Abstract
Significant losses in agricultural production are due to abiotic stresses, such as herbicide phytotoxicity. Dicamba (diglycolamine salt) is a herbicide used for post-emergent control of broadleaf weeds. It has a possibility to vapor-spread into neighboring fields causing damage to other crops. However, not every stress can be easily identified. Therefore, remote sensing has the potential as a new tool in early injury detection. This study evaluated the effects of simulated dicamba drift on the occurrence of phytotoxicity in soybeans (Glycine max). Soybean was assessed in seven dicamba doses (0, 0.056, 0.56, 5.6, 11.2, 28, 112 g ha−1) for changes in plant injury (scale of notes), spectral aspects (triangular greenness index (TGI), and shoot dry mass. The plants were photographed using a digital camera positioned at 1.2 m above the planting media level. The results indicate a positive effect of low dicamba doses (0.56 and 0.056 g a.e. ha−1) on TGI canopy distinction and shoot dry mass. Soybean TGI canopy distinction and the injury scale estimated at 45 days after sowing, and the soybean shoot dry mass observed at 99 days after sowing, presented significant and moderate Pearson’s r coefficient of correlations (r = −0.609 and 0.625), indicating TGI as a valid and practical spectral index for plant dicamba-injured evaluations.
1. Introduction
Herbicide technology is widely used in agriculture due to its characteristics, such as control efficiency and environmental security when properly applied and a broad control spectrum. This technology has been singly used for many years [1,2]. However, the exclusive use of herbicides with only a single mode of action (e.g., glyphosate) has led to the development of herbicide resistance in some regions and weed species [3]. This situation has become of severe concern to growers due to the lowering efficiency of herbicide control.
The development of crop resistance to multiple herbicides can be an option for integrated weed management since herbicide applications can be rotated. Crop breeding focuses on the development of crops resistant to herbicides that are plant growth regulators, such as 2,4-dichlorophenoxyacetic acid (2.4-D) and dicamba, which can also assist programs of integrated weed management [4].
In this context, at least 339 weeds are resistant to glyphosate globally, and only 19 are resistant to dicamba [5]. This herbicide is a synthetic auxin (3,6-dichloro-2-methoxybenzoic acid) that has been used in the control of dicot weeds (broadleaf plants) in many crops [6]. Dicamba-resistant soybean (Glycine max (L.) Merrill) allows the use of this herbicide to control dicot weeds that are hard to control, including those resistant to glyphosate [7].
Soybean is an important and strategic crop for protein production used as animal feed and for human consumption. Moreover, soybean is a significant source of five essential fatty acids for humans [8]. Brazil is a main soybean producer globally, harvesting about 123 million tons per year [9]. This great grain production is a consequence of combining factors, such as genetically improved cultivars, crop management, and the use of modern technologies. However, even with the most advanced techniques applied for crops, agricultural production is constantly subjected to stresses that can reduce grain yield.
One of the main causes of yield reduction is the occurrence of diseases in plants, biological (biotic diseases) or edaphoclimatic (abiotic diseases). Biological diseases are those caused by plant pathogenic microorganisms [10]. In contrast, diseases caused by edaphoclimatic conditions are those that are not caused by microorganisms and are fundamentally due to nutritional disturbances (excesses or deficiencies), insolation, extremes of soil pH, temperature, water restriction, salinity, and the phytotoxicity caused by chemical products (e.g., physiological disorders caused by herbicide drift) [11].
A consequence of the increased number of dicamba applications on tolerant plants was the appearance of phytotoxic symptoms (abiotic disease) in herbicide-susceptible crops located close to the applied area. It is estimated that 1.4 million hectares of soybean cultivated in 2018 were damaged by physical drift, volatility, and spray tank contamination from dicamba applications [12], as non-tolerant soybeans are very susceptible to this herbicide. The meta-analysis presented by Kniss [13] estimated that the dicamba dose required for reducing soybean yield by 5% was 1.9 g e.a. ha−1 for V1-V3 soybean phenological stage.
The damage of dicamba in soybean is already well documented, mainly in the USA. However, this assessment is usually performed using visual scales 14 days after dicamba application (or drift contamination), when damage symptoms can be better visualized [14]. However, this is an extensive period for crop management regarding decisions and procedures aiming to reduce the damage caused to soybeans. In this sense, image analysis of images shows great potential. Additionally, Foster et al. [15] observed that the visual injury was not a proper parameter to estimate soybean yield losses since it is variable and subjective to characterization.
However, the detection of stressed plants can be performed accurately and precisely through images composed of several bands of the electromagnetic spectrum. These bands are used to arithmetically calculate spectral indices of vegetation capable of expressing early physiological and morphological alterations in the vegetation analyzed [16]. These indexes can improve the agronomic decisions by (i) distinguishing vegetation from the other targets on the surface, (ii) indicating physiological processes, (iii) identifying vegetation vigor and health, and (iv) discriminating healthy vegetation from vegetation under stressed conditions [17,18].
Spectral vegetation indices allow the monitoring of plant stresses through the symptoms observed in the shoots, as well as the identification of underlying phytotoxicity (not visible to the bare eye) caused by dicamba vapor drift. This early identification of stresses in soybeans caused by dicamba herbicide might help in the management of this yield-reducing factor. Most spectral vegetation indexes, such as the normalized difference vegetation index (NDVI), the soil adjusted vegetation index (SAVI), and the atmospherically resistant vegetation index (ARVI), are calculated using ratios or normalized differences of two or three electromagnetic spectrum bands [19,20].
Broge and Leblanc [21] proposed the triangular vegetation index (TVI) based on the area of a triangle with vertices at the green, red, and near infrared (NIR) wavelengths, which are sensitive to both chlorophyll content and leaf area index. It utilized the area embraced by the green peak, the chlorophyll absorption minimum, and the NIR shoulder. However, red-edge bands are generally not available on low-cost multispectral sensors, which have broad bands at visible wavelengths. Therefore, a visible-band index called the triangular greenness index (TGI) was developed [22]. Hunt et al. [23] also demonstrated that TGI was among the best spectral indexes, including those using the red-edge bands.
Since drift contamination (phytotoxicity) caused by dicamba affects leaf chlorophyll content [24], it is hypothesized that TGI—a chlorophyll sensitive index—could indicate the occurrence of this physiological disorder, which is a new way to detect injuries early. Therefore, this study evaluated the application of dicamba herbicide and the occurrence and intensity of underlying phytotoxicity through biometric parameters (damage scale and shoot dry mass) and vegetation spectral index (TGI) in soybean.
2. Materials and Methods
2.1. Experimental Area and Soybean Cultivar
The experiment was implemented in a greenhouse covered with clear low-density polyethylene (LDPE) and sides protected with a thin mesh screen at the Universidade Federal de Uberlândia, Brazil, located at 18°53.00″ S, 48°15.25″ W, at 938 m above sea level. The climate of the region is Aw (tropical with dry winter and rainy summer), by Koppen’s Climate Classification [25].
The soybean cultivar evaluated was M7198 IPRO (MONSOY®). Four seeds were sown per pot (4 L capacity, with four 1-cm diameter holes in the bottom) in October 2019. Nine days after sowing, the seedlings were thinned to two per pot. Average air temperature varied between 25 and 29 °C (298 and 302 K), and maximum daily temperatures were below 34 °C (307 K) during the experiment (Weatherspark.Com accessed on 20 June 2021).
2.2. Planting Media and Fertilization
The planting media (substrate) used for growing soybean was a compost prepared with subsoil and sand (1:1 volume), homogenized, and sieved through a 0.4-cm mesh. Texture analysis of the planting media indicated 847, 8, and 145 g kg−1 of sand, silt, and clay, respectively. Approximately 4 kg of the planting media were used to fill each pot lined with filter paper (250 g m−2). Nematological analysis of the planting media did not indicate the presence of any plant-parasitic nematodes.
Ten days before sowing, a dose corresponding to 500 kg ha−1 of 20-5-15 N-P-K (20% of N, 5% of P2O5, 15% of K2O) fertilizer, and 1100 kg ha−1 dolomitic lime were mixed with the planting media, filled the pots, and watered until saturation. Five days before sowing and every 15 days after sowing, 30 mL of a nutrient solution containing macro and micronutrients (N, P, K, Ca, Mg, S: 15, 1, 6, 5, 2, 2 mmol L−1, respectively; B, Cu, Fe, Mn, Mo, Zn: 46, 0.3, 90, 12.6, 0.1, 1.3 μmol L−1, respectively) [26] were added to each pot. The pots were maintained over plates to retain excess solution and were watered daily to maintain moisture, but not soaked.
2.3. Herbicide Treatments
The herbicide treatments were applied 40 days after sowing using a handheld boom and a CO2-pressurized backpack sprayer. The equipment was calibrated to deliver a spray volume of 150 L ha−1 at 200 kPa, with AIXR110015 nozzles (TeeJet® Technologies, Weathon, USA).
To simulate herbicide drift, decreasing doses of dicamba (diglycolamine salt, Atectra®, BASF, Guaratinguetá, Brazil) were applied at 1/5× (112 g a.e. ha−1 dicamba), 1/20× (28 g a.e. ha−1 dicamba), 1/50× (11.2 g a.e. ha−1 dicamba), 1/100× (5.6 g a.e. ha−1 dicamba), 1/1000× (0.56 g a.e. ha−1 dicamba), and at 1/10,000× (0.056 g a.e. ha−1 dicamba) of the recommended rate (560 g a.e. ha−1 dicamba), in addition to a “zero dose” (0 g a.e. ha−1 dicamba) or a control treatment. Each treatment was implemented with 12 replications (n = 84 parcels or pots).
2.4. Evaluations
Each parcel (pot) was photographed once from the top at 45 days after sowing (DAS) (5 days after dicamba application) (beginning of flowering), during a clear day (no clouds), between 10:00 and 13:00 h inside the greenhouse covered with LDPE. An easily accessible tool (digital camera) was used from a Motorola XT1965-2 G7 Plus (RGB—red-green-blue camera with 4608 × 3456 pixel resolution) mobile phone, which was positioned at 1.2 m above the planting media level and fixed horizontally flat over the soybeans plant canopy.
Despite the relative homogeneity of light availability during the plant evaluation (capturing the photos), the histogram of the RGB images was equalized using the program Photoshop Lightroom® v.5.7 (adobe.com accessed on 20 January 2017). Figure 1 shows an example of an equalized histogram from a control plant (0 g a.e. ha−1 dicamba) and a dicamba affected plant. This procedure was intended to reduce the effects of any variation on natural lighting conditions over each of the 84 photographs captured.
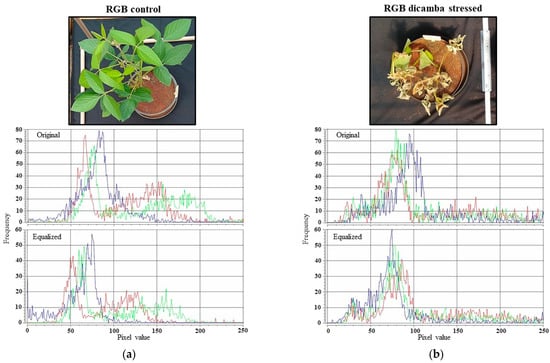
Figure 1.
Equalized histogram of a RGD image of a control plant (a) and an injured plant (b) by simulated dicamba drift.
Equalized images were used for the composition of the images based on the vegetation spectral index TGI (Equation (1)) [22,23] using the program ENVI® v.5.5 (envi.com.br accessed on 1 January 2018), as follows:
where TGI is the triangular greenness index; RGREEN is the reflectance measured in the spectral range of green; RRED is the reflectance measured in the spectral range of red; and RBLUE is the reflectance measured in the spectral range of blue.
The TGI images of the soybean shoots were classified in five distinct colors using Quant® (Viçosa, Brazil) [27], a widely used software [28], which also allows the quantification of the image relative areas (cm2) of the resulting colors.
The resulting colors of the images were grouped into five distinct sets, which allow the distinction of at least two shades of green on each plant leaf. Therefore, according to discriminant analysis, the predominant green color of the soybean leaflet’s blade was quantified (Quant®) and used as a reference for leaf effective integrity and activity across dicamba treatments. Image treatment procedure and results obtained are demonstrated in Figure 2.
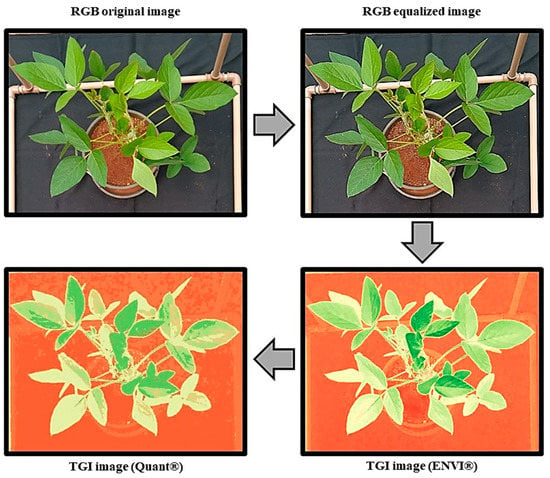
Figure 2.
The sequence of image composition from the original image to estimate soybean canopy area (cm2) of the predominant green shade (dark-green), expressed by the vegetation triangular greenness index (TGI). It can be observed that image treatment removes the background colors that are not plant-related. RGB: Red, green, blue.
On the same day of plant photography, visual evaluations of soybean injury caused by the dicamba treatments was performed and based on a scale proposed by Behrens and Lueschen [29], as follows:
- 0%—no effect, normal plant;
- 10%—slight crinkle of leaflets of the terminal leaf;
- 20%—cupping of terminal leaflets, slight crinkle of leaflets of the second leaf, growth rate normal;
- 30%—leaflets of two terminal leaves cupped, expansion of terminal leaf suppressed slightly;
- 40%—malformation and growth suppression of two terminal leaves, terminal leaf size less than one-half of control plants. New axillary leaves developing at a substantially reduced rate;
- 50%—no expansion of terminal leaf, second leaf size one-half of control plants. Axillary leaf buds unable to open and develop;
- 60%—slight terminal growth, necrosis of terminal leaf, and axillary bud apparent, chlorosis, and necrosis in axillary leaf clusters;
- 70%—terminal bud dead, substantial, strongly malformed axillary shoot growth;
- 80%—limited axillary shoot growth, leaves present at the time of treatment chlorotic with slight necrosis;
- 90%—plant dying, leaves mostly necrotic;
- 100%—dead plant.
Leaves and the remaining parts of the shoots of the plants were harvested at 99 DAS from each pot and dried in a forced-air oven at 68 °C for 96 h to determine total shoot dry mass. Correlations between the evaluated variables were computed to determine whether there was a linear relation between them [30].
2.5. Statistical Analysis
Extreme values (outliers) in the data of each variable studied were identified by boxplot graphs of the residues [31] generated in the SPSS Statistics® software (ibm.com accessed on 23 March 2013). The identified outliers were treated as lost parcels and replaced by values estimated using the method of minimum sum of residue squares. Then, for each outlier (lost parcel) replaced, one (1) degree-of-freedom was removed from the experimental error in the subsequent analysis of variance (ANOVA—F-test) [32].
SPSS Statistics® software was also used to confirm the normality of residue distribution by Shapiro-Wilk (basic assumptions for the ANOVA, p > 0.01) and Pearson’s correlation coefficients between variables. It is fundamental that the data are normally distributed and with no outliers to avoid errors associated with computation and interpretation of Pearson’s correlation [33].
Subsequent to the confirmation of the assumptions or data transformation by ‘√x + 1′ (square root of ‘x’ added to one, with ‘x’ as the original value of the respective parcel), the analysis of variance (F-test) was performed, and, when significant differences were observed among treatments (dicamba doses), regressions were analyzed (0.05 significance and R2 > 70%). These analyses were performed using SISVAR® statistical program (des.ufla.br accessed on 8 June 2017). Graphs were generated by Sigma Plot® v.12 software (sigmaplot.co.uk accessed on 13 January 2011).
3. Results
3.1. Analysis of Variance
Significant differences (p < 0.05) were found for the herbicide treatments (dicamba doses) on soybean responses—injury scale, TGI (cm2) at 45 DAS, and shoot dry mass (g) at 99 DAS—(Table 1).

Table 1.
Statistic of the normality of residues, analysis of variance (F-test statistic), and regressions of the variables evaluated for soybean treated with dicamba herbicide.
3.2. Triangular Greenness Index
The distinctive color of the leaf blade highlighted by the TGI spectral index represented the relative health plant tissue area (cm2) on the soybean canopy and is significantly associated with the dicamba dose applied (Figure 3). As dicamba dose increased, healthy leaf area on soybean canopy consistently decreased and followed a quadratic model (second-order regression) with a high prediction (R2 = 85.0%).
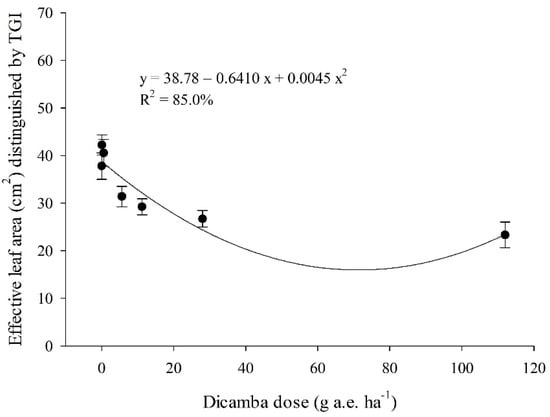
Figure 3.
Effective soybean leaf area (cm2) shown by the triangular greenness index (TGI) and estimated by the Quant® software at 5 days after the application of the dicamba doses (0, 0.056, 0.56, 5.6, 11.2, 28, 112 g.e. ha−1). Error lines in each point denote standard deviation.
The lowest dicamba doses of 0.056 and 0.56 g a.e. ha−1 presented slightly superior TGI canopy color distinction compared to the control treatment (0 g a.e. ha−1 of dicamba).
3.3. Injury Scale
The non-occurrence of normality of residue distribution for injury data on soybean—even after replacing the outliers—indicated the need for data transformation. However, data transformation did not change the result for normality of residues of the soybean injury scale data.
The regression analysis of soybean injury scale by the dicamba doses indicated that dicamba doses cause a significant effect on leaf injury. This response was highly predictable (R2 = 98.6%) using a second-order regression model (Figure 4).
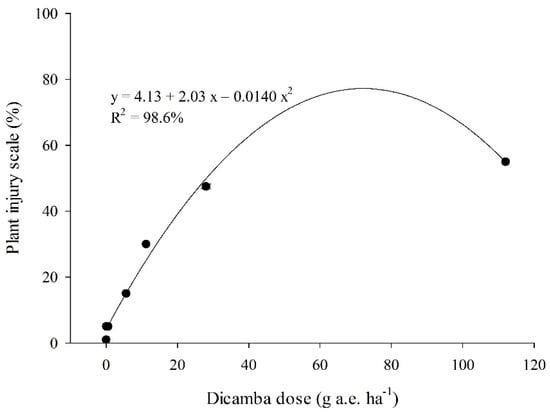
Figure 4.
Soybean injury scale for different doses of dicamba at 5 days after application of the dicamba doses (0, 0.056, 0.56, 5.6, 11.2, 28, 112 g.e. ha−1). Error bars in each point denote standard deviation.
3.4. Shoot Mass
Similar to TGI canopy color distinction, soybean shoot dry mass also reduced with increasing dicamba doses, following a quadratic model (second-order regression) with a high prediction (R2 = 94.8%). According to the regression model, no shoot dry mass will be observed for dicamba doses above 70.1 g a.e. ha−1, as it only happened with the dicamba dose of 112 g a.e. ha−1, but not the dose of 28 g a.e. ha−1.
The lowest dicamba doses of 0.056 and 0.56 g a.e. ha−1 also presented a peculiar greater shoot dry mass response compared to the control group (0 g a.e. ha−1 of dicamba) (Figure 5).
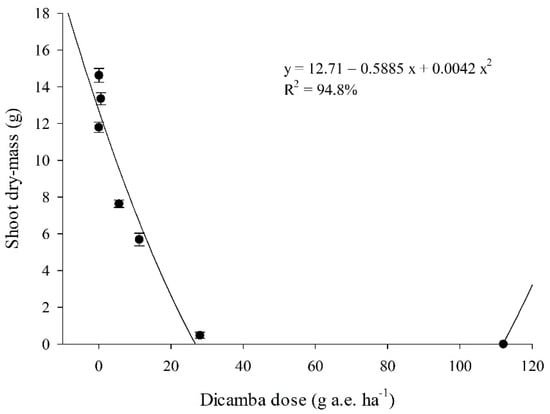
Figure 5.
Soybean shoot mass (g) for different doses of dicamba at 99 days after soybean sowing. Error lines in each point denote standard deviation.
3.5. Correlations
Pearson’s correlation coefficient (r) expresses the association of quantitative variables described by a linear function, which is calculated by the reason between the grouped variance (covariance) of the two variables and the product of their respective standard deviations.
To increase precision of the data computation and the interpretation of Pearson’s correlation, it is important that the data present normally distributed residues and that the data outliers (extreme values) be removed [33]. The coefficients of correlation between all pairs of variables evaluated in this study are presented in Table 2.

Table 2.
Pearson’s correlation (r) between the variables studied.
Soybean injury scale and TGI estimated at 45 days after sowing, and the soybean shoot dry mass observed at 99 days after sowing presented significant Pearson’s r coefficient (p < 0.01). Pearson’s coefficients observed between the variables are considered moderate (0.5 to 0.7) to very strong (>0.9) correlations, according to the scale proposed by Callegari-Jacques [34].
Soybean injury scale and shoot dry mass data dispersion according to dicamba doses are presented in Figure 6.
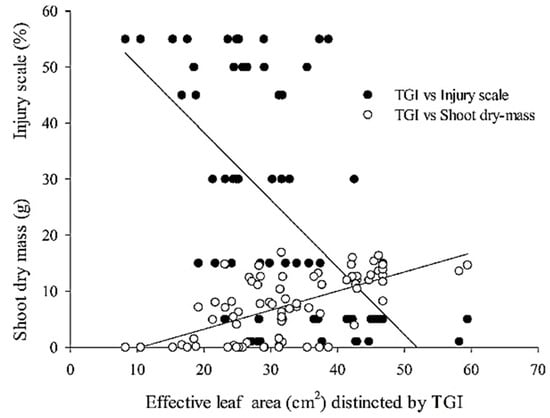
Figure 6.
Scatterplot between triangular greenness index (TGI) and soybean injury scale at 45 days after sowing and soybean shoot dry mass at 99 days after sowing.
The responses observed also indicated how TGI soybean canopy color distinction correlates to soybean shoot dry mass (positively skewed) and to the injury scale (negatively skewed) for soybean affected by dicamba herbicide.
4. Discussion
The dicamba injury rating scale proposed by Behrens and Lueschen [29] and still used in other studies [35,36] was well correlated to dicamba doses evaluated, to soybean shoot dry mass, and to canopy area highlighted by the TGI index in the present study. Therefore, the correlations of soybean quantified response (injury scale, shoot dry mass) with the TGI index were significant (p < 0.01) and expressive [34].
TGI index can be used to predict soybean plant injury and shoot dry mass. This trend can be observed in the dispersion graphs (scatter plots), where soybean plant injury increases with corresponding decreases in quantified TGI responses (cm2) and decreased TGI observations resulting in low soybean shoot dry mass. This index development was based on the triangle outlining the spectral plant signature for the wavelengths red (R), green (G), and blue (B) [22,23], and it is very sensitive to leaf chlorophyll content.
An accessible tool (digital camera) and the fast application of a RGB vegetation index can be used to evaluate soybean shoots affected by dicamba vapor drift. Applying a spectral vegetation index can allow early distinction of healthy vegetation from stressed one [37]. The present study made it possible to confirm the difference 5 days after dicamba application. Results found by Huang et al. [38] indicated that it is a challenge to clearly differentiate dicamba dose-responses in soybean using spectral index evaluations within the first days after plant intoxication. This report demonstrates the importance of procedures and results presented in the current study. It is important to note that these authors used a spectroradiometer; while, in the present study, a simple digital camera was used.
Spectral indices of vegetation evaluate plants allowing early acquisition of data on plant development, physiology, nutrition, and health status, directing to a proper indication of crop management for situations, such as early identification of phytotoxicity [37]. This tool has the potential to aid in decision-making based on precise information regarding crop conditions, leading to better chances of success of the management strategy adopted, thus reducing yield losses and environmental impacts due to agricultural activity.
The quantified (cm2) response of soybean shoots by TGI and shoot dry mass for 0.56 and 0.056 g a.e. ha−1 of dicamba were greater than the results observed for the control treatment (0 g a.e. ha−1 dicamba); the plant responses decreased for doses equal or greater than 5.6 g a.e ha−1 of dicamba, similar to the observations reported by Al-Khatib and Peterson [39]. Silva et al. [40] observed that 3.7 g a.e ha−1 of dicamba, applied in soybean at V5 or R2 phenological stage, was enough to cause significant decreases in plant height, plant injury (%), and yield (kg ha−1). Foster and Griffin [15] observed that 0.6 g a.e. ha−1 dicamba reduced soybean stem height at V3-V4 phenological stage and increased the number of lateral branches and lateral branch pod production. However, the dose did not affect total seed production or yield—similar to the control 0 g a.e. ha−1 dicamba.
The studies of Weidenhamer et al. [41] and Robinson et al. [42], reported greater soybean yield when low dicamba doses were applied, suggesting that an hormesis response was possible. In contrast, according to Kniss [13], the current published information presents little evidence to support significant increases in soybean yield at low dicamba doses.
The results observed in the present study indicated that an effect of low doses of dicamba on plant physiology and morphology occurred. However, to determine if a hormesis effect does exist at low dicamba doses on soybean performance, future studies should include low and very low dicamba doses and the evaluation of physiological responses, such as chlorophyll contents and activity. Many studies have presented results indicating that chlorophylls are a good parameter for herbicide injury evaluations, especially herbicides with auxinic effects, such as dicamba [43,44].
The present study was carefully implemented with 12 replications to observe how dicamba drift can affect soybean development and how dicamba effects could be tracked on-field using an unmanned aerial vehicle (UAV). The studies including plant responses to field accidental dicamba drift and plant responses to low dicamba doses, meet recent concerns regarding the upraised uses of dicamba in modern agriculture [45].
As the development of dicamba-resistant biotechnology proceeds, more research is needed to understand the impacts of low dicamba doses from vapor drift on crops and non-target crops. Although dicamba drift should be avoided at all costs [36], crop plants with resistance to dicamba will bring new herbicide resistance technologies and can extend the useful lifetime of many other technologies to control weeds. These technologies can be used jointly and can be supported by information from injury monitoring using vegetation spectral indexes, such as TGI.
5. Conclusions
TGI index can be used to predict soybean plant injury caused by dicamba herbicide. A spectral vegetation index can allow early distinction of healthy vegetation from stressed ones to aid in decision-making based on precise information regarding crop conditions. This leads to better chances of success of management strategies, thus reducing yield losses and environmental impacts due to agricultural activity.
Author Contributions
Conceptualization, E.M.L., L.C. and J.P.A.R.d.C.; investigation, A.d.S.O., M.G.M. and F.M.A.d.N.; methodology, E.M.L. and S.L.d.A.; project administration, A.d.S.O., M.G.M. and F.M.A.d.N.; resources, L.C. and J.P.A.R.d.C.; fund acquisition, J.P.A.R.d.C.; supervision, E.M.L.; writing—original draft, E.M.L.; writing—review and editing, E.M.L., L.C. and J.P.A.R.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, J.M.; Owen, M.D. Herbicide-resistant crops: Utilities and limitations for herbicide-resistant weed management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M. The rise and future of glyphosate and glyphosate-resistant crops: Glyphosate and glyphosate-resistant crops. Pest Manag. Sci. 2018, 74, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Clay, S.A. Near-term challenges for global agriculture: Herbicide-resistant weeds. Agron. J. 2021, 113, 4463–4472. [Google Scholar] [CrossRef]
- Service, R.F. What happens when weed killers stop killing? Science 2013, 341, 1329. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Herbicide-Resistant Weed Database. 2022. Available online: http://www.weedscience.com (accessed on 10 January 2022).
- Egan, J.F.; Mortensen, D.A. Quantifying vapor drift of dicamba herbicides applied to soybean. Environ. Toxicol. Chem. 2012, 31, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Byker, H.P.; Soltani, N.; Robinson, D.E.; Tardif, F.J.; Lawton, M.B.; Sikkema, P.H. Control of glyphosate-resistant horseweed (Conyza canadensis) with dicamba applied preplant and postemergence in dicamba-resistant soybean. Weed Technol. 2013, 27, 492–496. [Google Scholar] [CrossRef]
- Hamurcu, M.; Arslan, D.; Hakki, E.E.; Ozcan, M.M.; Pandey, A.; Khan, M.K.; Gezgin, S. Boron application affecting the yield and fatty acid composition of soybean genotypes. Plant Soil Environ. 2019, 65, 238–243. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento (Conab). Acompanhamento da Safra Brasileira de Grãos. 6 Levantamento, Março 2022. 2022. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 6 April 2022).
- Lemes, E.; Castro, L.; Assis, R. Doenças da Soja: Melhoramento Genético e Técnicas de Manejo; Editora Milenium: Campinas, Brazil, 2015. [Google Scholar]
- Ogle, H.J. Abiotic diseases of plants. In Plant Pathogens and Plant Diseases; Brown, J.F., Ogle, H.J., Eds.; University of New England Printery: Armidale, NSW, Australia, 1997; pp. 156–171. [Google Scholar]
- USDA. The Use of Genetically Engineered Dicamba-Tolerant Soybean Seeds has Increased Quickly, Benefiting Adopters but Damaging Crops in Some Fields. 2019. Available online: https://www.ers.usda.gov/amber-waves/2019/october/the-use-of-genetically-engineered-dicamba-tolerant-soybean-seeds-has-increased-quickly-benefiting-adopters-but-damaging-crops-in-some-fields/ (accessed on 1 August 2022).
- Kniss, A.R. Soybean response to dicamba: A meta-analysis. Weed Technol. 2018, 32, 507–512. [Google Scholar] [CrossRef]
- Egan, J.F.; Barlow, K.M.; Mortesen, D.A. A meta-analysis on the effects of 2,4-d and dicamba drift on soybean and cotton. Weed Sci. 2014, 62, 193–206. [Google Scholar] [CrossRef]
- Foster, M.R.; Griffin, J.L. Changes in soybean yield components in response to dicamba. Agrosyst. Geosci. Environ. Agrosyst. 2019, 2, 190026. [Google Scholar] [CrossRef] [Green Version]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1978, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.G.; Cunha, J.P.A.R.; Lemes, E.M. Dicamba injury on soybean assessed visually and with spectral vegetation index. AgriEngineering 2021, 3, 240–250. [Google Scholar] [CrossRef]
- Polivova, M.; Brook, A. Detailed investigation of spectral vegetation indices for fine field-scale phenotyping. In Vegetation Index and Dynamics; Carmona, E.C., Ortiz, A.C., Canas, R.Q., Musarella, C.M., Eds.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/76442 (accessed on 12 May 2022).
- Bhagat, V.S.; Kadam, A.; Kumar, S. Analysis of remote sensing based vegetation indices (VIs) for unmanned aerial system (UAS): A review. Remote Sens. Land. 2019, 3, 58–73. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and future of plant stress detection: An overview from remote sensing to positron emission tomography. Front. Plant Sci. 2021, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Broge, N.H.; Leblanc, E. Comparing predictive power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Hunt, E.R.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote sensing leaf chlorophyll content using a visible band index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Christoffoleti, P.J.; Figueiredo, M.R.A.; Peres, L.E.P.; Nissen, S.; Gaines, T. Auxinic herbicides mechanisms of action and weed resistance: A look into recent plant science advances. Sci. Agric. 2015, 72, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes, G.; Leonardo, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Tuite, J. Plant Pathological Methods: Fungi and Bacteria; Burgess Publishing: Minneapolis, MN, USA, 1969. [Google Scholar]
- Vale, F.X.R.; Filho, E.F.; Liberato, J.R. Quant—Quantificação de Doenças; Editora UFV: Viçosa, Brazil, 2001. [Google Scholar]
- Del Ponte, E.M.; Pethybridge, S.J.; Bock, C.H.; Michereff, S.J.; Machado, F.J.; Spolti, P. Standard area diagrams for aiding severity estimation: Scientometrics, pathosystems and methodological trends in the last 25 years. Phytopathology 2017, 107, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Behrens, R.; Lueschen, W.E. Dicamba volatility. Weed Sci. 1979, 27, 486–493. [Google Scholar] [CrossRef]
- Pearson, K. The grammar of science. Nature 1982, 46, 199–200. [Google Scholar] [CrossRef]
- Chambers, J.M.; Cleveland, W.S.; Kleiner, B.; Tukey, P.A. Graphical Methods for Data Analysis; Wadsworth and Brooks/Cole: Belmont, CA, USA, 1983. [Google Scholar]
- Pimentel-Gomes, F.; Garcia, C.H. Estatística Aplicada a Experimentos Agronômicos e Florestais: Exposição Com Exemplos e Orientações Para Uso de Aplicativos; FEALQ Editora: Piracicaba, Brazil, 2002. [Google Scholar]
- Filho, D.B.F.; Silva, J.A., Jr. Desvendando os mistérios do coeficiente de correlação de Pearson (r). R. Política Hoje 2009, 18, 1–33. [Google Scholar]
- Callegari-Jacques, S.M. Bioestatística: Princípios e Aplicações; Artmed Editora: Porto Alegre, Brazil, 2009. [Google Scholar]
- Andersen, S.M.; Clay, S.A.; Wrage, L.J.; Matthees, D. Soybean foliage residues of dicamba and 24-D and correlation to application rates and yield. Agron. J. 2004, 96, 750–760. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Scott, J.E.; Knezevic, S.Z. Glyphosate-resistant soybean response to micro-rates of three dicamba-based herbicides. Agrosyst. Geosci. Environ. 2019, 2, 180052. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.K. Plant disease detection by imaging sensors—Parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yuan, L.; Reddy, K.N.; Zhang, J. In-situ plant hyperspectral sensing for early detection of soybean injury from dicamba. Biosyst. Eng. 2016, 149, 51–59. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Peterson, D. Soybean (Glycine max) response to simulated drift from selected sulfonylurea herbicides, dicamba, glyphosate, and glufosinate. Weed Technol. 1999, 13, 264–270. [Google Scholar] [CrossRef]
- Silva, D.R.O.; Silva, E.D.N.; Aguiar, A.C.M.; Novello, B.D.; Silva, A.A.; Basso, C.J. Drift of 2,4-D and dicamba applied to soybean at vegetative and reproductive growth stage. Cienc. Rural. 2018, 48, e20180179. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Triplett, G.B.; Sobotka, F.E. Dicamba injury to soybean. Agron. J. 1989, 81, 637–643. [Google Scholar] [CrossRef]
- Robinson, A.P.; Simpson, D.M.; Johnson, W.G. Response of glyphosate-tolerant soybean yield components to dicamba exposure. Weed Sci. 2013, 61, 526–536. [Google Scholar] [CrossRef]
- Weber, J.; Kunz, C.; Peteinatos, G.; Santel, H.J.; Gerhards, R. Utilization of chlorophyll fluorescence imaging technology to detect plant injury by herbicides in sugar beet and soybean. Weed Technol. 2017, 31, 523–535. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Weber, J.F.; Gerhards, R. Early identification of herbicide stress in soybean (Glycine max (L.) Merr.) using chlorophyll fluorescence imaging technology. Sensors 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gage, K.L.; Krausz, R.F.; Walters, S.A. Emerging challenges for weed management in herbicide-resistant crops. Agriculture 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).