Validation of Relation between SPAD and Rice Grain Protein Content in Farmer Fields in the Coastal Area of Sendai, Japan
Abstract
1. Introduction
2. Materials and Methods
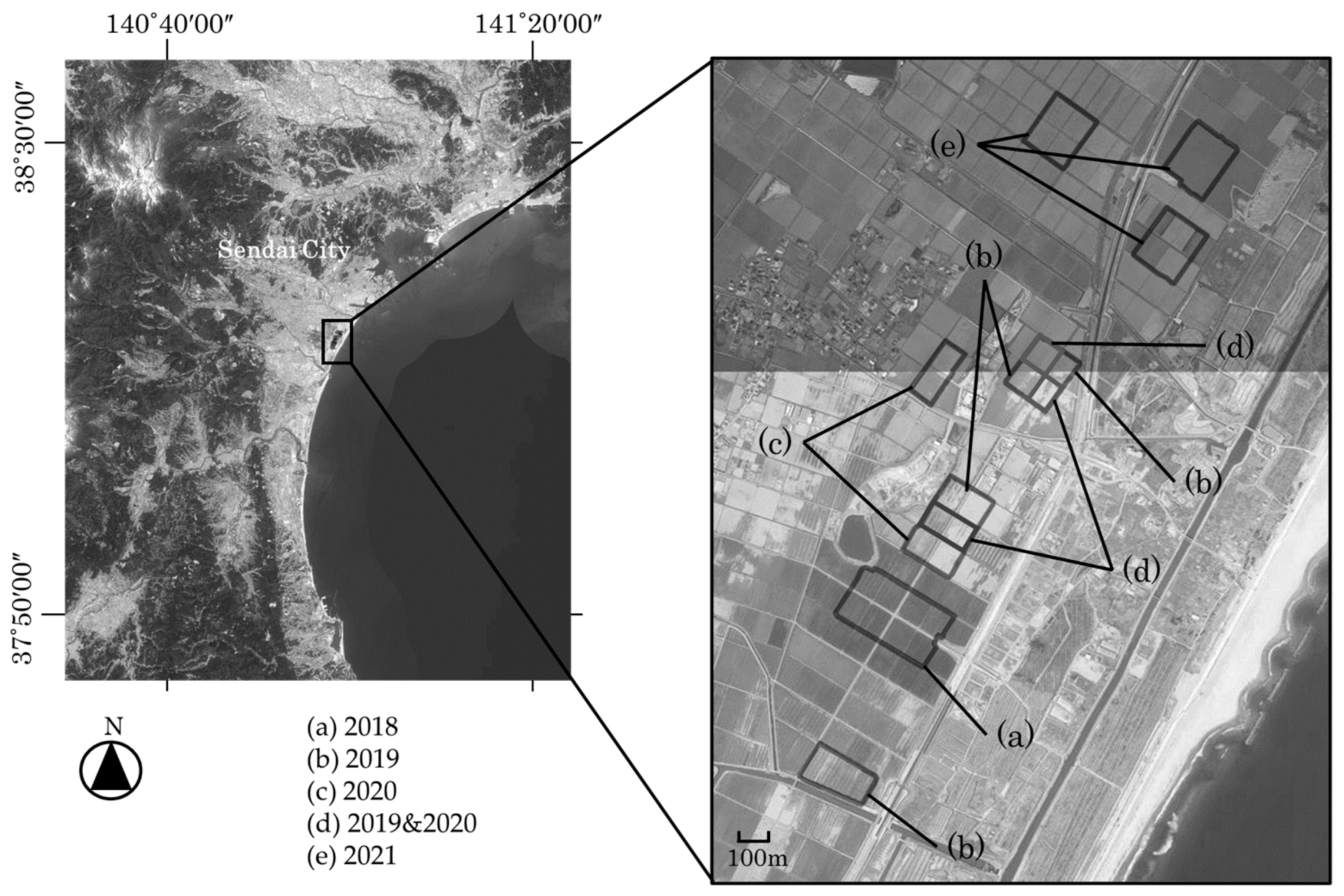
2.1. Research Fields
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Effect of Year, Cultivar and Planting Method on SPAD and Grain Protein Content
3.2. Estimation of Grain Protein Content Based on SPAD Value
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maclean, J.; Hardy, B.; Hettel, G. Rice Almanac: Source Book for One of the Most Important Economic Activities on Earth; IRRI: Los Baños, Philippines, 2013. [Google Scholar]
- Zhang, L.X.; Zhang, C.Q.; Yan, Y.; Hu, Z.J.; Wang, K.; Zhou, J.H.; Zhou, Y.; Cao, L.M.; Wu, S.J. Influence of starch fine structure and storage proteins on the eating quality of rice varieties with similar amylose contents. J. Sci. Food Agric. 2020, 101, 3811–3818. [Google Scholar] [CrossRef]
- Martin, M.; Fitzgerald, M.A. Proteins in rice grains influence cooking properties. J. Cereal Sci. 2002, 36, 285–294. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Z.W. Prediction model for eating property of Indica rice. J. Food Qual. 2014, 37, 274–280. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; He, L.H.; Wang, T.; Lu, H.; Yang, F.; Deng, F.; Chen, Y.; Tao, Y.F.; Li, M.; et al. Correlation of taste values with chemical compositions and Rapid Visco Analyser profiles of 36 indica rice (Oryza sativa L.) varieties. Food Chem. 2021, 349, 129176. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, E.T.; Li, C.X.; Cai, M.L.; Cheng, B.; Cao, C.G.; Jiang, Y. Use of protein content, amylose content, and RVA parameters to evaluate the taste quality of rice. Front. Nutr. 2022, 8, 758547. [Google Scholar] [CrossRef]
- Wang, G.Y.; Abe, T.; Sasahara, T. Concentrations of Kjeldahl-digested nitrogen, amylose, and amino acids in milled grains of rice (Oryza sativa L.) cultivated under organic and customary farming practices. Jpn. J. Crop Sci. 1998, 67, 307–311. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Gouis, J.L.; Moreau, D.; Bogard, M. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, J.H.; Liu, L.Y.; Huang, W.J.; Zhao, C.J.; Wang, C.Z. Prediction of grain protein content in winter wheat (Triticum aestivum L.) using plant pigment ratio (PPR). Field Crops Res. 2004, 90, 311–321. [Google Scholar] [CrossRef]
- Wang, H.; McCaig, T.; DePauw, R.; Clarke, J. Flag leaf physiological traits in two high-yielding Canada Western Red Spring wheat cultivars. Can. J. Plant Sci. 2008, 88, 35–42. [Google Scholar] [CrossRef]
- Thomas, J.R.; Gausman, H.W. Leaf reflectance vs. Leaf Chlorophyll and Carotenoid Concentrations for Eight Crops1. Agron. J. 1997, 69, 799–802. [Google Scholar] [CrossRef]
- Himelblau, E.; Amasino, R.M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 2001, 158, 1317–1323. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Song, X.; Gu, X.; Yang, G.; Chen, L.; Ma, Q.; Li, Z. Comparison of Leaf and Canopy Parameters for Estimating Wheat Nitrogen and Grain Protein Content. In Proceedings of the International Conference on Agro-Geoinformatics, Hangzhou, China, 6–9 August 2018; pp. 1–6. [Google Scholar]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf Chlorophyll Content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Ellsworth, J.W.; Koehler, K.J. Sensitivity of Chlorophyll Meters for Diagnosing Nitrogen Deficiencies of Corn in Production Agriculture. Agron. J. 2008, 100, 543–550. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Hunt, E.R. Combined Spectral Index to Improve Ground-Based Estimates of Nitrogen Status in Dryland Wheat. Agron. J. 2008, 100, 1694–1702. [Google Scholar] [CrossRef]
- Balasubramanian, A.C. Adaptation of the chlorophyll meter (SPAD) technology for real time N management in rice: A review. Int. Rice Res. Notes 2000, 25, 4–8. [Google Scholar]
- Ghosh, M.; Swain, D.K.; Jha, M.K.; Tewari, V.K. Precision nitrogen management using chlorophyll meter for improving growth, productivity and N use efficiency of rice in subtropical climate. J. Agric. Sci. 2013, 5, 253–266. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Lv, Y.; He, J. SPAD values and nitrogen nutrition index for the evaluation of rice nitrogen status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cao, Q.; Zhu, Y. Non-destructive assessment of plant nitrogen parameters using leaf chlorophyll measurements in rice. Front. Plant Sci. 2016, 7, 1829. [Google Scholar] [CrossRef]
- Giamerti, Y.; Hongo, C.; Saito, D.; Caasi, O.; Susilawati, P.N.; Shishido, M.; Sudiarta, I.P.; Wijaya, I.M.; Homma, K.K. Evaluating Multispectral Imaging for Assessing Bacterial Leaf Blight Damage in Indonesian Agricultural Insurance. In Proceedings of the International Conference on Agribusiness and Rural Development, IConARD, Yogyakarta, Indonesia, 13–14 October 2020; Volume 232, p. 03008. [Google Scholar]
- Xue, L.H.; Cao, W.X.; Yang, L.Z. Predicting grain yield and protein content in winter wheat at different n supply levels using canopy reflectance spectra. Pedosphere 2007, 17, 646–653. [Google Scholar] [CrossRef]
- Nakano, H.; Tanaka, R.; Guan, S.; Okami, M.; Wada, H.; Hakata, M.; Ohdan, H. Identification of growth-related indicators affecting the appearance and protein content of rice grains. Agron. J. 2022, 114, 565–581. [Google Scholar] [CrossRef]
- Hirai, Y.; Keisuke, S.; Hamagami, K. Evaluation of an analytical method to identify determinants of rice yield components and protein content. Comput. Electron. Agric. 2012, 83, 77–84. [Google Scholar] [CrossRef]
- Mori, S.; Yokoyama, K.; Fujii, H. Classification of brown rice with different protein content using the diagnosis of leaf color during the ripening period in Shonai area of Yamagata prefecture. Jpn. J. Crop Sci. 2010, 79, 113–119. [Google Scholar] [CrossRef]
- Fukuyama, T.; Abe, N. Relationships between protein content of rice grain and leaf color in paddy field of Budokubo, Nagaoka city. Bull. Fac. Agric. Niigata Univ. 2011, 63, 55–62, (In Japanese, with English Summary). [Google Scholar]
- Wakamatsu, K.; Sasaki, O.; Uezono, I.; Tanaka, A. Effect of the amount of nitrogen application on occurrence of white-back kernels during ripening of rice under high-temperature conditions. Jpn. J. Crop Sci. 2008, 77, 424–433. [Google Scholar] [CrossRef]
- Wang, Z.L.; Cheng, Y.B.; Gong, R.; Zhou, S.C.; Wang, C.R.; Li, H.; Huang, D.Q.; Zhou, D.G.; Zhao, L.; Pan, Y.Y.; et al. Correlation Between SPAD Value of Flag Leaf and Rice Quality of High Quality indica Rice. Chin. J. Rice Sci. 2021, 35, 89–97, (In Chinese, with English Summary). [Google Scholar]
- Matsue, Y. Studies on palatability of rice in northern Kyushu. VI. Effects of seedling characteristics under abnormal weather in 1993 on the palatability and physiochemical characteristics of rice. Jpn. J. Crop Sci. 1995, 64, 714–716. [Google Scholar] [CrossRef]
- Wada, T.; Oosato, K.F.; Hamachi, Y. Effects of high air temperature and insufficient solar radiation during ripening period on the palatability and physicochemical properties of rice in 1999 in Kyushu regions. Jpn. J. Crop Sci. 2002, 71, 349–354. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, G.; Zhao, G.; Yao, H.; Xu, H. Variation in rice quality of different cultivars and grain positions as affected by water management. Field Crops Res. 2003, 80, 245–252. [Google Scholar] [CrossRef]
- Ueda, K.; Kusutani, A.; Asanuma, K.; Ichii, M. Effect of transplanting timing on growth of rice cultivar kinuhikari in Kagawa Prefecture. Jpn. J. Crop Sci. 1998, 67, 289–296. [Google Scholar] [CrossRef]
- Matsue, Y.; Mizuta, K.; Furuno, K.; Yoshida, T. Studies on palatability of rice in northern Kyushu. II. Effects of harvest time on palatability and physiochemical properties of milled rice. Jpn. J. Crop Sci. 1991, 60, 497–503. [Google Scholar] [CrossRef]
- Saito, K.; Miyama, M.; Yamamoto, J.; Katsukita, H. Diagnosis of nitrogen nutrition of rice cv. koshihikari using chlorophyllmeter. Bull. Chiba Agric. Exp. Stn. 1992, 33, 27–35. [Google Scholar]
- Crop Investigation Criteria Editing Committee. Crop Investigation Criteria; Kyushu Branch, Crop Science Society of Japan: Fukuoka, Japan, 2013. (In Japanese) [Google Scholar]
- Causton, D.; Grafen, A.; Hails, R. Modern Statistics for the Life Sciences; Oxford University Press: London, UK, 2002. [Google Scholar]
- Wang, G.Y.; Bronson, K.F.; Thorp, K.R. Multiple leaf measu rements improve effectiveness of chlorophyll meter for durum wheat nitrogen management. Crop Sci. 2014, 54, 817–826. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crops Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Endo, T.; Nagano, K.; Sasaki, K.; Chiba, B.; Wagatsuma, K.; Hayashi, H.; Saeki, K.; Sato, H.; Sakai, M.; Nakagomi, Y. New rice cultivar “Datemasayume”. Bull. Miyagi Pref. Furukawa Agric. Exp. Stn. 2018, 13, 19–44. [Google Scholar]
- Matsunaga, K.; Sasaki, T.; Nagano, K.; Okamoto, E.; Abe, S.; Uematsu, K.; Kano, A.; Takizawa, H.; Hayasaka, H.; Usuki, S.; et al. New rice cultivar “Manamusume”. Bull. Miyagi Pref. Furukawa Agric. Exp. Stn. 2002, 3, 53–68. [Google Scholar]
- Kobayashi, A.; Hori, K.; Yamamoto, T.; Yano, M. Koshihikari: A premium short-grain rice cultivar its expansion and breeding in Japan. Rice 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Abe, S.; Matsunaga, K.; Okamoto, E.; Nagano, K.; Tanno, K.; Chiba, Y.; Kano, A.; Uematsu, K. A new rice cultivar “Hitomebore”. Bull. Miyagi Pref. Furukawa Agric. Exp. Stn. 1993, 2, 1–17. [Google Scholar]
- Ozaki, K.; Kobayashi, T.; Ohashi, Y.; Kawase, K. Establishment of predict method to regulate desirable spikelet numbers and protein contents by growth diagnosis production, and application to ‘Koshihikari’ cultivated in Tango area, Kyoto prefecture. Bull. Agric. For. Tech. Dept. Kyoto Pref. 2012, 35, 1–10. [Google Scholar]
- Horie, T. Global warming and rice production in Asia: Modeling, impact prediction and adaptation. Proc. Jpn. Acad. Ser. B 2019, 95, 211–245. [Google Scholar] [CrossRef]
- Shimoyanagi, R.; Abo, M.; Shiotsu, F. Higher temperatures during grain filling affect grain chalkiness and rice nutrient contents. Agronomy 2021, 11, 1360. [Google Scholar] [CrossRef]
- Masutomi, Y.; Takimoto, T.; Shimamura, M.; Manabe, T.; Arakawa, M.; Shibota, N.; Ooto, A.; Azuma, S.; Imai, Y.; Tamura, M. Rice grain quality degradation and economic loss due to global warming in Japan. Environ. Res. Commun. 2019, 19, 121003. [Google Scholar] [CrossRef]
- Tsukaguchi, T.; Iida, Y. Effects of assimilate supply and high temperature during grain-filling period on the occurrence of various types of chalky kernels in rice plants (Oryza sativa L.). Plant Prod. Sci. 2008, 11, 203–210. [Google Scholar] [CrossRef]
- Yoshida, H.; Takehisa, K.; Kojima, T.; Ohno, H.; Sasaki, K.; Nakagawa, H. Modeling the effects of N application on growth, yield and plant properties associated with the occurrence of chalky grains of rice. Plant Prod. Sci. 2016, 19, 30–42. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Zhang, Z. A remote sensing-based scheme to improve regional crop model calibration at sub-model component level. Agric. Syst. 2020, 181, 102814. [Google Scholar] [CrossRef]
- Maki, M.; Sekiguchi, K.; Homma, K.; Hirooka, Y.; Oki, K. Estimation of rice yield by SIMRIW-RS, a model that integrates remote sensing data into a crop growth model. J. Agric. Meteorol. 2017, 73, 2–8. [Google Scholar] [CrossRef]
- Ma, J.J.; Zheng, B.Y.; He, Y. Applications of a Hyperspectral Imaging System Used to Estimate Wheat Grain Protein: A Review. Front. Plant Sci. 2022, 13, 837200. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, Y.; Sigit, G.; Utoyo, B.; Lubis, I.; Junaedi, A.; Trisasongko, B.H.; Wijaya, I.M.A.S.; Maki, M.; Hongo, C.; Homma, K. Drought damage assessment for crop insurance based on vegetation index by unmanned aerial vehicle (UAV) multispectral images of paddy fields in Indonesia. Agriculture 2023, 13, 113. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nomoto, S.; Hashimoto, N.; Maki, M.; Hongo, C.; Shiraiwa, T.; Homma, K. Monitoring spatial and time-series variations in red crown rot damage of soybean in farmer fields based on UAV remote sensing. Plant Prod. Sci. 2023. [Google Scholar] [CrossRef]
- Hirooka, Y.; Homma, K.; Shiraiwa, T. A leaf area-based non-destructive approach to predict productivity. Agron. J. 2021, 113, 3922–3934. [Google Scholar] [CrossRef]
- Hashimoto, N.; Saito, Y.; Yamamoto, S.; Ishibashi, T.; Ito, R.; Maki, M.; Homma, K. Feasibility of yield estimation based on leaf area dynamicsmeasurements in rice paddy fields of farmers. Field Crops Res. 2022, 286, 108609. [Google Scholar] [CrossRef]
- Botha, E.J.; Leblon, B.; Zebarth, B.J.; Watmough, J. Non-Destructive Estimation of Wheat Leaf Chlorophyll Content from Hyperspectral Measurements through Analytical Model Inversion. Int. J. Remote Sens. 2010, 31, 1679–1697. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the Methods for Estimating Leaf Chlorophyll Content with SPAD Chlorophyll Meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Obara, K.; Homma, K.; Tajima, R.; Maki, M.; Saito, Y.; Hashimoto, N.; Yamamoto, S.; Hongo, C. Analysis of RGB images to estimate SPAD values in rice for UAV remote sensing. Jpn. J. Crop Sci. 2020, 89, 50–51. [Google Scholar] [CrossRef]

| Year | Cultivar | Planting Method | Number of Points | Planting Density (Row × Column) | Fertilizer | |
|---|---|---|---|---|---|---|
| Basal (g m−2) | Additional (g m−2) | |||||
| 2018 | Hitomebore | Transplanting | 40 | 0.3 m × 0.21 m | 40 a | 5 b |
| Manamusume | Transplanting | 20 | 0.3 m × 0.21 m | 40 a | 5 b | |
| Datemasayume | Transplanting | 20 | 0.3 m × 0.21 m | 40 a | 5 b | |
| Hitomebore | Direct sowing c (flooded) | 80 | 0.3 m × 0.21 m | 40 a | - | |
| 2019 | Hitomebore | Direct sowing c (flooded) | 48 | 0.3 m × 0.2 m | 40 d | - |
| Hitomebore | Transplanting e (dense) | 48 | 0.3 m × 0.2 m | 40 d | - | |
| Hitomebore | Transplanting | 48 | 0.3 m × 0.2 m | 40 d | - | |
| 2020 | Manamusume | Transplanting e (dense) | 8 | 0.3 m × 0.18 m | 40 c | - |
| Manamusume | Direct sowing | 8 | 0.3 m × 0.18 m | 40 d | - | |
| Hitomebore | Transplanting | 16 | 0.3 m × 0.18 m | 40 d | - | |
| 2021 | Manamusume | Direct sowing | 48 | 0.3 m × 0.18 m | 40 d | 8 b |
| Hitomebore | Transplanting | 48 | 0.3 m × 0.2 m | 40 d | 8 b | |
| Manamusume | Transplanting e (dense) | 48 | 0.3 m × 0.2 m | 40 d | 8 b | |
| Month | Period | Solar Radiation (MJ m−2) | Temperature (°C) | Precipitation (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2018 | 2019 | 2020 | 2021 | 2018 | 2019 | 2020 | 2021 | ||
| May | early | 14.2 | 23.8 | 20.3 | 20.9 | 15.4 | 15.4 | 17.4 | 15.9 | 3.3 | 1.5 | 0.3 | 1.9 |
| late | 20.2 | 23.5 | 15.8 | 16.0 | 18.5 | 19.3 | 16.3 | 18.0 | 3.3 | 3.7 | 6.3 | 2.8 | |
| June | early | 18.4 | 19.4 | 22.4 | 20.4 | 19.0 | 18.5 | 21.8 | 20.5 | 5.0 | 5.3 | 1.2 | 1.8 |
| late | 16.9 | 13.6 | 14.1 | 15.5 | 21.5 | 19.5 | 20.6 | 20.7 | 1.7 | 6.0 | 1.7 | 2.3 | |
| July | early | 15.9 | 11.7 | 8.5 | 9.5 | 24.6 | 20.3 | 20.9 | 21.8 | 3.6 | 4.0 | 14.7 | 9.1 |
| late | 18.6 | 15.0 | 10.7 | 21.3 | 26.4 | 24.5 | 21.5 | 26.2 | 0.3 | 3.2 | 11.1 | 3.3 | |
| August | early | 15.9 | 17.1 | 18.0 | 13.8 | 25.8 | 27.4 | 26.3 | 24.6 | 7.5 | 0.3 | 2.5 | 7.3 |
| late | 13.3 | 12.3 | 18.0 | 13.6 | 24.2 | 25.1 | 26.9 | 25.0 | 10.0 | 5.3 | 2.2 | 4.5 | |
| September | early | 9.9 | 14.1 | 11.9 | 12.3 | 21.9 | 24.0 | 24.7 | 20.7 | 4.9 | 3.9 | 7.5 | 5.8 |
| late | 11.6 | 13.1 | 10.6 | 13.9 | 19.7 | 20.9 | 20.2 | 21.0 | 7.6 | 1.0 | 5.3 | 2.4 | |
| Avg. | 15.5 | 16.4 | 15.0 | 15.7 | 21.7 | 21.5 | 21.7 | 21.5 | 4.7 | 3.4 | 5.3 | 4.1 | |
| Cultivar | Planting Method | Year | Booting Stage | Heading Stage | Milking Stage | Maturity Stage | PC (%) |
|---|---|---|---|---|---|---|---|
| Hitomebore | Transplanting | 2018 | 43.91 | 34.31 | 31.15 | 26.15 | 7.7 |
| Transplanting | 2019 | 40.83 | 32.55 | 31.27 | 24.42 | 7.0 | |
| Transplanting | 2020 | 41.77 | 35.35 | 32.34 | 24.12 | 7.0 | |
| Transplanting | 2021 | 41.18 | 34.38 | 34.42 | 28.05 | 7.8 | |
| Transplanting (dense) | 2019 | 40.38 | 31.93 | 30.53 | 24.57 | 7.3 | |
| Direct sowing (flooded) | 2018 | 41.44 | 36.37 | 27.99 | 26.16 | 7.8 | |
| Direct sowing (flooded) | 2019 | 38.99 | 34.00 | 29.06 | 30.11 | 7.8 | |
| Manamusume | Transplanting | 2018 | 46.97 | 34.35 | 30.05 | 26.76 | 7.8 |
| Transplanting (dense) | 2020 | 42.98 | 39.89 | 37.75 | 34.47 | 8.8 | |
| Transplanting (dense) | 2021 | 39.88 | 35.86 | 34.58 | 28.90 | 8.6 | |
| Direct sowing | 2020 | 37.97 | 36.36 | 33.35 | 28.29 | 7.7 | |
| Direct sowing | 2021 | 35.89 | 31.04 | 32.80 | 30.90 | 8.2 | |
| Datemasayume | Transplanting | 2018 | 44.05 | 34.09 | 31.94 | 28.37 | 8.2 |
| Average | |||||||
| Year | 2018 | 43.13 | 34.92 | 30.36 | 26.89 | 7.9 | |
| 2019 | 40.07 | 32.83 | 30.29 | 26.37 | 7.4 | ||
| 2020 | 40.91 | 37.20 | 34.48 | 28.96 | 7.8 | ||
| 2021 | 38.98 | 33.76 | 33.93 | 29.28 | 8.2 | ||
| Cultivar | Hitomebore | 41.21 | 34.13 | 30.97 | 26.23 | 7.5 | |
| Manamusume | 40.74 | 35.50 | 33.71 | 29.86 | 8.2 | ||
| Datemasayume | 44.05 | 34.09 | 31.94 | 28.37 | 8.2 | ||
| Planting method | Transplanting | 43.12 | 34.17 | 31.86 | 26.31 | 7.6 | |
| Transplanting (dense) | 40.38 | 31.93 | 30.53 | 24.57 | 7.3 | ||
| Direct sowing | 36.93 | 33.70 | 33.08 | 29.60 | 8.0 | ||
| Direct sowing (flooded) | 40.22 | 35.19 | 28.53 | 28.14 | 7.80 | ||
| Overall | 41.25 | 34.65 | 32.09 | 27.79 | 7.8 | ||
| ANOVA results | |||||||
| Year | - | *** | *** | *** | *** | *** | |
| Cultivar | - | *** | *** | *** | *** | *** | |
| Planting method | - | *** | *** | *** | *** | *** | |
| Year × Cultivar | - | *** | *** | *** | *** | *** | |
| Year × Planting method | - | ** | *** | ** | *** | *** | |
| Cultivar × Planting method | - | ** | *** | ** | *** | *** | |
| Year × Cultivar × Planting method | - | * | ns | ns | ** | ns | |
| Regression coefficients a | ANOVA results | |||||
| Intercept | ×SPAD | |||||
| Main effect | 0.701 | 0.222 | *** | Intercept | ns | |
| SPAD | *** | |||||
| Year | Year | *** | ||||
| 2018 | 0.000 | 0.000 | Year × SPAD | *** | ||
| 2019 | 3.328 | *** | −0.125 | *** | ||
| 2020 | −0.326 | −0.017 | ||||
| 2021 | 1.767 | −0.066 | ||||
| Cultivar | Cultivar | ** | ||||
| Hitomebore | 0.000 | 0.000 | Cultivar × SPAD | ** | ||
| Manamusume | 0.118 | 0.012 | ||||
| Datemasayume | 8.243 | ** | −0.245 | ** | ||
| Planting method | Planting method | ** | ||||
| Transplanting | 0.000 | 0.000 | Planting method × SPAD | ** | ||
| Transplanting (dense) | 1.068 | −0.040 | ||||
| Direct sowing (flooded) | 1.564 | −0.005 | ||||
| Direct sowing | −3.661 | * | 0.113 | * | ||
| R2 | 0.662 | *** | ||||
| Regression Coefficients a | ANOVA Results | |||||
|---|---|---|---|---|---|---|
| Intercept | x SPAD | |||||
| main effect | 4.695 | ** | 0.117 | * | Intercept | ** |
| SPAD | * | |||||
| Year | Year | ns | ||||
| 2018 | 0.000 | 0.000 | Year × SPAD | ns | ||
| 2019 | −1.262 | 0.020 | ||||
| 2020 | −0.774 | 0.016 | ||||
| 2021 | −1.457 | 0.044 | ||||
| Cultivar | Cultivar | ns | ||||
| Hitomebore | 0.000 | 0.000 | Cultivar × SPAD | ns | ||
| Manamusume | −0.896 | 0.037 | ||||
| Datemasayume | 0.162 | −0.001 | ||||
| Planting method | Planting method | * | ||||
| Transplanting | 0.000 | 0.000 | Planting method × SPAD | ns | ||
| Transplanting (dense) | 2.368 | −0.070 | ||||
| Direct sowing (flooded) | −1.013 | 0.033 | ||||
| Direct sowing | −1.311 | 0.032 | ||||
| R2 | 0.683 | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Hashimoto, N.; Saito, Y.; Obara, K.; Ishibashi, T.; Ito, R.; Yamamoto, S.; Maki, M.; Homma, K. Validation of Relation between SPAD and Rice Grain Protein Content in Farmer Fields in the Coastal Area of Sendai, Japan. AgriEngineering 2023, 5, 369-379. https://doi.org/10.3390/agriengineering5010024
Zhang L, Hashimoto N, Saito Y, Obara K, Ishibashi T, Ito R, Yamamoto S, Maki M, Homma K. Validation of Relation between SPAD and Rice Grain Protein Content in Farmer Fields in the Coastal Area of Sendai, Japan. AgriEngineering. 2023; 5(1):369-379. https://doi.org/10.3390/agriengineering5010024
Chicago/Turabian StyleZhang, Lina, Naoyuki Hashimoto, Yuki Saito, Kasumi Obara, Taro Ishibashi, Ruito Ito, Shuhei Yamamoto, Masayasu Maki, and Koki Homma. 2023. "Validation of Relation between SPAD and Rice Grain Protein Content in Farmer Fields in the Coastal Area of Sendai, Japan" AgriEngineering 5, no. 1: 369-379. https://doi.org/10.3390/agriengineering5010024
APA StyleZhang, L., Hashimoto, N., Saito, Y., Obara, K., Ishibashi, T., Ito, R., Yamamoto, S., Maki, M., & Homma, K. (2023). Validation of Relation between SPAD and Rice Grain Protein Content in Farmer Fields in the Coastal Area of Sendai, Japan. AgriEngineering, 5(1), 369-379. https://doi.org/10.3390/agriengineering5010024








