Neuromuscular Control in Postural Stability: Insights into Myoelectric Activity Involved in Postural Sway During Bipedal Balance Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment
2.3. Experimental Procedure
2.4. Data Analysis
2.4.1. EMG and COP Data Pre-Processing
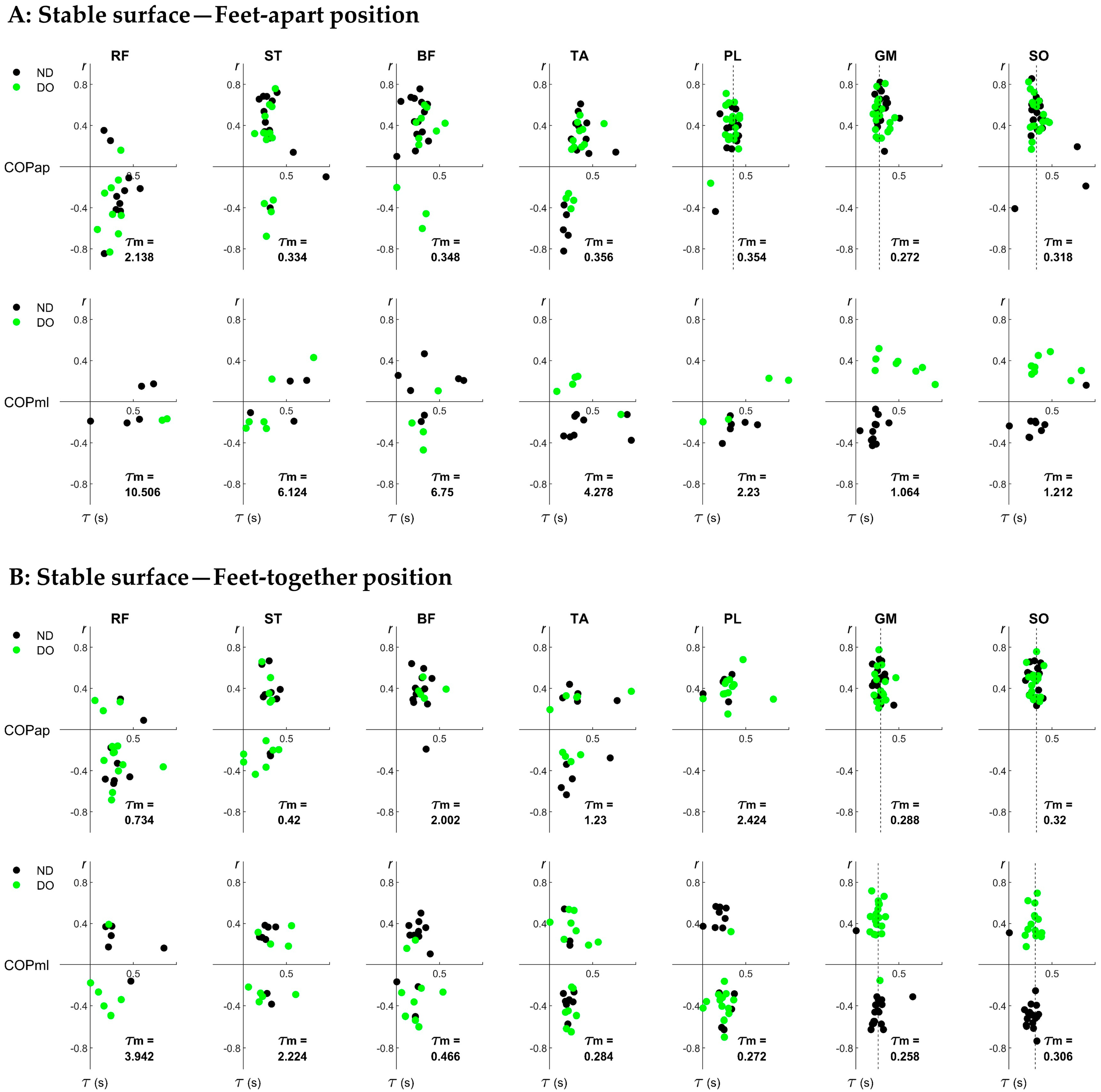
2.4.2. Determining the EMG–COP Coordination
2.5. Statistical Analysis
3. Results
3.1. Overview of EMG–COP Correlation
3.2. Individual Pairs of EMG–COP Correlations
4. Discussion
Limitations and Future Research
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamoorthy, V.; Goodman, S.; Zatsiorsky, V.; Latash, M.L. Muscle Synergies during Shifts of the Center of Pressure by Standing Persons: Identification of Muscle Modes. Biol. Cybern. 2003, 89, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zech, A.; Hübscher, M.; Vogt, L.; Banzer, W.; Hänsel, F.; Pfeifer, K. Balance Training for Neuromuscular Control and Performance Enhancement: A Systematic Review. J. Athl. Train. 2010, 45, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Horlings, C.G.; van Engelen, B.G.; Allum, J.H.; Bloem, B.R. A Weak Balance: The Contribution of Muscle Weakness to Postural Instability and Falls. Nat. Clin. Pract. Neurol. 2008, 4, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Freitas, S. Revision of Posturography Based on Force Plate for Balance Evaluation. Rev. Bras. Fisioter. 2010, 14, 183–192. [Google Scholar] [CrossRef]
- Quijoux, F.; Nicolaï, A.; Chairi, I.; Bargiotas, I.; Ricard, D.; Yelnik, A.; Oudre, L.; Bertin-Hugault, F.; Vidal, P.P.; Vayatis, N.; et al. A Review of Center of Pressure (COP) Variables to Quantify Standing Balance in Elderly People: Algorithms and Open-Access Code. Physiol. Rep. 2021, 9, e15067. [Google Scholar] [CrossRef]
- Quijoux, F.; Vienne-Jumeau, A.; Bertin-Hugault, F.; Zawieja, P.; Lefèvre, M.; Vidal, P.-P.; Ricard, D. Center of Pressure Displacement Characteristics Differentiate Fall Risk in Older People: A Systematic Review with Meta-Analysis. Ageing Res. Rev. 2020, 62, 101117. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. BioMed Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Federolf, P.; Angulo-Barroso, R.M.; Busquets, A.; Ferrer-Uris, B.; Gløersen, Ø.; Mohr, M.; Ó’ Reilly, D.; Promsri, A.; van Andel, S.; Wachholz, F.; et al. Letter to the Editor Regarding “The Assessment of Center of Mass and Center of Pressure during Quiet Stance: Current Applications and Future Directions”. J. Biomech. 2021, 128, 110729. [Google Scholar] [CrossRef]
- Boonstra, T.W.; Danna-Dos-Santos, A.; Xie, H.B.; Roerdink, M.; Stins, J.F.; Breakspear, M. Muscle Networks: Connectivity Analysis of EMG Activity during Postural Control. Sci. Rep. 2015, 5, 17830. [Google Scholar] [CrossRef]
- Kumai, K.; Ikeda, Y.; Sakai, K.; Goto, K.; Morikawa, K.; Shibata, K. Brain and Muscle Activation Patterns during Postural Control Affect Static Postural Control. Gait Posture 2022, 96, 102–108. [Google Scholar] [CrossRef]
- Gatev, P.; Thomas, S.; Kepple, T.; Hallett, M. Feedforward Ankle Strategy of Balance during Quiet Stance in Adults. J. Physiol. 1999, 514, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Croft, J.L.; Von Tscharner, V.; Zernicke, R.F. Movement Variability and Muscle Activity Relative to Center of Pressure during Unipedal Stance on Solid and Compliant Surfaces. Motor Control 2008, 12, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Lemos, T.; Imbiriba, L.A.; Vargas, C.D.; Vieira, T.M. Modulation of Tibialis Anterior Muscle Activity Changes with Upright Stance Width. J. Electromyogr. Kinesiol. 2015, 25, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Lemos, T.; Rodrigues, E.C.; Vargas, C.D. Motor Imagery Modulation of Postural Sway Is Accompanied by Changes in the EMG–COP Association. Neurosci. Lett. 2014, 577, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, S.; Honeine, J.L.; Do, M.C.; Schieppati, M. Leg Muscle Activity during Tandem Stance and the Control of Body Balance in the Frontal Plane. Clin. Neurophysiol. 2013, 124, 1175–1186. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Howarth, S.; Winter, D.A.; Callaghan, J.P. Application of Autocorrelation and Cross-Correlation Analyses in Human Movement and Rehabilitation Research. J. Orthop. Sports Phys. Ther. 2009, 39, 287–295. [Google Scholar] [CrossRef]
- Edwards, W.B.; Derrick, T.R.; Hamill, J. Time Series Analysis in Biomechanics. In Handbook of Human Motion; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–24. [Google Scholar]
- Swinnen, S.P.; Gooijers, J. Bimanual Coordination. In Brain Mapping; Elsevier: Amsterdam, The Netherlands, 2015; pp. 475–482. ISBN 9780123970251. [Google Scholar]
- Promsri, A.; Haid, T.; Werner, I.; Federolf, P. Leg Dominance Effects on Postural Control When Performing Challenging Balance Exercises. Brain Sci. 2020, 10, 128. [Google Scholar] [CrossRef]
- Promsri, A.; Longo, A.; Haid, T.; Doix, A.-C.M.; Federolf, P. Leg Dominance as a Risk Factor for Lower-Limb Injuries in Downhill Skiers—A Pilot Study into Possible Mechanisms. Int. J. Environ. Res. Public Health 2019, 16, 3399. [Google Scholar] [CrossRef]
- Brophy, R.; Silvers, H.J.; Gonzales, T.; Mandelbaum, B.R.; Holly, M.; Silvers, J.; Mandelbaum, B.R. Gender Influences: The Role of Leg Dominance in ACL Injury among Soccer Players. Br. J. Sports Med. 2010, 44, 694–697. [Google Scholar] [CrossRef]
- Steidl-Müller, L.; Hildebrandt, C.; Müller, E.; Fink, C.; Raschner, C. Limb Symmetry Index in Competitive Alpine Ski Racers: Reference Values and Injury Risk Identification According to Age-Related Performance Levels. J. Sport Health Sci. 2018, 7, 405–415. [Google Scholar] [CrossRef]
- Ruedl, G.; Webhofer, M.; Helle, K.; Strobl, M.; Schranz, A.; Fink, C.; Gatterer, H.; Burtscher, M. Leg Dominance Is a Risk Factor for Noncontact Anterior Cruciate Ligament Injuries in Female Recreational Skiers. Am. J. Sports Med. 2012, 40, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.F.; Connolly, D.A.J.; Beynnon, B.D. Risk Factors for Lower Extremity Injury: A Review of the Literature. Br. J. Sports Med. 2003, 37, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Muehlbauer, T.; Kibele, A.; Granacher, U. Effects of Strength Training Using Unstable Surfaces on Strength, Power and Balance Performance Across the Lifespan: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1645–1669. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Saeys, W.; Vereeck, L.; De Hertogh, W.; Truijen, S. Are Unstable Support Surfaces Superior to Stable Support Surfaces during Trunk Rehabilitation after Stroke? A Systematic Review. Disabil. Rehabil. 2018, 40, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Cimadoro, G.; Paizis, C.; Alberti, G.; Babault, N. Effects of Different Unstable Supports on EMG Activity and Balance. Neurosci. Lett. 2013, 548, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A. Modulation of Lower-Limb Muscle Activity in Maintaining Unipedal Balance According to Surface Stability, Sway Direction, and Leg Dominance. Sports 2022, 10, 155. [Google Scholar] [CrossRef]
- Müller, M.L.T.M.; Redfern, M.S. Correlation between EMG and COP Onset Latency in Response to a Horizontal Platform Translation. J. Biomech. 2004, 37, 1573–1581. [Google Scholar] [CrossRef]
- Borg, F.; Finell, M.; Hakala, I.; Herrala, M. Analyzing Gastrocnemius EMG-Activity and Sway Data from Quiet and Perturbed Standing. J. Electromyogr. Kinesiol. 2007, 17, 622–634. [Google Scholar] [CrossRef]
- Tomita, H.; Kuno, S.; Kawaguchi, D.; Nojima, O. Limits of Stability and Functional Base of Support While Standing in Community-Dwelling Older Adults. J. Mot. Behav. 2021, 53, 83–91. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Federolf, P. Complexity, Composition, and Control of Bipedal Balancing Movements as the Postural Control System Adapts to Unstable Support Surfaces or Altered Feet Positions. Neuroscience 2020, 430, 113–124. [Google Scholar] [CrossRef]
- Bosquée, J.; Werth, J.; Epro, G.; Hülsdünker, T.; Potthast, W.; Meijer, K.; Ellegast, R.; Karamanidis, K. The Ability to Increase the Base of Support and Recover Stability Is Limited in Its Generalisation for Different Balance Perturbation Tasks. Eur. Rev. Aging Phys. Act. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Kwon, Y.; Jeon, H.-M.; Bang, M.-J.; Jun, J.-H.; Eom, G.-M.; Lim, D.-H. Feet Distance and Static Postural Balance: Implication on the Role of Natural Stance. Biomed. Mater. Eng. 2014, 24, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Ganz, D.A.; Latham, N.K. Prevention of Falls in Community-Dwelling Older Adults. N. Engl. J. Med. 2020, 382, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.; Melnikov, A.; Skvortsov, D.; Akhmerova, K.; Vavaev, A.; Golov, A.; Draugelite, V.; Nikolaev, R.; Chechelnickaia, S.; Zhuk, D.; et al. Postural Stability in Athletes: The Role of Sport Direction. Gait Posture 2021, 89, 120–125. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B. SENIAM Project. Available online: http://www.seniam.org/ (accessed on 8 June 2016).
- Kiers, H.; Brumagne, S.; van Dieën, J.; van der Wees, P.; Vanhees, L. Ankle Proprioception Is Not Targeted by Exercises on an Unstable Surface. Eur. J. Appl. Physiol. 2012, 112, 1577–1585. [Google Scholar] [CrossRef]
- Huurnink, A.; Fransz, D.P.; Kingma, I.; van Dieën, J.H. Comparison of a Laboratory Grade Force Platform with a Nintendo Wii Balance Board on Measurement of Postural Control in Single-Leg Stance Balance Tasks. J. Biomech. 2013, 46, 1392–1395. [Google Scholar] [CrossRef]
- De Luca, C.J. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Promsri, A. Modulation of Bilateral Lower-Limb Muscle Coordination When Performing Increasingly Challenging Balance Exercises. Neurosci. Lett. 2022, 767, 136299. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Paillard, T.; Noé, F. Does Monopedal Postural Balance Differ between the Dominant Leg and the Non-Dominant Leg? A Review. Hum. Mov. Sci. 2020, 74, 102686. [Google Scholar] [CrossRef]
- Rinaldin, C.D.P.; Avila de Oliveira, J.; Ribeiro de Souza, C.; Scheeren, E.M.; Coelho, D.B.; Teixeira, L.A. Compensatory Control between the Legs in Automatic Postural Responses to Stance Perturbations under Single-Leg Fatigue. Exp. Brain Res. 2021, 239, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A.; Bangkomdet, K.; Jindatham, I.; Jenchang, T. Leg Dominance—Surface Stability Interaction: Effects on Postural Control Assessed by Smartphone-Based Accelerometry. Sports 2023, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A. A Potential Mechanism Involved in the Regularity of Center-of-Pressure Displacements During Achieving Unipedal Equilibrium on Stable and Unstable Surfaces. J. Appl. Biomech. 2024, 40, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Penedo, T.; Polastri, P.F.; Rodrigues, S.T.; Santinelli, F.B.; Costa, E.d.C.; Imaizumi, L.F.I.; Barbieri, R.A.; Barbieri, F.A. Motor Strategy during Postural Control Is Not Muscle Fatigue Joint-Dependent, but Muscle Fatigue Increases Postural Asymmetry. PLoS ONE 2021, 16, e0247395. [Google Scholar] [CrossRef] [PubMed]
- Parrington, L.; Ball, K. Biomechanical Considerations of Laterality in Sport. In Laterality in Sports: Theories and Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 279–308. ISBN 9780128014264. [Google Scholar]
- Prentice, W. Rehabilitation Techniques for Sports Medicine and Athletic Training; Routledge: New York, NY, USA, 2024; ISBN 9781003526308. [Google Scholar]
- Wrisley, D.M.; Whitney, S.L. The Effect of Foot Position on the Modified Clinical Test of Sensory Interaction and Balance. Arch. Phys. Med. Rehabil. 2004, 85, 335–338. [Google Scholar] [CrossRef]
- Kędziorek, J.; Błażkiewicz, M. Nonlinear Measures to Evaluate Upright Postural Stability: A Systematic Review. Entropy 2020, 22, 1357. [Google Scholar] [CrossRef]
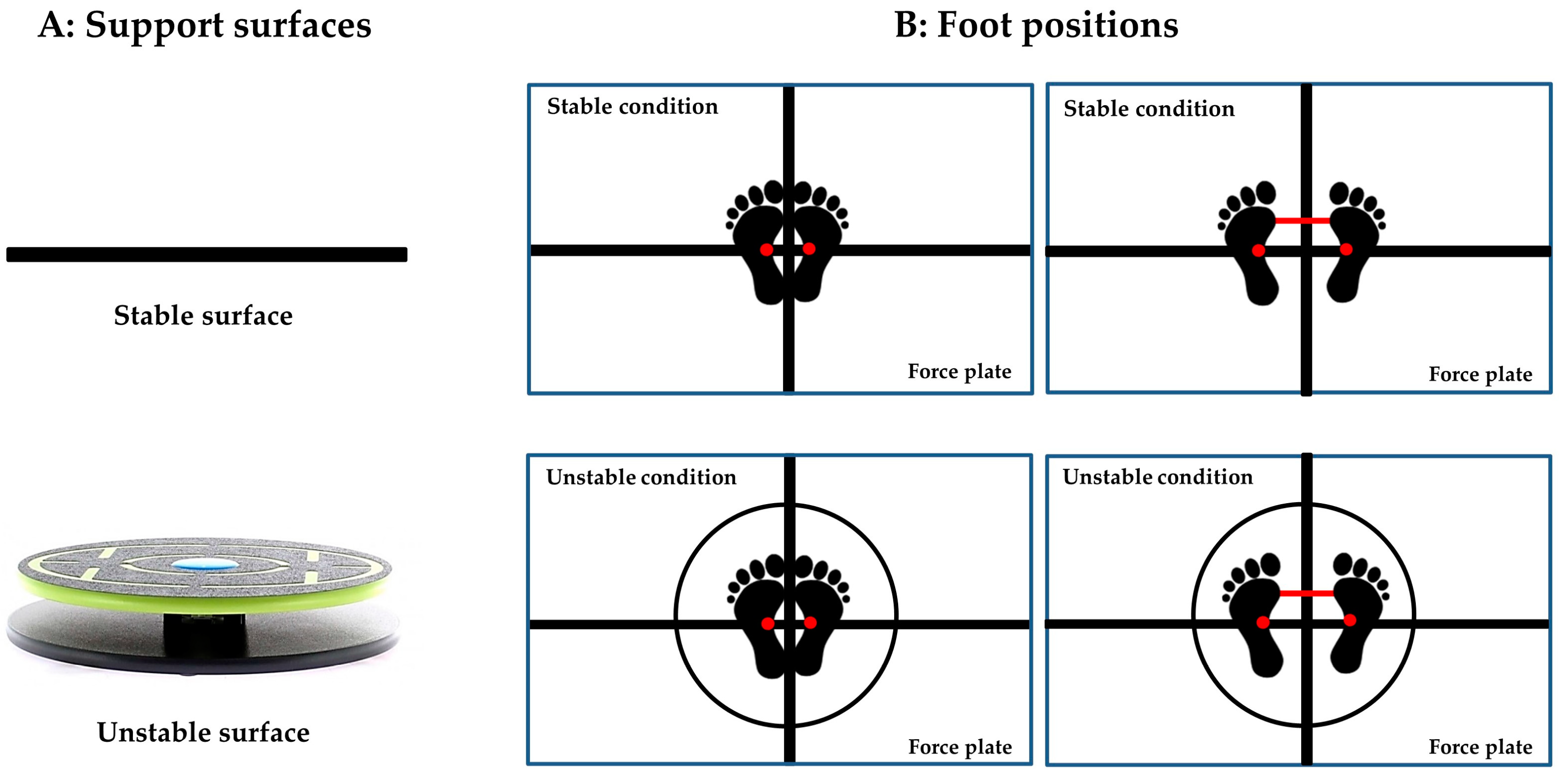

| Total (n = 20) | Male (n = 10) | Female (n = 10) | |
|---|---|---|---|
| Age (yrs.) | 25.2 ± 4.0 | 25.4 ± 2.2 | 25.0 ± 5.3 |
| Weight (kg) | 68.7 ± 11.0 | 75.3 ± 11.4 | 62.1 ± 5.2 * |
| Height (cm) | 174.7 ± 8.9 | 181.3 ± 7.7 | 168.2 ± 4.0 * |
| Body mass index (kg/m2) | 22.4 ± 2.1 | 22.8 ± 1.8 | 22.0 ± 2.4 |
| Physical activity participation (hours/week) | 9.1 ± 5.3 | 9.2 ± 6.0 | 9.1 ± 4.9 |
| A: Leg Dominance | |||||
| Muscle | ND | DO | p-Value | 2 | Power |
| RF | 0.20 ± 0.01 | 0.20 ± 0.02 | 0.669 | 0.009 | 0.070 |
| ST | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.933 | 0.000 | 0.051 |
| BF | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.367 | 0.039 | 0.142 |
| TA | 0.30 ± 0.02 | 0.23 ± 0.01 | 0.004 * | 0.325 | 0.859 |
| PL | 0.28 ± 0.02 | 0.30 ± 0.02 | 0.433 | 0.030 | 0.119 |
| GM | 0.46 ± 0.02 | 0.43 ± 0.02 | 0.190 | 0.080 | 0.253 |
| SO | 0.41 ± 0.02 | 0.42 ± 0.02 | 0.824 | 0.002 | 0.055 |
| B: Surface Stability | |||||
| Muscle | SS | US | p-Value | 2 | Power |
| RF | 0.23 ± 0.02 | 0.16 ± 0.01 | 0.001 * | 0.439 | 0.971 |
| ST | 0.29 ± 0.03 | 0.17 ± 0.01 | <0.001 * | 0.485 | 0.988 |
| BF | 0.30 ± 0.02 | 0.18 ± 0.01 | <0.001 * | 0.492 | 0.990 |
| TA | 0.25 ± 0.02 | 0.28 ± 0.01 | 0.238 | 0.066 | 0.213 |
| PL | 0.32 ± 0.02 | 0.25 ± 0.02 | 0.012 # | 0.263 | 0.743 |
| GM | 0.54 ± 0.03 | 0.34 ± 0.02 | <0.001 * | 0.596 | 1 |
| SO | 0.50 ± 0.03 | 0.33 ± 0.02 | <0.001 * | 0.569 | 0.999 |
| C: Sway Direction | |||||
| Muscle | AP | ML | p-Value | 2 | Power |
| RF | 0.22 ± 0.02 | 0.18 ± 0.01 | 0.081 | 0.138 | 0.418 |
| ST | 0.28 ± 0.03 | 0.18 ± 0.01 | 0.001 * | 0.402 | 0.948 |
| BF | 0.26 ± 0.02 | 0.22 ± 0.01 | 0.085 | 0.135 | 0.407 |
| TA | 0.32 ± 0.02 | 0.22 ± 0.01 | <0.001 * | 0.519 | 0.995 |
| PL | 0.30 ± 0.02 | 0.28 ± 0.02 | 0.356 | 0.041 | 0.147 |
| GM | 0.47 ± 0.02 | 0.42 ± 0.02 | 0.060 | 0.158 | 0.475 |
| SO | 0.45 ± 0.02 | 0.39 ± 0.02 | 0.027 # | 0.211 | 0.618 |
| D: Foot Position | |||||
| Muscle | FA | FT | p-Value | 2 | Power |
| RF | 0.20 ± 0.02 | 0.19 ± 0.01 | 0.608 | 0.013 | 0.079 |
| ST | 0.25 ± 0.03 | 0.21 ± 0.02 | 0.138 | 0.102 | 0.313 |
| BF | 0.24 ± 0.02 | 0.23 ± 0.02 | 0.600 | 0.013 | 0.080 |
| TA | 0.25 ± 0.01 | 0.29 ± 0.01 | 0.056 | 0.163 | 0.488 |
| PL | 0.26 ± 0.01 | 0.31 ± 0.02 | 0.057 | 0.162 | 0.484 |
| GM | 0.43 ± 0.02 | 0.46 ± 0.02 | 0.317 | 0.048 | 0.165 |
| SO | 0.39 ± 0.02 | 0.45 ± 0.02 | 0.021 # | 0.229 | 0.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promsri, A. Neuromuscular Control in Postural Stability: Insights into Myoelectric Activity Involved in Postural Sway During Bipedal Balance Tasks. Signals 2025, 6, 6. https://doi.org/10.3390/signals6010006
Promsri A. Neuromuscular Control in Postural Stability: Insights into Myoelectric Activity Involved in Postural Sway During Bipedal Balance Tasks. Signals. 2025; 6(1):6. https://doi.org/10.3390/signals6010006
Chicago/Turabian StylePromsri, Arunee. 2025. "Neuromuscular Control in Postural Stability: Insights into Myoelectric Activity Involved in Postural Sway During Bipedal Balance Tasks" Signals 6, no. 1: 6. https://doi.org/10.3390/signals6010006
APA StylePromsri, A. (2025). Neuromuscular Control in Postural Stability: Insights into Myoelectric Activity Involved in Postural Sway During Bipedal Balance Tasks. Signals, 6(1), 6. https://doi.org/10.3390/signals6010006






