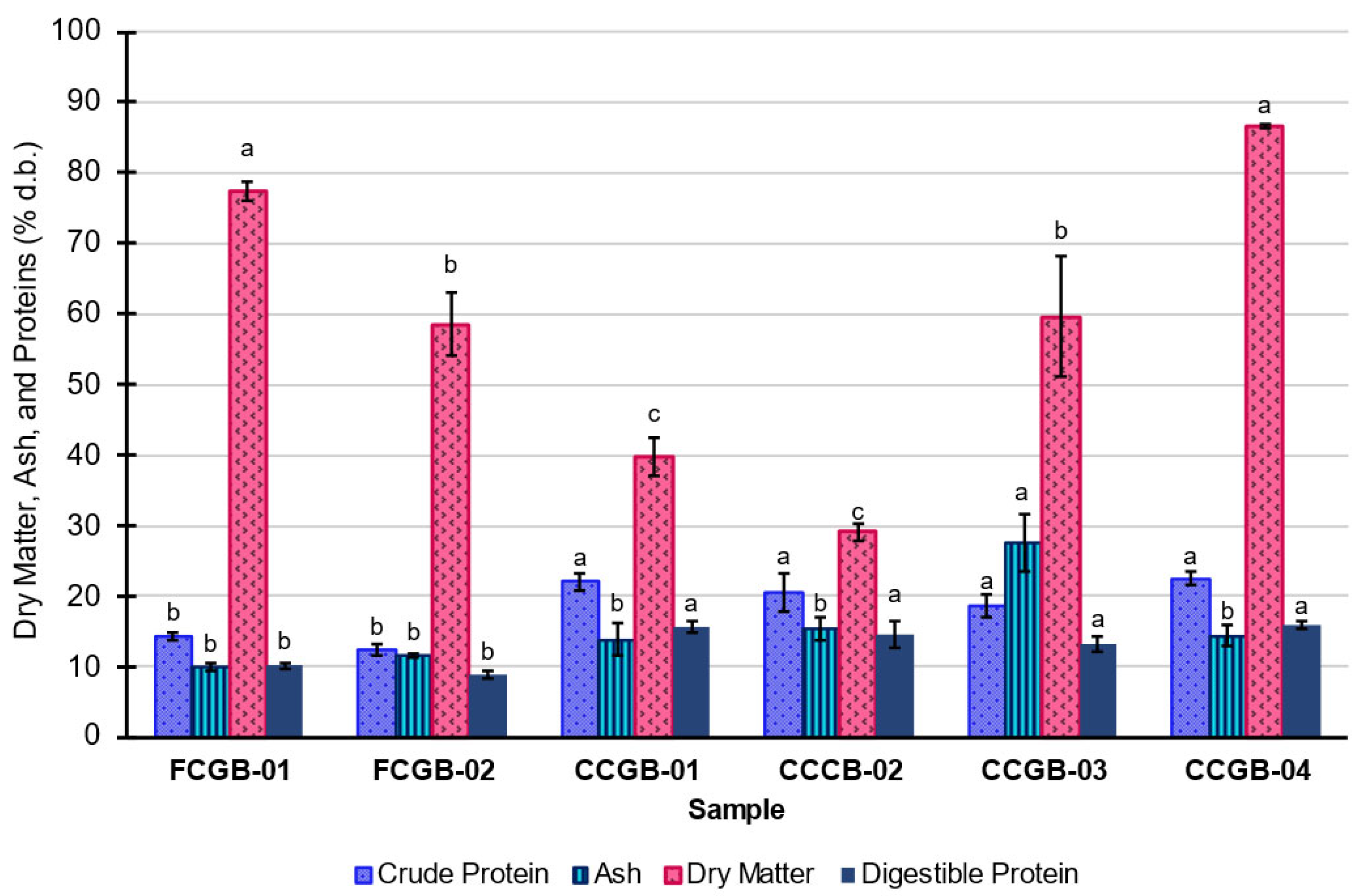
3.1. Crude Protein, Ash, and Fat
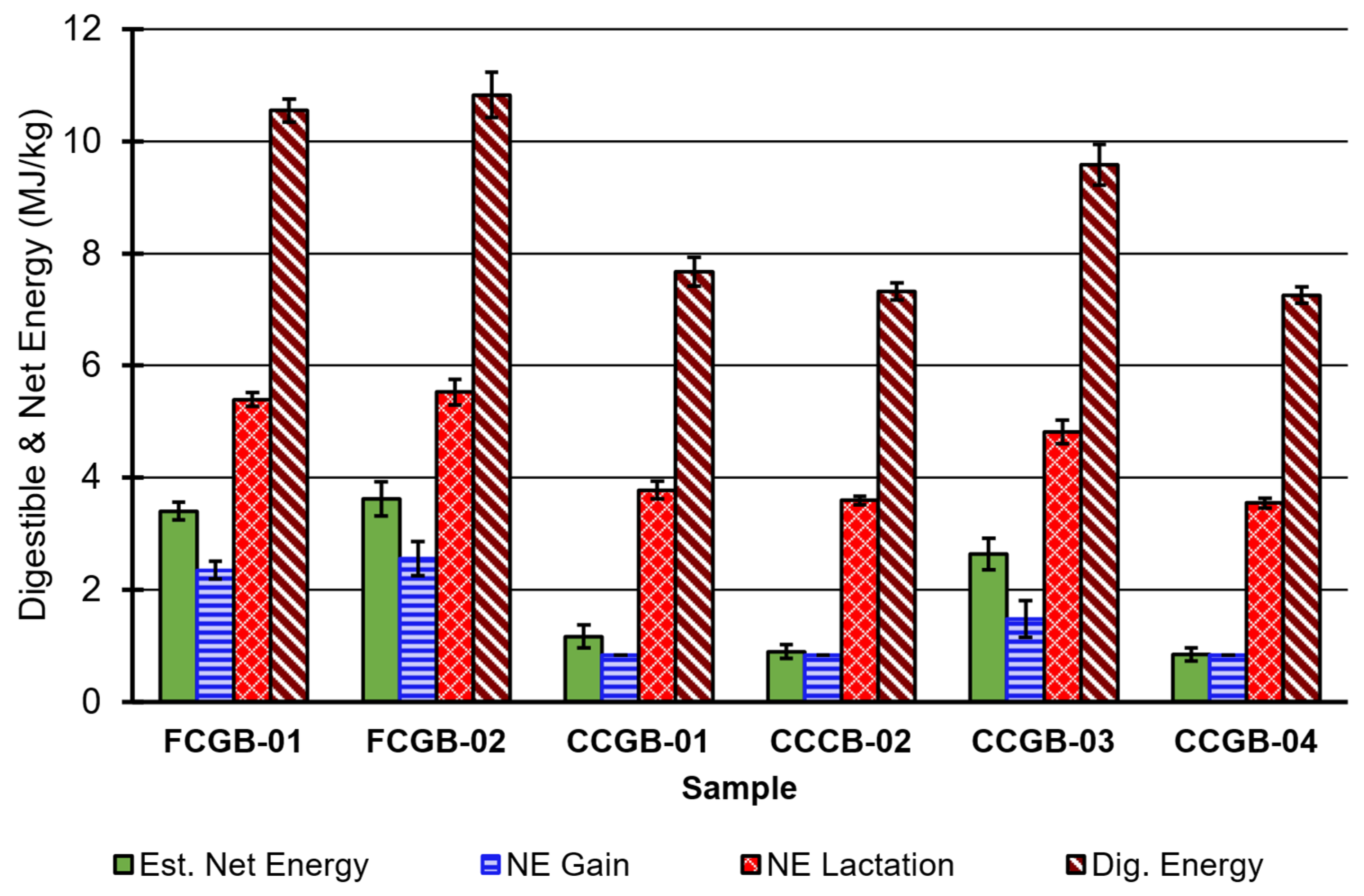
3.2. Fiber, Total Digestible Nutrients, and Estimated Energy
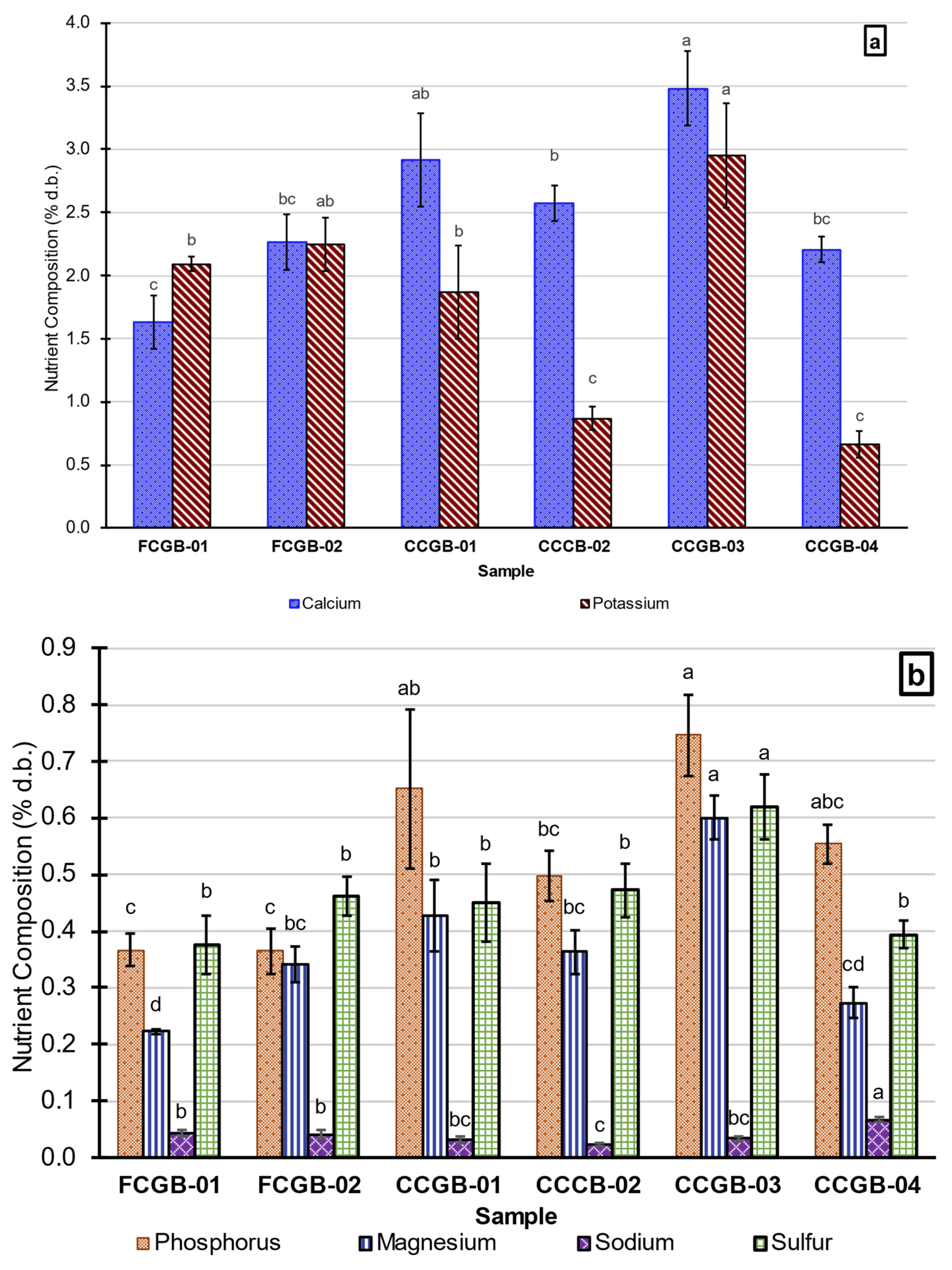
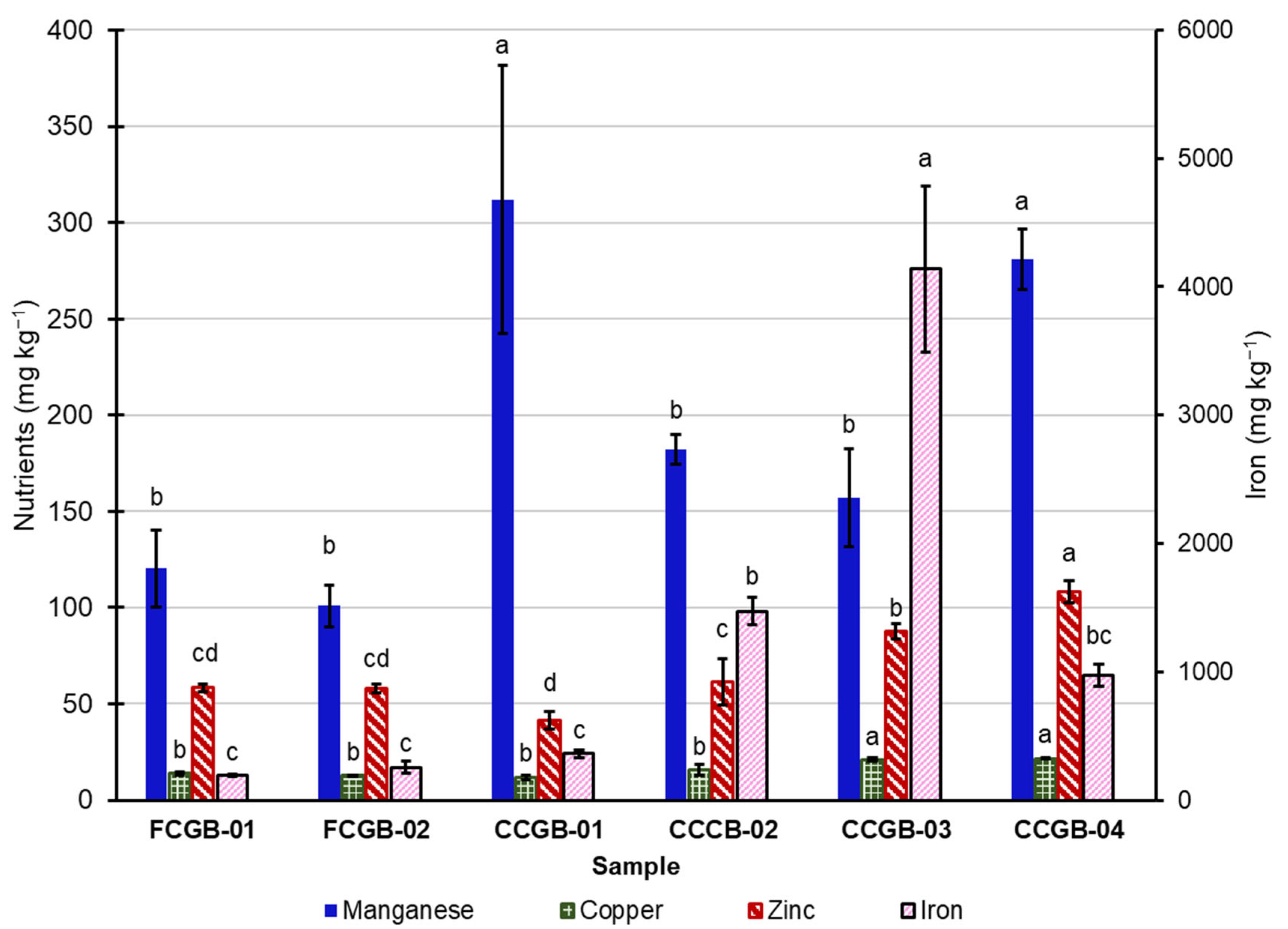
3.3. Macronutrients, Micronutrients, and Trace Elements
3.3.1. Macronutrients
3.3.2. Micronutrients
4. Discussion
4.1. Dry Matter/Moisture and Ash
4.2. Overall Quality Assessment: Fiber, Estimated Energy, and Nutrient Digestibility
4.3. Nutrients, Diet Formulation, Net Energy System, and Minerals
4.3.1. Diet Formulation
4.3.2. Nutrient Variabilities
4.4. Other Formulation Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- USDA-NASS. Crop Production 2022 Summary; USDA, National Agricultural Statistics Service: Washington, DC, USA, 2023. [Google Scholar]
- Stewart, L.; Rossi, J. Using Cotton Byproducts in Beef Cattle Diets; University of Georgia: Athens, GA, USA, 2022. [Google Scholar]
- Wilde, C.; Johnson, J.; Farmer, M. Inventory of Cotton Gin Trash on the Texas High Plains and Bio-Energy Feedstock Potentials. Tex. J. Agric. Nat. Resour. 2010, 23, 42–49. [Google Scholar]
- Misra, S.; Gembler, J. Synthesis of Gin Trash Utilization Research; Texas Tech University: Lubbock, TX, USA, 1996. [Google Scholar]
- Holt, G.A.; Barker, G.L.; Baker, R.V.; Brashears, A. Various Parameters of Cotton Gin Byproducts Produced from the Gin Processing Machinery. In Proceedings of the Beltwide Cotton Conference, San Antonio, TX, USA, 4–8 January 2000; pp. 1595–1602. [Google Scholar]
- Holt, G.A.; Barker, G.L.; Baker, R.V.; Brashears, A. Characterization of Cotton Gin Byproducts Produced by Various Machinery Groups Used in the Ginning Operation. Trans. ASAE 2000, 43, 1393–1400. [Google Scholar] [CrossRef]
- Hamawand, I.; Sandell, G.; Pittaway, P.; Chakrabarty, S.; Yusaf, T.; Chen, G.; Seneweera, S.; Al-Lwayzy, S.; Bennett, J.; Hopf, J. Bioenergy from Cotton Industry Wastes: A review and potential. Renew. Sustain. Energy Rev. 2016, 66, 435–448. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Naebe, M. A review on cotton gin trash: Sustainable commodity for material fabrication. J. Clean. Prod. 2021, 281, 125300. [Google Scholar] [CrossRef]
- Myer, R.O. Cotton Gin Trash: Alternative Roughage Feed for Beef Cattle; University of Florida: Gainesville, FL, USA, 2007. [Google Scholar]
- Hamed, A.H.M.; Osman, A.A.B.; Elimam, M.E. Effects of Cotton Gin Trash Level on the Performance of Desert Lamb in New Halfa Area, Kassala State, Sudan. Anim. Rev. 2014, 1, 11–16. [Google Scholar]
- Hill, G.M.; Rivera, J.D.; Franklin, A.N.; Stone, G.W.; Tillman, D.R.; Mullinix Jr, B.G. Evaluation of Cotton-Gin Trash Blocks Fed to Beef Cattle. Prof. Anim. Sci. 2013, 29, 260–270. [Google Scholar] [CrossRef]
- Rogers, G.M.; Poore, M.H.; Paschal, J.C. Feeding Cotton Products to Cattle. Vet. Clin. Food Anim. Pract. 2002, 18, 267–294. [Google Scholar] [CrossRef]
- Thomasson, J.A.; Willcutt, M.H. Wetting Method for Initiating Composting in Cotton Gin Waste. Appl. Eng. Agric. 1996, 12, 417–425. [Google Scholar] [CrossRef]
- Rankins, D.L. The Importance of By-products to the US Beef Industry. Vet. Clin. Food Anim. Pract. 2002, 18, 207–211. [Google Scholar] [CrossRef]
- Thomasson, J.; Willcut, M.H. Water Application Method for Composting Cotton Gin Waste. In Proceedings of the National Cotton Council Beltwide Cotton Conference, Nashville, TN, USA, 9–12 January 1996; pp. 1597–1604. [Google Scholar]
- Arndt, D.L.; Richardson, C.R.; Albin, R.C.; Sherrod, L.B. Digestibility of Chemically Treated Cotton Plant by-Product and Effect on Mineral Balance, Urine Volume and Ph. J. Anim. Sci. 1980, 51, 215–223. [Google Scholar] [CrossRef]
- Andrade, A.P.; de Figueiredo, M.P.; de Quadros, D.G.; Ferreira, J.Q.; Whitney, T.R.; Luz, Y.S.; Santos, H.R.O.; Souza, M.N.S. Chemical and biological treatment of cotton gin trash for fattening Santa Ines lambs. Livest. Sci. 2020, 240, 104146. [Google Scholar] [CrossRef]
- Mullenix, M.K.; Stewart Jr, R.L.; Jacobs, J.L.; Davis, D.L. Invited Review: Using whole cottonseed and cotton harvest residue in southeastern US beef cattle diets: Quality, intake, and changes in feed characteristics. Appl. Anim. Sci. 2022, 38, 447–455. [Google Scholar] [CrossRef]
- Villalobos, C.; Britton, C.M.; Avila, M.; Richardson, R.; Holt, G.; Bezanilla, G. Cotton By-products Supplementation for Steers Grazing Tobosagrass (Hilaria mutica [Buckl.] Benth.) Rangeland. Tex. J. Agric. Nat. Resour. 2009, 22, 17–31. [Google Scholar]
- Hills, D.J.; Curley, R.G.; Knutson, J.K.; Seiber, J.N.; Winterlin, W.L.; Rauschkolb, R.S.; Pullman, G.S.; Elmore, C.L. Composting Treatment for Cotton Gin Trash Fines. Trans. ASABE 1981, 24, 14–19. [Google Scholar] [CrossRef]
- Weather Underground Incorporated. Historical Weather. Available online: https://www.wunderground.com/history (accessed on 20 July 2023).
- Pognani, M.; Barrena, R.; Font, X.; Sanchez, A. Effect of freezing on the conservation of the biological activity of organic solid wastes. Bioresour. Technol. 2012, 104, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.L. Freezing: An Underutilized Food Safety Technology? Int. J. Food Microbiol. 2004, 90, 127–138. [Google Scholar] [CrossRef]
- Soukup, D.A.; Buck, B.J.; Harris, W. Chapter 2: Preparing Soils for Mineralogical Analyses. In Methods of Soil Analysis Part. 5—Mineralogical Methods; Soil Science Society of America, Inc.: Maddison, WI, USA, 2008; Volume 5, pp. 13–31. [Google Scholar]
- Zeng, J.F.; Yin, H.J.; Shen, X.L.; Liu, N.; Ge, J.Y.; Han, L.J.; Huang, G.Q. Effect of Aeration Interval on Oxygen Consumption and GHG Emission during Pig Manure Composting. Bioresour. Technol. 2018, 250, 214–220. [Google Scholar] [CrossRef]
- Peters, J.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; University of Minnesota Libraries Publishing: Minneapolis, MN, USA, 2003. [Google Scholar]
- US Composting Council. The Test Method for the Examination of Composting and Compost (TMECC); US Composting Council: Bethesda, MD, USA, 2002. [Google Scholar]
- Schindelbeck, R.R.; Moebius-Clune, B.N.; Moebius-Clune, D.J.; Kurtz, K.S.; van Es, H.M. (Eds.) Cornell University Comprehensive Assessment of Soil Health Laboratory Standard Operating Procedures; Cornell University: Ithaca, NY, USA, 2016. [Google Scholar]
- Culman, S.W.; Hurriso, T.T. Procedure for Autoclaved-Citrate Extractable (ACE) Soil Protein; Soil Science Society of America, Inc.: Maddison, WI, USA, 2020. [Google Scholar]
- U.S. EPA. Method. 3051A (SW-846): Microwave Assisted Acid. Digestion of Sediments, Sludges, and Oils; U.S. EPA: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition, 2001; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Moe, P.W.; Tyrell, H.F. Metabolizable Energy Requirements of Pregnant Dairy Cows. J. Dairy Sci. 1972, 55, 480–483. [Google Scholar] [CrossRef]
- Tyrell, H.F.; Moe, P.W. Net Energy Value for Lactation of a High and Low Concentrate Ration Containing Corn Silage. J. Dairy Sci. 1972, 55, 1106–1112. [Google Scholar] [CrossRef]
- Rivera, D.; Parish, J. Interpreting Forage and Feed Analysis Reports; Mississippi State University Extension Service: Verona, MS, USA, 2010. [Google Scholar]
- Elliott, J.P.; Drackley, J.K.; Fahey, G.C.; Shanks, R.D. Utilization of Supplemental Fat by Dairy-Cows Fed Diets Varying in Content of Nonstructural Carbohydrates. J. Dairy Sci. 1995, 78, 1512–1525. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Scientific Research Publishing: Wuhan, China, 2023. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. Available online: https://CRAN.R-project.org/package=agricolae2021 (accessed on 9 August 2023).
- Brookside Laboratories. Brookside Laboratories Manure/Compost Methods. Available online: https://scripts.blinc.com/worksheet_pdf/CompostManureMethods.pdf (accessed on 31 May 2023).
- Agblevor, F.A.; Cundiff, J.S.; Mingle, C.; Li, W. Storage and characterization of cotton gin waste for ethanol production. Resour. Conserv. Recycl. 2006, 46, 198–216. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Roussos, A.I.; Koroneos, C.J. A laboratory study of cotton gin waste pyrolysis. J. Anal. Appl. Pyrolysis 2000, 56, 47–59. [Google Scholar] [CrossRef]
- Bernard, J.K.; Woldeghebriel, A.; Mueller, T.C. Nutrient Intake and Digestibility of Cotton Gin Trash Treated with Hypochlorite Oxidant or Extruded. J. Appl. Anim. Res. 2001, 19, 165–176. [Google Scholar] [CrossRef]
- Hills, D.J. Composting Gin Trash in California. In Proceedings of the Symposium Cotton Gin Trash Utilization Alternatives, Texas Cotton Ginners’ Association, Austin, TX, USA, 1982; pp. 63–86. [Google Scholar]
- Axe, D.; Addis, D.; Clark, J.; Dunbar, J.; Garrett, W.; Hinman, N.; Zinn, R. Feeding Value of Cleaned and Uncleaned Cotton Gin Trash. In Proceedings of the American Society for Animal Science Western Section, Virtual, 1982; Volume 33, pp. 57–59. [Google Scholar]
- Warner, A. Utilization of Cotton Byproducts in Cattle Finishing Diets: Impacts on Performance, Carcass Traits, and Ruminal Degradability of Diet Components; Oklahoma State University: Stillwater, OK, USA, 2020. [Google Scholar]
- Buser, M. Extruding Cotton Gin Byproducts to Reduce Chemical Residues. J. Cotton Sci. 2001, 5, 92–102. [Google Scholar]
- Ozkan, Ç.O.; Boga, M.; Atalay, A.I.; Guven, I.; Kaya, E. Determination of potential nutritive value of cotton gin trash produced from different feed companies. J. Appl. Anim. Res. 2014, 43, 474–478. [Google Scholar] [CrossRef]
- Kennedy, J.B. Evaluation of Cotton Gin Trash as a Roughage Source for Stocker Cattle; Auburn University: Auburn, AL, USA, 2006. [Google Scholar]
- Preston, R.L. Feed Composition Table: Here Are the Latest Calculations on the Nutrient Composition of a Wide Range of Feedstuffs Fed to Cattle and Sheep. Beef Magazine, March 2016. pp. 16–34.
- Stewart, R.L.; Bader, M.J.; Harris, G.H. Evaluation of Cotton Gin Trash as a Cattle Feed; Science Annual Report; University of Georgia, Animal & Dairy: Athens, GA, USA, 1998; pp. 28–30. [Google Scholar]
- Conner, M.C. Utilization of Chemically Treated Cotton Gin Trash by Ruminants. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1985. [Google Scholar]
- Bader, M.J.; Bramwell, R.K.; Stewart, R.L.; Hill, G.M. Gin Trash Studies Conducted in Georgia. In Proceedings of the Beltwide Cotton Conference, San Diego, CA, USA, 5–9 January 1998; pp. 1698–1699. [Google Scholar]
- Ball, D.; Collins, M.; Lacefield, G.; Martin, N.; Mertens, D.; Olson, K.; Putnam, D.; Undersander, D.; Wolf, M. Understanding Forage Quality; American Farm Bureau Federation Publication: Washington, DC, USA, 2001; pp. 1–15. [Google Scholar]
- Heeg, A. Breaking Down a Feed Analysis Report. Progressive Dairy, 30 June 2016.
- Sguizzato, A.L.L.; Marcondes, M.I.; Dijkstra, J.; Valadares, S.D.; Campos, M.M.; Machado, F.S.; Silva, B.C.; Rotta, P.P. Energy requirements for pregnant dairy cows. PLoS ONE 2020, 15, e0235619. [Google Scholar] [CrossRef] [PubMed]
- Lalman, D.; Richards, C. Nutrient Requirements of Beef Cattle; Oklahoma State University: Stillwater, OK, USA, 2017. [Google Scholar]
- Blair, R. Nutrition and Feeding of Organic Cattle, 2nd ed.; CAB International: Boston, MA, USA, 2021. [Google Scholar]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y.N. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Irshad, M.; Eneji, A.E.; Hussain, Z.; Ashraf, M. Chemical characterization of fresh and composted livestock manures. J. Soil. Sci. Plant Nut 2013, 13, 115–121. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Lanno, M.; Kriipsalu, M.; Shanskiy, M.; Silm, M.; Kisand, A. Distribution of phosphorus forms depends on compost source material. Resources 2021, 10, 102. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2022. [Google Scholar]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compost. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Ksheem, A.M.; Bennett, J.M.; Antille, D.L.; Raine, S.R. Towards a method for optimized extraction of soluble nutrients from fresh and composted chicken manures. Waste Manag. 2015, 45, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.O.; Abdelbagi, A.O.; Abdalla, M.A.; Ishag, A.E.S.A.; Hammad, A.M.A.; Gadallah, N.A.H.; Hur, J.-H. Insecticide Residues in Cotton, Sorghum and Fallow Soil from the Nuba Mountains Cotton Corporation of South Kordofan State, Sudan. J. Health Pollut. 2021, 11, 210608. [Google Scholar] [CrossRef] [PubMed]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of agro-industrial wastes into animal feed through microbial fermentation: A review of the global and Ghanaian case. Heliyon 2023, 9, e14814. [Google Scholar] [CrossRef] [PubMed]

| Sample | Description | Source/Location | Composting/Storage Age |
|---|---|---|---|
| FCGB-01 | Fresh Cotton Gin Byproducts | Gin-01, Tennessee | <30 days |
| FCGB-02 | Fresh Cotton Gin Byproducts | Gin-02, Mississippi | <30 days |
| CCGB-01 | Composted Cotton Gin Byproducts | Gin-01, Tennessee | >8 months |
| CCGB-02 | Composted Cotton Gin Byproducts | Gin-02, Mississippi | >8 months |
| CCGB-03 | Composted Cotton Gin Byproducts | Gin-03, Arkansas | >8 months |
| CCGB-04 | Composted Cotton Gin Byproducts | Gin-04, Missouri | >8 months |
| Material | Ash | Crude Protein | ADF a | TDN c | Fat | Source |
|---|---|---|---|---|---|---|
| Fresh CGB | 10.8 | 13.4 | 37.9 b | 58.0 | 1.9 | The Current Study |
| 17.8 | 13.63 | 69.6 | - | - | [44] | |
| 23.9 | 7.3 | 65.9 | - | - | [44] | |
| 12.1 | 8.7 | - | 48.5 | 3.6 | [45] | |
| 5.9–20.9 | 7.4–16.6 | - | - | - | [12] | |
| - | 15.5 | 46.5 | 37.3 | - | [46] | |
| 4.2 | 7.7 | - | - | - | [43] | |
| 8.9–17.7 | 6.6–12.5 | 40.7–48.3 | - | 3.4–6.1 | [47] | |
| - | 12.4 | 60.8 | - | - | [48] | |
| 14.0 | 9.0 | 50.0 | 42.0 | 2.0 | [49] | |
| 9.9 | 16.4 | 44.7 | 47.7 | 5.2 | [11] | |
| 9.9 | 17.5 | 37.5 | 49.4 | 5.2 | [11] | |
| 11.1 | 11.7 | 41.2 | 46.6 | - | [50] d | |
| 10.0 | 7.0 | 46.0 | 44.0 | 2.0 | [9] | |
| - | 8.4 | 53.4 | - | - | [51] | |
| 13.4 | 14.0 | - | 44.9 | - | [52] | |
| - | 9.0 | 49.5 | - | - | [51] | |
| 8.2 | 38.35 | 22.7 b | [10] | |||
| 9.7 | 13.8 | 39.7 | - | - | [42] | |
| Composted CGB | 17.8 | 20.9 | 56.0 | 43.2 | 0.5 | This Study |
| CGB Compost (Aerobic) | 16.7–21.1 | - | - | - | - | [43] |
| CGB Compost (Anaerobic) | 13.87 | - | - | - | - | [43] |
| Sample | Digestible Dry Matter (%) | Dry Matter Intake (%) | Relative Feed Values |
|---|---|---|---|
| FCGB-01 | 59.7 ± 0.5 | 2.2 ± 0.1 | 100.0 ± 4.9 |
| FCGB-02 | 67.9 ± 2.2 | 2.5 ± 0.3 | 131.9 ± 16.1 |
| CCGB-01 | 66.5 ± 1.6 | 1.6 ± 0.0 | 81.6 ± 3.2 |
| CCCB-02 | 71.9 ± 0.6 | 1.5 ± 0.0 | 84.3 ± 1.4 |
| CCGB-03 | 62.5 ± 4.1 | 1.8 ± 0.0 | 87.0 ± 5.7 |
| CCGB-04 | 37.8 ± 3.8 | 1.5 ± 0.0 | 43.9 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alege, F.P.; Donohoe, S.P.; Tumuluru, J.S.; Delhom, C.D.; Blake, C.D.; Thomas, J.W. Forage Properties of Fresh and Composted Cotton Gin Byproducts as Feed Supplements. AgriEngineering 2023, 5, 1955-1970. https://doi.org/10.3390/agriengineering5040120
Alege FP, Donohoe SP, Tumuluru JS, Delhom CD, Blake CD, Thomas JW. Forage Properties of Fresh and Composted Cotton Gin Byproducts as Feed Supplements. AgriEngineering. 2023; 5(4):1955-1970. https://doi.org/10.3390/agriengineering5040120
Chicago/Turabian StyleAlege, Femi Peter, Sean Paul Donohoe, Jaya Shankar Tumuluru, Christopher D. Delhom, Cody D. Blake, and Joe W. Thomas. 2023. "Forage Properties of Fresh and Composted Cotton Gin Byproducts as Feed Supplements" AgriEngineering 5, no. 4: 1955-1970. https://doi.org/10.3390/agriengineering5040120
APA StyleAlege, F. P., Donohoe, S. P., Tumuluru, J. S., Delhom, C. D., Blake, C. D., & Thomas, J. W. (2023). Forage Properties of Fresh and Composted Cotton Gin Byproducts as Feed Supplements. AgriEngineering, 5(4), 1955-1970. https://doi.org/10.3390/agriengineering5040120







