Comparing Two Methods of Leaf Area Index Estimation for Rice (Oryza sativa L.) Using In-Field Spectroradiometric Measurements and Multispectral Satellite Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Area
Cultivar Selection
2.2. Sampling Dates
- Phase #1: vegetative, characterized by starting with germination and concluding with panicle initiation (days 1 to 55 after sowing).
- Phase #2: reproductive, characterized by panicle initiation and concluding in flowering (days 55 to 105 after sowing).
- Phase #3: maturity or ripening, which is characterized by grain filling and extending until maturity (days 105 to 120 after sowing).
2.3. In-Field Spectral Signature Collection
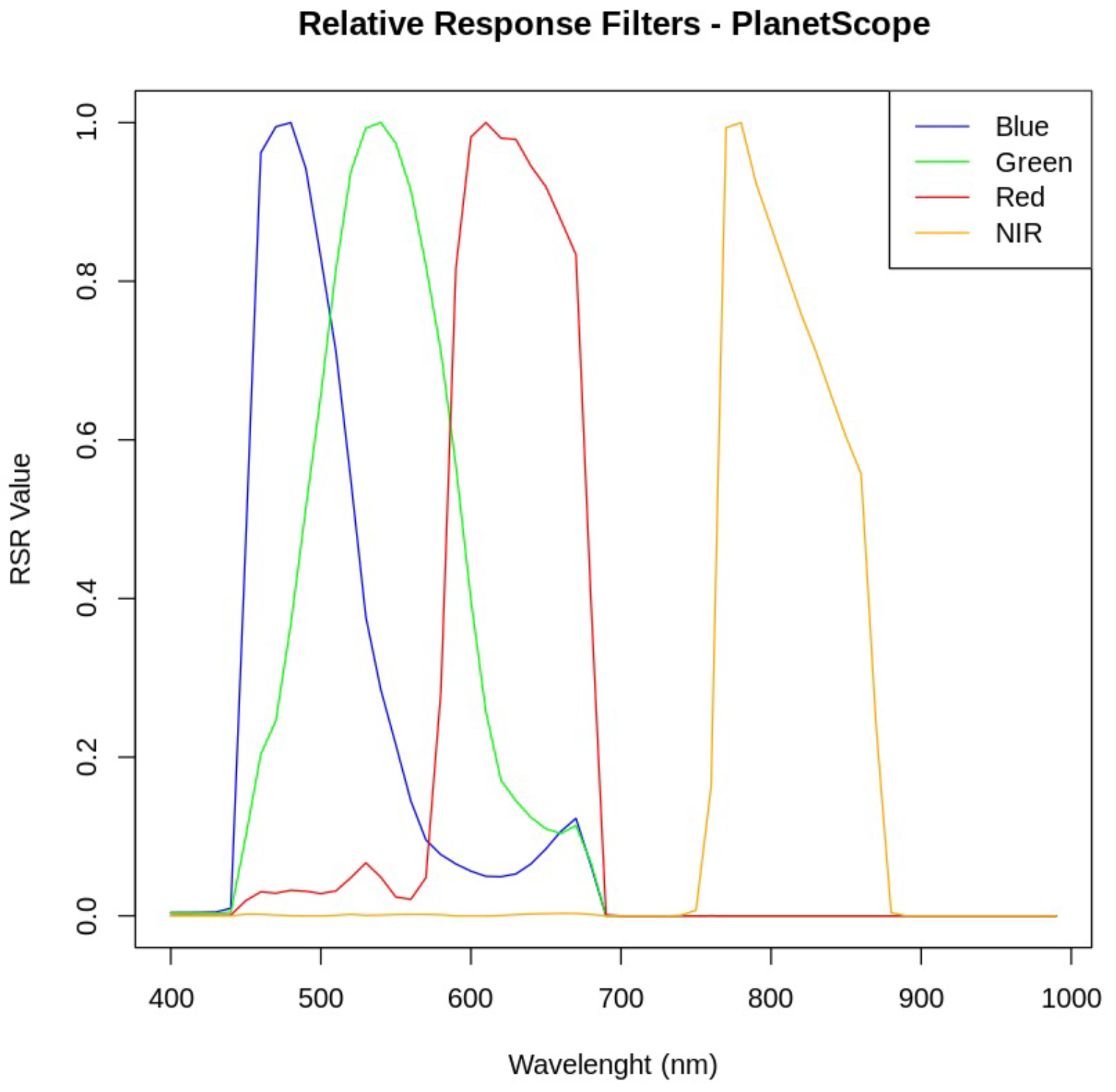
2.4. Transformation of the On-Site Spectral Signature to a Satellite Spectral Signature
2.5. Measurements of Green Leaf Area
2.6. Modeling the Relationship between In-Situ Reflectance and LAI
2.7. Spectral Estimation of LAI
2.8. Calibration Adjustment to PlanetScope Satellite Imagery
- 25 January 2018 (field and satellite measurement).
- 8 February 2018 (field and satellite measurement).
- 21 February 2018 (only field measurement, satellite measurement was acquired on 22 February 2018).
- 5 March 2018 (field and satellite measurement)
2.9. Software
3. Results
3.1. Analysis of Collected Spectral Signatures
3.2. True LAI Estimation
3.3. Model for In-Field Spectral Estimation of LAI
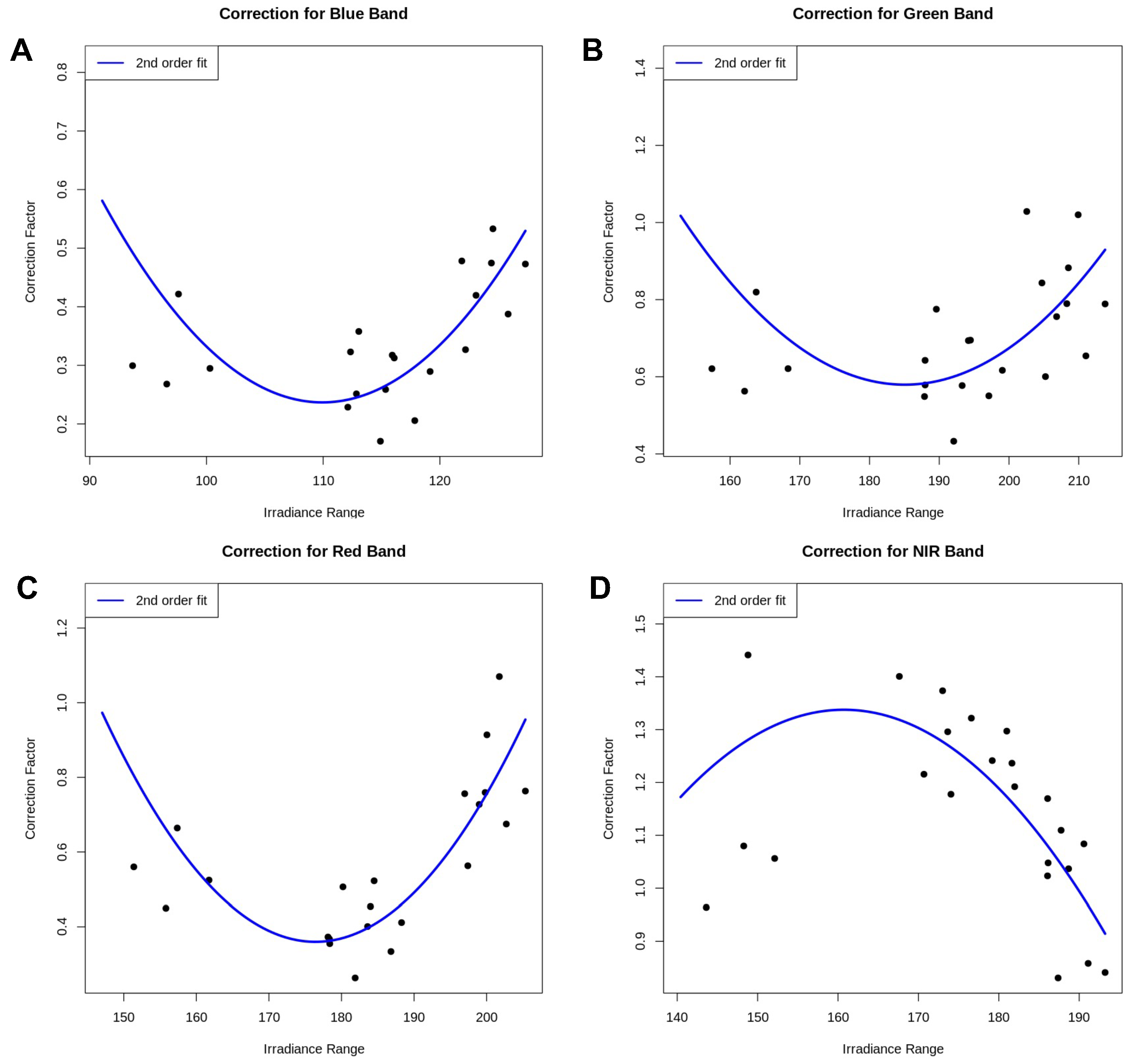
3.4. Relative Correction of the Satellite Image
- 920,757.68 N and 584,436.09 E
- 920,718.36 N and 584,545.32 E
- 920,822.46 N and 584,602.76 E
- 920,893.83 N and 584,531.64 E
3.5. Validating the Satellite Image Correction
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahzadi, F.; Ebrahimi, A.; Zarrinnia, V.; Azizinezhad, R. Evaluation of Genetic Diversity in Iranian Rice (Oryza sativa) Cultivars for Resistance to Blast Disease Using Microsatellite (SSR) Markers. Agric. Res. 2020, 9, 460–468. [Google Scholar] [CrossRef]
- Sharma, T.R.; Rai, A.K.; Gupta, S.K.; Vijayan, J.; Devanna, B.N.; Ray, S. Rice Blast Management Through Host-Plant Resistance: Retrospect and Prospects. Agric. Res. 2012, 1, 37–52. [Google Scholar] [CrossRef]
- Osinga, S.A.; Paudel, D.; Mouzakitis, S.A.; Athanasiadis, I.N. Big data in agriculture: Between opportunity and solution. Agric. Syst. 2022, 195, 103298. [Google Scholar] [CrossRef]
- Cravero, A.; Pardo, S.; Sepúlveda, S.; Mu noz, L. Challenges to Use Machine Learning in Agricultural Big Data: A Systematic Literature Review. Agronomy 2022, 12, 748. [Google Scholar] [CrossRef]
- García, S.A.; Martínez, L.J. Método para identificación de cultivos de arroz (Oryza sativa L.) con base en imágenes de satélite. Agron. Colomb. 2010, 38, 281–290. [Google Scholar]
- Xu, T.; Wang, F.; Yi, Q.; Xie, L.; Yao, X. A Bibliometric and Visualized Analysis of Research Progress and Trends in Rice Remote Sensing over the Past 42 Years (1980–2021). Remote Sens. 2022, 14, 3607. [Google Scholar] [CrossRef]
- Zheng, J.; Song, X.; Yang, G.; Du, X.; Mei, X.; Yang, X. Remote sensing monitoring of rice and wheat canopy nitrogen: A review. Remote Sens. 2022, 14, 5712. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.; Yang, R.; Chen, H.; Wang, Y.; Zhang, Y.; Huang, J.; Liu, F. Developing Novel Rice Yield Index Using UAV Remote Sensing Imagery Fusion Technology. Drones 2022, 6, 151. [Google Scholar] [CrossRef]
- San Bautista, A.; Fita, D.; Franch, B.; Casti neira-Ibá nez, S.; Arizo, P.; Sánchez-Torres, M.J.; Becker-Reshef, I.; Uris, A.; Rubio, C. Crop monitoring strategy based on remote sensing data (Sentinel-2 and Planet), Study case in a rice field after applying Glycinebetaine. Agronomy 2022, 12, 708. [Google Scholar] [CrossRef]
- Chuvieco, E. Teledetección Ambiental: La Observación de la Tierra Desde el Espacio; Ariel Ciencias, Editorial Ariel: Barcelona, Spain, 2010. [Google Scholar]
- Rouse, J.W.; Haas, R.W.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation NASA/GSFCT Type III Final Report. 1974. Available online: https://ntrs.nasa.gov/citations/19750020419 (accessed on 17 April 2023).
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with Crop Circle active crop canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Liang, L.; Di, L.; Zhang, L.; Deng, M.; Qin, Z.; Zhao, S.; Lin, H. Estimation of crop LAI using hyperspectral vegetation indices and a hybrid inversion method. Remote Sens. Environ. 2015, 165, 123–134. [Google Scholar] [CrossRef]
- Serrano Reyes, J.; Fábrega, J.R.; Quirós-McIntire, E.I.; Sánchez-Galán, J.E.; Jiménez, J.U. Análisis Prospectivo de la Detección Hiperespectral de Cultivos de Arroz (Oryza Sativa L.). KnE Eng. 2018, 3, 69. [Google Scholar] [CrossRef]
- Xie, Q.; Huang, W.; Liang, D.; Chen, P.; Wu, C.; Yang, G.; Zhang, J.; Huang, L.; Zhang, D. Leaf area index estimation using vegetation indices derived from airborne hyperspectral images in winter wheat. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3586–3594. [Google Scholar] [CrossRef]
- Jiménez, J.U.; Quirós-McIntire, E.I.; Camargo-García, V.; Serrano, J.; Sánchez-Galán, J.; Fábrega, J. Caracterización morfológica y espectral de 6 variedades criollas de arroz (Oryza sativa L.) en Panamá. In Proceedings of the Innovation in Education and Inclusion: Proceedings of the 16th LACCEI International Multi-Conference for Engineering, Education and Technology, Lima, Peru, 18–20 July 2018. [Google Scholar] [CrossRef]
- Monsi, M.; Saeki, T. On the factor light in plant communities and its importance for matter production. Ann. Bot. 2005, 95, 549. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, J.; Zhang, R.; Huang, S.; Chen, S.; Wang, H.; Luo, J.; Fan, Y. Intercomparison of three two-source energy balance models for partitioning evaporation and transpiration in semiarid climates. Remote Sens. 2018, 10, 1149. [Google Scholar] [CrossRef]
- Colaizzi, P.D.; Kustas, W.P.; Anderson, M.C.; Agam, N.; Tolk, J.A.; Evett, S.R.; Howell, T.A.; Gowda, P.H.; O’Shaughnessy, S.A. Two-source energy balance model estimates of evapotranspiration using component and composite surface temperatures. Adv. Water Resour. 2012, 50, 134–151. [Google Scholar] [CrossRef]
- Jarma, A.d.J.; Degiovanni, V.M.; Montoya, R.A. Índices fisiotécnicos, fases de crecimiento y etapas de desarrollo de la planta de arroz. In Producción Eco-Eficiente del Arroz en América Latina. Publicación CIAT No. 365; Degiovanni, B.V., Martínez, R.C.P., Motta, O.F., Eds.; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2010; Chapter 5; Volume Tomo I, 487p. [Google Scholar]
- Ross, J. The radiation Regime and Architecture of Plant Stands. Tasks Veg. Sci. 1981, 3, 391. [Google Scholar] [CrossRef]
- Casa, R.; Upreti, D.; Pelosi, F. Measurement and estimation of leaf area index (LAI) using commercial instruments and smartphone-based systems. IOP Conf. Ser. Earth Environ. Sci. 2019, 275, 012006. [Google Scholar] [CrossRef]
- Mora, D.; Jiménez, J.U.; Fábrega, J.R. Relación Entre el Índice de Área Foliar y el Índice Normalizado de Vegetación en el Bosque Húmedo Tropical de Panamá en Gamboa. I + D Tecnológico 2014, 10, 28–40. [Google Scholar]
- Wang, F.m.; Huang, J.f.; Lou, Z.h. A comparison of three methods for estimating leaf area index of paddy rice from optimal hyperspectral bands. Precis. Agric. 2011, 12, 439–447. [Google Scholar] [CrossRef]
- He, J.; Qin, Y.; Guo, C.; Zhao, L.; Zhou, X.; Yao, X.; Cheng, T.; Tian, Y. Monitoring leaf area index after heading stage using hyperspectral remote sensing data in rice. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 6284–6287. [Google Scholar] [CrossRef]
- Castrellón, M.G.; Pauloo, R.A.; Popescu, I.; Fábrega, J. ONASP: A web application for groundwater data visualization in Panama. IOP Conf. Ser. Earth Environ. Sci. 2023, 1136, 012028. [Google Scholar] [CrossRef]
- Buitrago, I.C. Las Variedades Mejoradas de Arroz del Idiap: Un Aporte al Desarrollo del Sector Arrocero Panameño 1975–2010; Instituto de Investigación Agropecuaria de Panamá, Departamento de Ediciones y Publicaciones: Panama City, Panama, 2012. [Google Scholar]
- Buitrago, I.C.; Quirós McIntire, E.I.; Zachrisson Salamina, B. Fenología de la Planta de Arroz y su Importancia en el Manejo Integrado del Cultivo; Instituto de Investigación Agropecuaria de Panamá, Departamento de Ediciones y Publicaciones: Panama City, Panama, 2012. [Google Scholar]
- Ariza, A.A. Machine Learning and Big Data Techniques for Satellite-Based Rice Phenology Monitoring. Master’s Thesis, The University of Manchester, Manchester, UK, 2019. [Google Scholar]
- Sánchez-Galán, J.E.; Serrano Reyes, J.; Jiménez, J.U.; Quirós-McIntire, E.I.; Fábrega, J.R. Supervised Classification of Spectral Signatures from Agricultural Land-Cover in Panama Using the Spectral Angle Mapper Algorithm. In Proceedings of the 2019 XLV Latin American Computing Conference (CLEI), Panama City, Panama, 30 September–4 October 2019; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Sánchez-Galán, J.E.; Barranco, F.R.; Reyes, J.S.; Quirós-McIntire, E.I.; Jiménez, J.U.; Fábrega, J.R. Using Supervised Classification Methods for the Analysis of Multi-spectral Signatures of Rice Varieties in Panama. Adv. Sci. Technol. Eng. Syst. J. 2021, 6, 552–558. [Google Scholar] [CrossRef]
- Planet Labs Inc. Planet Imagery Product Specifications; Planet Labs Inc.: San Francisco, CA, USA, 2021; Available online: https://assets.planet.com/docs/Combined-Imagery-Product-Spec-Dec-2018.pdf (accessed on 26 April 2023).
- Melillos, G.; Hadjimitsis, D.G. Detection Underground Structures in Cyprus Using Landsat-8 Bands. In Proceedings of the IGARSS 2020–2020 IEEE International Geoscience and Remote Sensing Symposium, Waikoloa, HI, USA, 26 September–2 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1181–1184. [Google Scholar]
- Trishchenko, A.P.; Cihlar, J.; Li, Z. Effects of spectral response function on surface reflectance and NDVI measured with moderate resolution satellite sensors. Remote Sens. Environ. 2002, 81, 1–18. [Google Scholar] [CrossRef]
- Trishchenko, A.P. Effects of spectral response function on surface reflectance and NDVI measured with moderate resolution satellite sensors: Extension to AVHRR NOAA-17, 18 and METOP-A. Remote Sens. Environ. 2009, 113, 335–341. [Google Scholar] [CrossRef]
- Agapiou, A.; Hadjimitsis, D.G.; Alexakis, D.D. Evaluation of broadband and narrowband vegetation indices for the identification of archaeological crop marks. Remote Sens. 2012, 4, 3892–3919. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Nguyen, H.T.; Lee, B.W. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. QGIS Association. Available online: https://www.qgis.org (accessed on 26 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Spiess, A.N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chen, R.K. Modeling rice growth with hyperspectral reflectance data. Crop Sci. 2004, 44, 1283–1290. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crop. Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Collison, A.; Wilson, N. Planet Surface Reflectance Product; Version 1.0.; Planet Labs, Inc.: San Francisco, CA, USA, 2018. [Google Scholar]



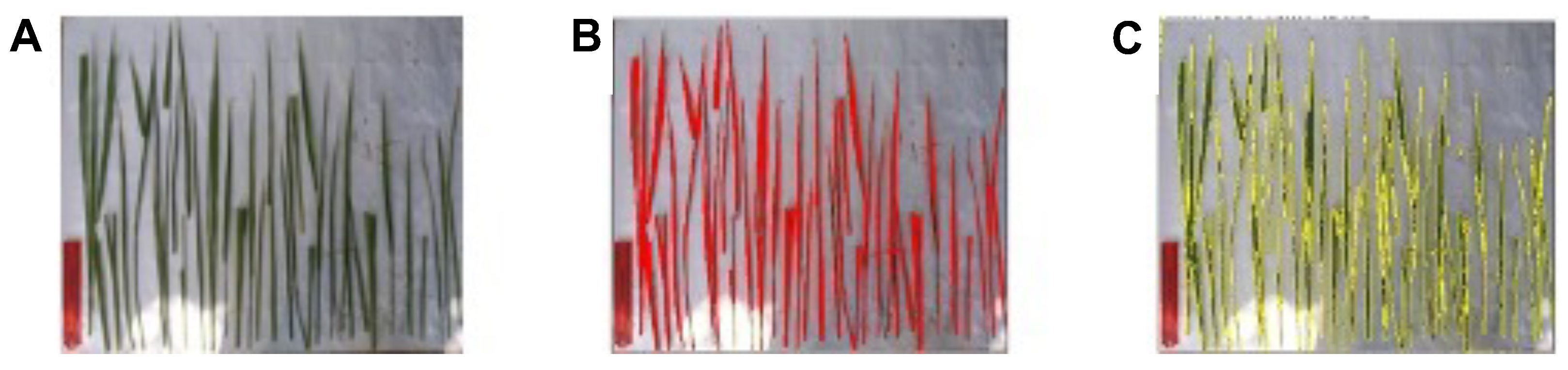



| Phase | Days after Sowing | Measured LAI | Mean Absolute Deviation for LAI |
|---|---|---|---|
| Vegetative | 47 | 3.48, 4.20, 6.36 | 1.12 |
| Reproductive | 67 | 5.71, 6.45, 9.33 | 1.44 |
| 82 | 5.71, 6.20, 9.36 | 1.51 | |
| Maturity | 97 | 8.25, 9.54, 9.94 | 0.66 |
| 116 | 4.00, 4.96, 5.05 | 0.45 |
| Band | Final Model | Residual Standard Error | Signifcance | Pseudo |
|---|---|---|---|---|
| Blue | Y = 0.2127 × x + 11.92 | 0.1005 | p < 0.001 | 0.53 |
| Green | Y = 0.1568 × x + 15.09 | 0.1739 | p < 0.001 | 0.36 |
| Red | Y = 0.2506 × x + 22.46 | 0.1485 | p < 0.001 | 0.68 |
| NIR | Y = + 0.1288 × x − 9.0127 | 0.1528 | p < 0.05 | 0.41 |
| Irradiance | ||||
|---|---|---|---|---|
| Sample | Blue Band | Green Band | Red Band | NIR Band |
| 1 | 106.137 | 177.2489 | 169.5444 | 161.3283 |
| 2 | 106.1878 | 177.241 | 169.2943 | 158.1286 |
| 3 | 105.7683 | 176.4078 | 168.4679 | 159.3418 |
| 4 | 104.2549 | 173.8378 | 166.0449 | 159.3632 |
| 5 | 107.4158 | 179.0375 | 170.9401 | 161.7234 |
| 6 | 105.515 | 175.912 | 167.9908 | 160.3049 |
| 7 | 104.5517 | 174.196 | 166.2761 | 158.6433 |
| Average | 105.6901 | 176.2687 | 168.3655 | 159.8334 |
| Model | Calculated NDVI | Measured LAI | Estimated LAI | Percentual Error (%) |
|---|---|---|---|---|
| 1 | 0.9189 | 7.71 | 8.03 | 4.15 |
| 0.9164 | 6.51 | 8.00 | 22.09 |
| Model | Calculated MTVI2 | Measured LAI | Estimated LAI | Percentual Error (%) |
|---|---|---|---|---|
| 2 | 0.8057 | 7.71 | 8.41 | 9.03 |
| 0.8009 | 6.51 | 8.35 | 28.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano Reyes, J.; Jiménez, J.U.; Quirós-McIntire, E.I.; Sanchez-Galan, J.E.; Fábrega, J.R. Comparing Two Methods of Leaf Area Index Estimation for Rice (Oryza sativa L.) Using In-Field Spectroradiometric Measurements and Multispectral Satellite Images. AgriEngineering 2023, 5, 965-981. https://doi.org/10.3390/agriengineering5020060
Serrano Reyes J, Jiménez JU, Quirós-McIntire EI, Sanchez-Galan JE, Fábrega JR. Comparing Two Methods of Leaf Area Index Estimation for Rice (Oryza sativa L.) Using In-Field Spectroradiometric Measurements and Multispectral Satellite Images. AgriEngineering. 2023; 5(2):965-981. https://doi.org/10.3390/agriengineering5020060
Chicago/Turabian StyleSerrano Reyes, Jorge, José Ulises Jiménez, Evelyn Itzel Quirós-McIntire, Javier E. Sanchez-Galan, and José R. Fábrega. 2023. "Comparing Two Methods of Leaf Area Index Estimation for Rice (Oryza sativa L.) Using In-Field Spectroradiometric Measurements and Multispectral Satellite Images" AgriEngineering 5, no. 2: 965-981. https://doi.org/10.3390/agriengineering5020060
APA StyleSerrano Reyes, J., Jiménez, J. U., Quirós-McIntire, E. I., Sanchez-Galan, J. E., & Fábrega, J. R. (2023). Comparing Two Methods of Leaf Area Index Estimation for Rice (Oryza sativa L.) Using In-Field Spectroradiometric Measurements and Multispectral Satellite Images. AgriEngineering, 5(2), 965-981. https://doi.org/10.3390/agriengineering5020060






