Neuromuscular Control in Incline and Decline Treadmill Running: Insights into Movement Synergies for Training and Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Procedure
2.2. Signal Processing and Movement Component Analysis
2.3. Computing PCA-Based Variables
2.4. Statistical Analysis
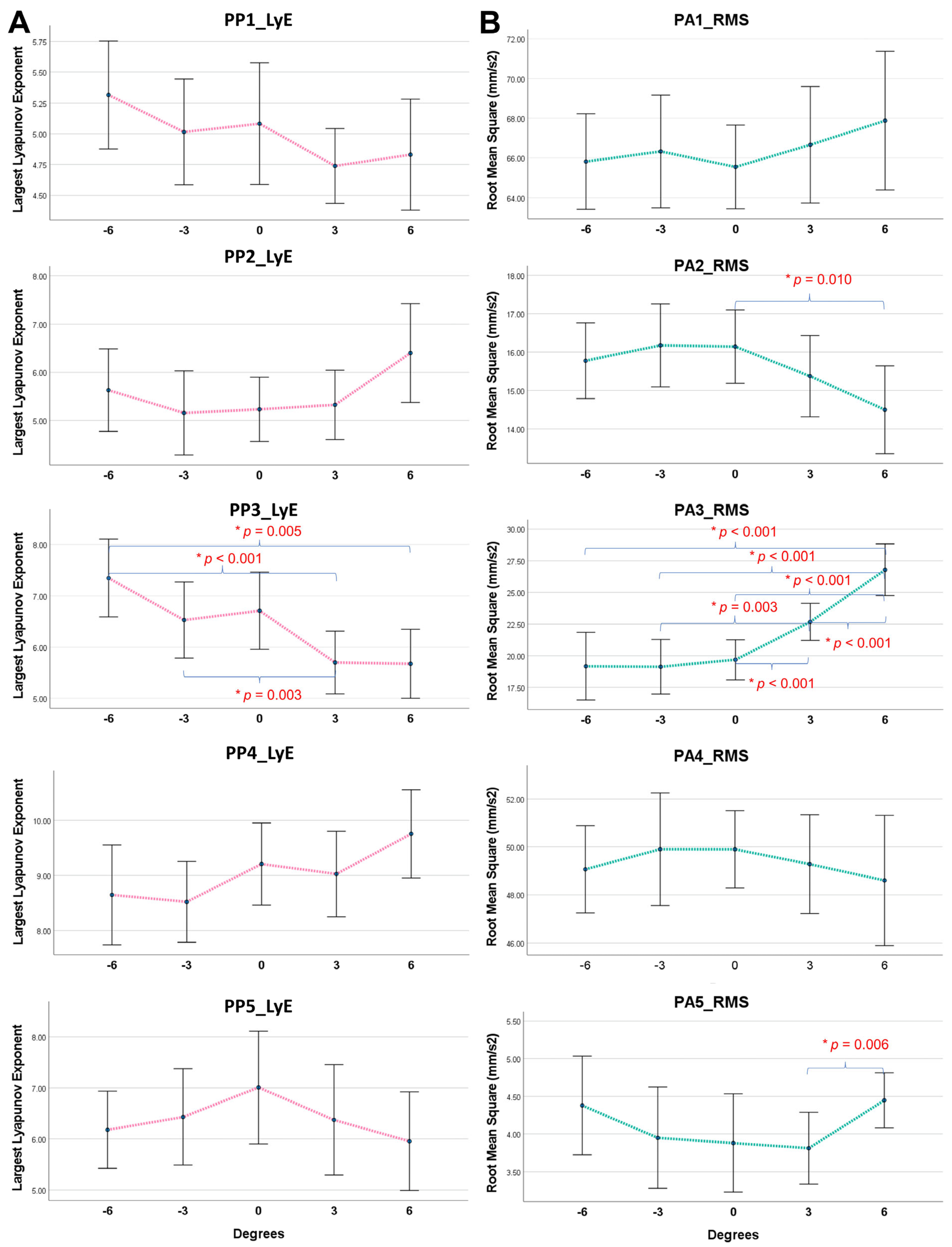
3. Results
3.1. Movement Components of Treadmill Running
3.2. Treadmill Gradient Effects on Local Dynamic Stability and Acceleration Magnitude
4. Discussion
Limitations and Future Research
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federolf, P.; Tecante, K.; Nigg, B. A Holistic Approach to Study the Temporal Variability in Gait. J. Biomech. 2012, 45, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.; Federolf, P.; von Tscharner, V.; Stirling, L.; Nigg, B.M. Discrimination of Gender-, Speed-, and Shoe-Dependent Movement Patterns in Runners Using Full-Body Kinematics. Gait Posture 2012, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.S.; Ferris, D.P. Biomechanics and Energetics of Running on Uneven Terrain. J. Exp. Biol. 2015, 218, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Self, Z.T.; Spence, A.J.; Wilson, A.M. Speed and Incline during Thoroughbred Horse Racing: Racehorse Speed Supports a Metabolic Power Constraint to Incline Running but Not to Decline Running. J. Appl. Physiol. 2012, 113, 602–607. [Google Scholar] [CrossRef]
- Garnier, Y.M.; Lepers, R.; Dubau, Q.; Pageaux, B.; Paizis, C. Neuromuscular and Perceptual Responses to Moderate-Intensity Incline, Level and Decline Treadmill Exercise. Eur. J. Appl. Physiol. 2018, 118, 2039–2053. [Google Scholar] [CrossRef]
- Vernillo, G.; Giandolini, M.; Edwards, W.B.; Morin, J.-B.; Samozino, P.; Horvais, N.; Millet, G.Y. Biomechanics and Physiology of Uphill and Downhill Running. Sports Med. 2017, 47, 615–629. [Google Scholar] [CrossRef]
- Promsri, A.; Mohr, M.; Federolf, P. Principal Postural Acceleration and Myoelectric Activity: Interrelationship and Relevance for Characterizing Neuromuscular Function in Postural Control. Hum. Mov. Sci. 2021, 77, 102792. [Google Scholar] [CrossRef]
- Ferley, D.D.; Osborn, R.W.; Vukovich, M.D. The Effects of Incline and Level-Grade High-Intensity Interval Treadmill Training on Running Economy and Muscle Power in Well-Trained Distance Runners. J. Strength Cond. Res. 2014, 28, 1298–1309. [Google Scholar] [CrossRef]
- Harrison, K.; Holmes, H.H.; Finley, E.B.; Guzman, K.S.; Kimbrough, K.C.; Roper, J.A. Incline and Decline Running Alters Joint Moment Contributions but Not Peak Support Moments in Individuals with an Anterior Cruciate Ligament Reconstruction and Controls. Front. Sports Act. Living 2023, 5, 1217783. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K. The Neural Origin of Muscle Synergies. Front. Comput. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Federolf, P. Complexity, Composition, and Control of Bipedal Balancing Movements as the Postural Control System Adapts to Unstable Support Surfaces or Altered Feet Positions. Neuroscience 2020, 430, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.A. A Novel Approach to Study Human Posture Control: “Principal Movements” Obtained from a Principal Component Analysis of Kinematic Marker Data. J. Biomech. 2016, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Haid, T.H.; Zago, M.; Promsri, A.; Doix, A.-C.M.; Federolf, P.A. PManalyzer: A Software Facilitating the Study of Sensorimotor Control of Whole-Body Movements. Front. Neuroinform. 2019, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A. Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study. Sports 2022, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.A.; Boyer, K.A.; Andriacchi, T.P. Application of Principal Component Analysis in Clinical Gait Research: Identification of Systematic Differences Between Healthy and Medial Knee-Osteoarthritic Gait. J. Biomech. 2013, 46, 2173–2178. [Google Scholar] [CrossRef]
- Hoerzer, S.; Federolf, P.A.; Maurer, C.; Baltich, J.; Nigg, B.M. Footwear Decreases Gait Asymmetry during Running. PLoS ONE 2015, 10, e0138631. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Cusumano, J.P.; Cavanagh, P.R.; Sternad, D. Local Dynamic Stability Versus Kinematic Variability of Continuous Overground and Treadmill Walking. J. Biomech. Eng. 2001, 123, 27–32. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Marin, L.C. Kinematic Variability and Local Dynamic Stability of Upper Body Motions When Walking at Different Speeds. J. Biomech. 2006, 39, 444–452. [Google Scholar] [CrossRef]
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieen, J.H. Assessing the Stability of Human Locomotion: A Review of Current Measures. J. R. Soc. Interface 2013, 10, 20120999. [Google Scholar] [CrossRef]
- England, S.A.; Granata, K.P. The Influence of Gait Speed on Local Dynamic Stability of Walking. Gait Posture 2007, 25, 172–178. [Google Scholar] [CrossRef]
- Hamacher, D.; Singh, N.B.; Van Dieën, J.H.; Heller, M.O.; Taylor, W.R. Kinematic Measures for Assessing Gait Stability in Elderly Individuals: A Systematic Review. J. R. Soc. Interface 2011, 8, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Matcuk, G.R.; Mahanty, S.R.; Skalski, M.R.; Patel, D.B.; White, E.A.; Gottsegen, C.J. Stress Fractures: Pathophysiology, Clinical Presentation, Imaging Features, and Treatment Options. Emerg. Radiol. 2016, 23, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, T.; Tenforde, A.S.; Strahl, A.; Rolvien, T.; Hollander, K. Does Magnetic Resonance Imaging Grading Correlate with Return to Sports After Bone Stress Injuries? A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2022, 50, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Meijer, K. Dataset of Running Kinematics, Kinetics and Muscle Activation at Different Speeds, Surface Gradients, Cadences and with Forward Trunk Lean. Data Brief 2024, 54, 110312. [Google Scholar] [CrossRef]
- Van Hooren, B.; van Rengs, L.; Meijer, K. Per-Step and Cumulative Load at Three Common Running Injury Locations: The Effect of Speed, Surface Gradient, and Cadence. Scand. J. Med. Sci. Sports 2024, 34, e14570. [Google Scholar] [CrossRef]
- Federolf, P. A Novel Approach to Solve the “Missing Marker Problem” in Marker-Based Motion Analysis That Exploits the Segment Coordination Patterns in Multi-Limb Motion Data. PLoS ONE 2013, 8, e78689. [Google Scholar] [CrossRef]
- Longo, A.; Haid, T.; Meulenbroek, R.; Federolf, P. Biomechanics in Posture Space: Properties and Relevance of Principal Accelerations for Characterizing Movement Control. J. Biomech. 2019, 82, 397–403. [Google Scholar] [CrossRef]
- Longo, A.; Meulenbroek, R.; Haid, T.; Federolf, P. Postural Reconfiguration and Cycle-to-Cycle Variability in Patients with Work-Related Musculoskeletal Disorders Compared to Healthy Controls and in Relation to Pain Emerging during a Repetitive Movement Task. Clin. Biomech. 2018, 54, 103–110. [Google Scholar] [CrossRef]
- Longo, A.; Federolf, P.; Haid, T.; Meulenbroek, R. Effects of a Cognitive Dual Task on Variability and Local Dynamic Stability in Sustained Repetitive Arm Movements Using Principal Component Analysis: A Pilot Study. Exp. Brain Res. 2018, 236, 1611–1619. [Google Scholar] [CrossRef]
- Promsri, A. Assessing Walking Stability Based on Whole-Body Movement Derived from a Depth-Sensing Camera. Sensors 2022, 22, 7542. [Google Scholar] [CrossRef]
- Mehdizadeh, S. The Largest Lyapunov Exponent of Gait in Young and Elderly Individuals: A Systematic Review. Gait Posture 2018, 60, 241–250. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Cusumano, J.P. Nonlinear Time Series Analysis of Normal and Pathological Human Walking. Chaos Interdiscip. J. Nonlinear Sci. 2000, 10, 848–863. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Effects of Walking Speed, Strength and Range of Motion on Gait Stability in Healthy Older Adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov Exponents from a Time Series. Phys. D 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Kantz, H. A Robust Method to Estimate the Maximal Lyapunov Exponent of a Time Series. Phys. Lett. A 1994, 185, 77–87. [Google Scholar] [CrossRef]
- Ó’Reilly, D.; Federolf, P. Identifying Differences in Gait Adaptability across Various Speeds Using Movement Synergy Analysis. PLoS ONE 2021, 16, e0244582. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. BioMed Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Bontemps, B.; Vercruyssen, F.; Gruet, M.; Louis, J. Downhill Running: What Are The Effects and How Can We Adapt? A Narrative Review. Sports Med. 2020, 50, 2083–2110. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Patterns in Human Walking and Running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef]
- Umberger, B.R. Stance and Swing Phase Costs in Human Walking. J. R. Soc. Interface 2010, 7, 1329–1340. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait Variability: Methods, Modeling and Meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Common Muscle Synergies for Balance and Walking. Front. Comput. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, R.; Thelen, D.; Heiderscheit, B. Hip Muscle Loads During Running at Various Step Rates. J. Orthop. Sports Phys. Ther. 2014, 44, 766–774, A1–4. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Moore, W.; Guillet, M.; Beaulieu, C. High Hamstring Tendinopathy in Runners Meeting the Challenges of Diagnosis, Treatment, and Rehabilitation. Physician Sportsmed. 2005, 33, 32–43. [Google Scholar] [CrossRef]
- Pohl, M.B.; Kendall, K.D.; Patel, C.; Wiley, J.P.; Emery, C.; Ferber, R. Experimentally Reduced Hip-Abductor Muscle Strength and Frontal-Plane Biomechanics During Walking. J. Athl. Train. 2015, 50, 385–391. [Google Scholar] [CrossRef]

| Total | Female (n = 9) | Male (n = 10) | |
|---|---|---|---|
| Age (yrs.) | 23.6 ± 3.7 | 23.3 ± 3.9 | 23.8 ± 3.7 |
| Weight (kg) | 67.2 ± 10.4 | 61.5 ± 9.1 | 72.2 ± 9.0 * |
| Height (cm) | 174.9 ± 9.2 | 168.2 ± 7.4 | 180.9 ± 5.9 * |
| Body mass index (kg/m2) | 21.9 ± 2.0 | 21.6 ± 1.9 | 22.0 ± 2.1 |
| PMk | Movement Characteristics | PPk_rVAR (%) | PAk_rVAR (%) |
|---|---|---|---|
| k = 1 | Swing-phase movement coupled with trunk rotation: One leg moves forward while the trunk rotates to balance the body’s momentum. | 72.5 ± 7.3 | 49.0 ± 4.2 |
| 2 | Double-leg support coupled with anteroposterior sliding: Both feet are on the ground (double-leg support) and involve anteroposterior (forward–backward) movement. | 12.8 ± 6.6 | 2.8 ± 0.8 |
| 3 | Single-leg support coupled with trunk rotation: One leg supports the body while the other swings forward. Trunk rotation is also involved here, assisting in the balance during the single-leg support. | 7.3 ± 3.2 | 5.3 ± 2.1 |
| 4 | Double-leg support coupled with hip and knee flexion/extension: Hip and knee movements during double-leg support, capturing the flexion and extension of these joints as both feet are in contact with the ground. | 2.9 ± 0.5 | 27.0 ± 2.4 |
| 5 | Single-leg support coupled with mediolateral sliding: The mediolateral (side-to-side) movements during the single-leg support phase. | 2.2 ± 1.8 | 0.2 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promsri, A. Neuromuscular Control in Incline and Decline Treadmill Running: Insights into Movement Synergies for Training and Rehabilitation. Signals 2025, 6, 2. https://doi.org/10.3390/signals6010002
Promsri A. Neuromuscular Control in Incline and Decline Treadmill Running: Insights into Movement Synergies for Training and Rehabilitation. Signals. 2025; 6(1):2. https://doi.org/10.3390/signals6010002
Chicago/Turabian StylePromsri, Arunee. 2025. "Neuromuscular Control in Incline and Decline Treadmill Running: Insights into Movement Synergies for Training and Rehabilitation" Signals 6, no. 1: 2. https://doi.org/10.3390/signals6010002
APA StylePromsri, A. (2025). Neuromuscular Control in Incline and Decline Treadmill Running: Insights into Movement Synergies for Training and Rehabilitation. Signals, 6(1), 2. https://doi.org/10.3390/signals6010002






