Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques
Abstract
1. Problem Introduction
2. Related Works
3. Challenges in Electromyography
3.1. Phsical Principles
3.2. Signal Characteristics
4. Case Study: High-Frequency Tasks
5. Post-Processing EMG Signals: Proposed Supervised Learning Model
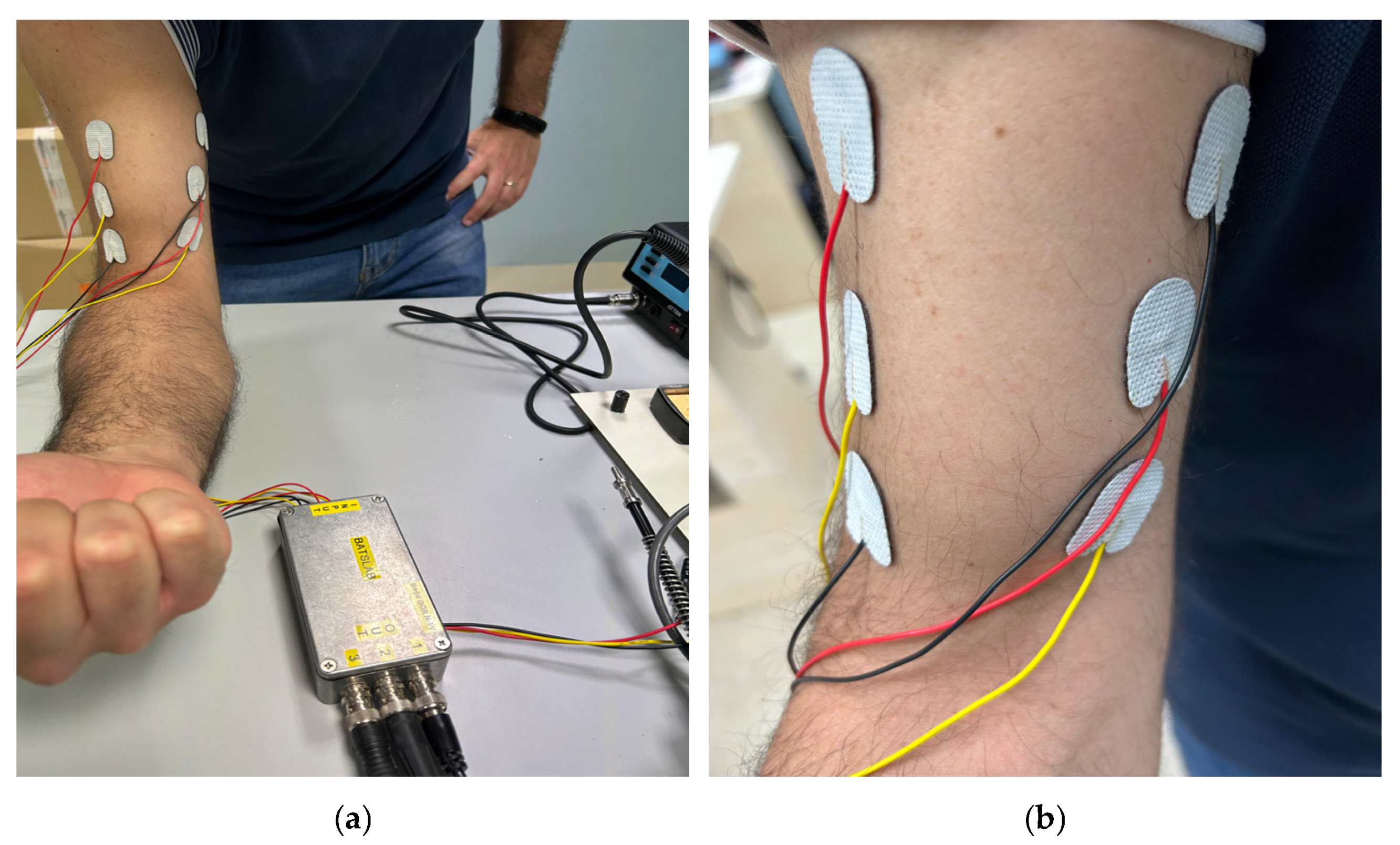
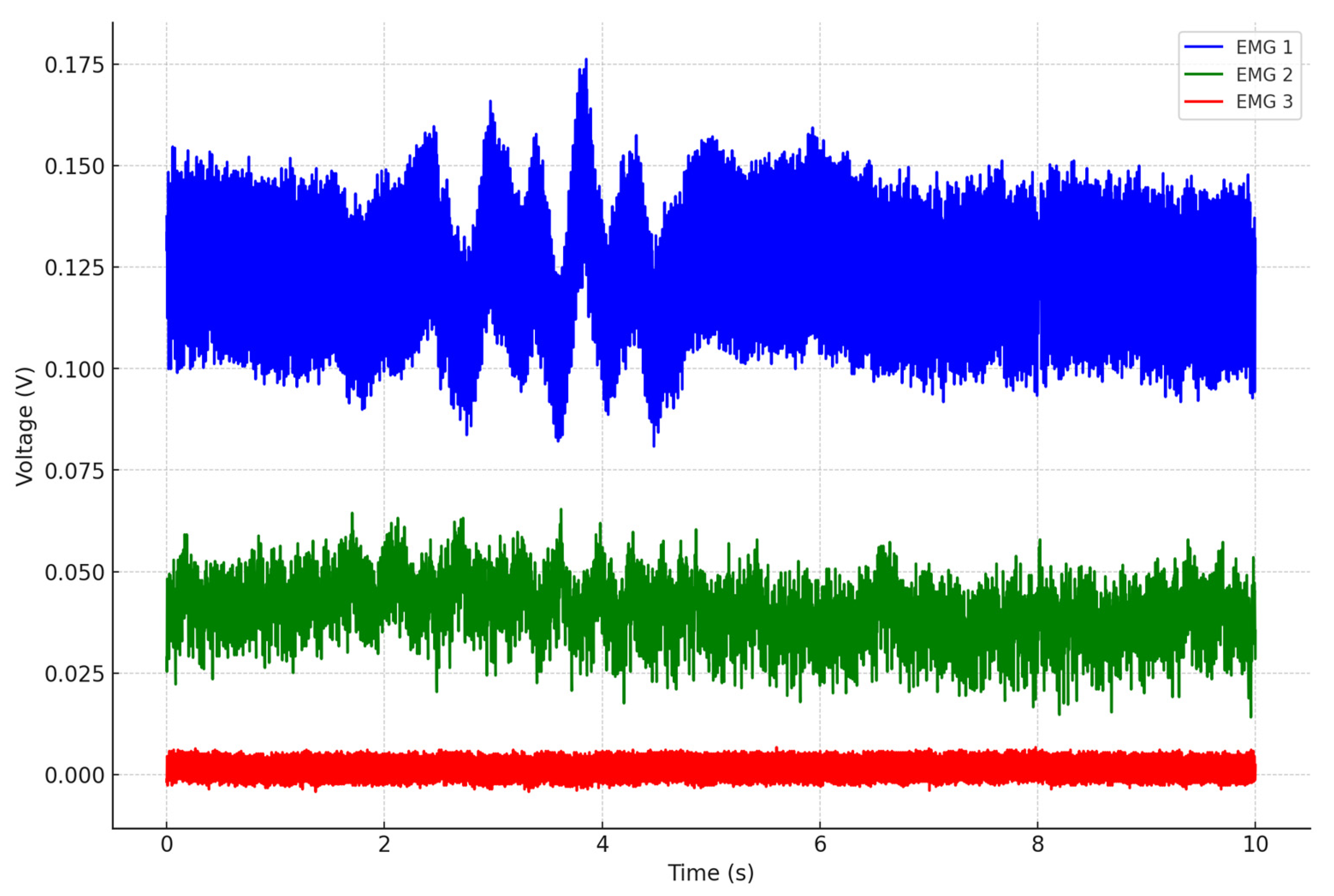
5.1. Data Assessment
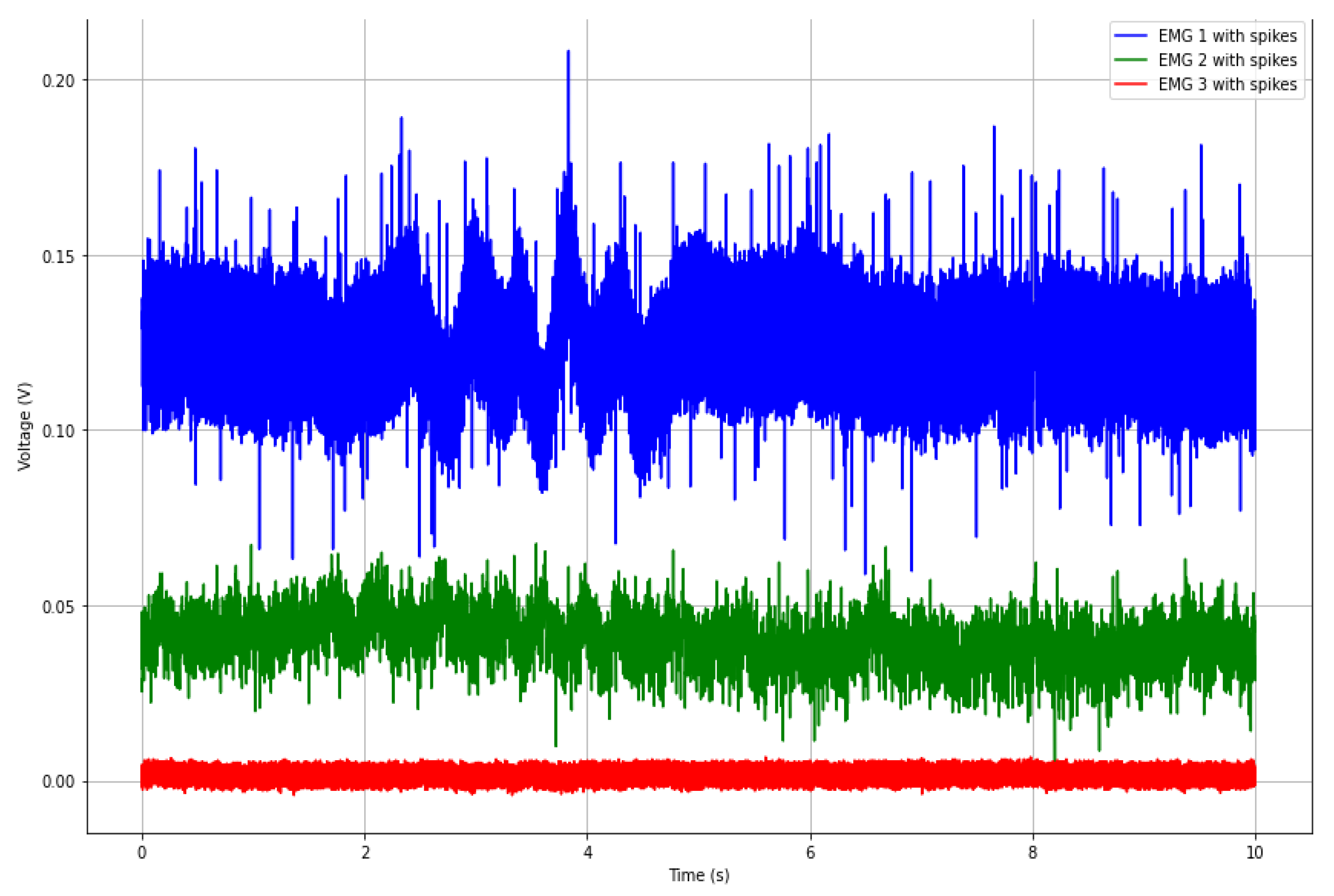
5.2. Data Pre-Processing
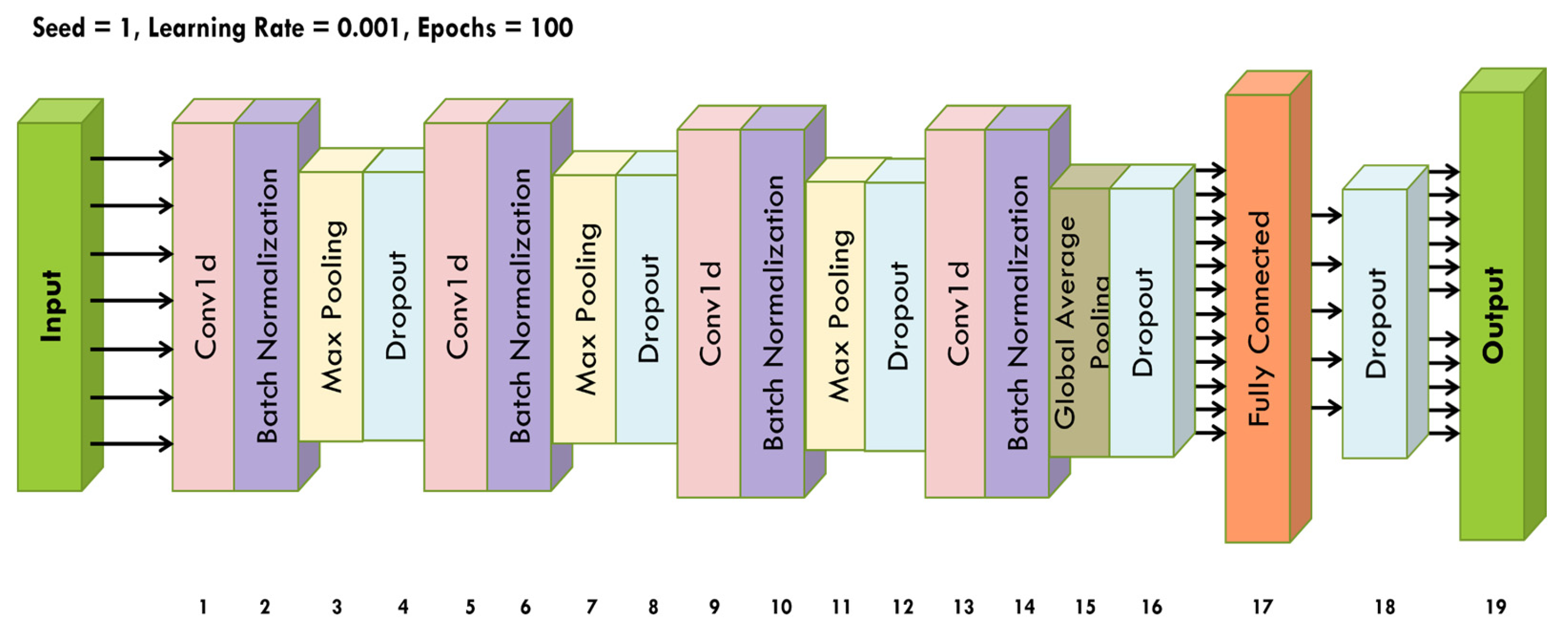
5.3. SLM Architecture
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nahavandi, D.; Alizadehsani, R.; Khosravi, A.; Acharya, U.R. Application of artificial intelligence in wearable devices: Opportunities and challenges. Comput. Methods Programs Biomed. 2022, 213, 106541. [Google Scholar] [CrossRef]
- Nova, S.N.; Rahman, M.S.; Hosen, A.S. Deep Learning in Biomedical Devices: Perspectives, Applications, and Challenges. In Rhythms in Healthcare; Springer: Singapore, 2022; pp. 13–35. [Google Scholar]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry electrodes for human bioelectrical signal monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef]
- Tasneem, N.T.; Pullano, S.A.; Critello, C.D.; Fiorillo, A.S.; Mahbub, I. A low-power on-chip ECG monitoring system based on MWCNT/PDMS dry electrodes. IEEE Sens. J. 2020, 20, 12799–12806. [Google Scholar] [CrossRef]
- Washington, P.; Park, N.; Srivastava, P.; Voss, C.; Kline, A.; Varma, M.; Tariq, Q.; Kalantarian, H.; Schwartz, J.; Patnaik, R.; et al. Data-driven diagnostics and the potential of mobile artificial intelligence for digital therapeutic phenotyping in computational psychiatry. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Menniti, M.; Laganà, F.; Oliva, G.; Bianco, M.G.; Fiorillo, A.S.; Pullano, S.A. Development of Non-Invasive Ventilator for Homecare and Patient Monitoring System. Electronics 2024, 13, 790. [Google Scholar] [CrossRef]
- Menniti, M.; Oliva, G.; Laganà, F.; Bianco, M.G.; Fiorillo, A.S.; Pullano, S.A. Portable Non-Invasive Ventilator for Homecare and Patients Monitoring System. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023. [Google Scholar]
- Gregorio, F.; González, G.; Schmidt, C.; Cousseau, J. Signal Processing Techniques for Power Efficient Wireless Communication Systems: Practical Approaches for RF Impairments Reduction; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wu, Y.; Guo, K.; Chu, Y.; Wang, Z.; Yang, H.; Zhang, J. Advancements and Challenges in Non-Invasive Sensor Technologies for Swallowing Assessment: A Review. Bioengineering 2024, 11, 430. [Google Scholar] [CrossRef]
- Vidhya, C.M.; Maithani, Y.; Singh, J.P. Recent advances and challenges in textile electrodes for wearable biopotential signal monitoring: A comprehensive review. Biosensors 2023, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Pulcinelli, M.; Pinnelli, M.; Massaroni, C.; Lo Presti, D.; Fortino, G.; Schena, E. Wearable Systems for Unveiling Collective Intelligence in Clinical Settings. Sensors 2023, 23, 9777. [Google Scholar] [CrossRef]
- Merces, L.; Ferro, L.M.M.; Nawaz, A.; Sonar, P. Advanced Neuromorphic Applications Enabled by Synaptic Ion-Gating Vertical Transistors. Adv. Sci. 2024, 11, 2305611. [Google Scholar] [CrossRef]
- Laganà, F.; De Carlo, D.; Calcagno, S.; Pullano, S.A.; Critello, D.; Falcone, F.; Fiorillo, A.S. Computational model of cell deformation under fluid flow based rolling. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019. [Google Scholar]
- Pandarinath, C.; Bensmaia, S.J. The science and engineering behind sensitized brain-controlled bionic hands. Physiol. Rev. 2022, 102, 551–604. [Google Scholar] [CrossRef]
- Arif, A.; Wang, Y.; Yin, R.; Zhang, X.; Helmy, A. EF-Net: Mental State Recognition by Analyzing Multimodal EEG-fNIRS via CNN. Sensors 2024, 24, 1889. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Fernandez, C.; Rusk, S.; Nygate, Y.; Turkington, F.; Mortara, J. 426 Clinical Validation of A.I. Analysis of Photoplethysmogram (PPG) Based Sleep-Wake Staging, Total Sleep Time, and Respiratory Rate. Sleep 2021, 44, A168–A169. [Google Scholar] [CrossRef]
- Rajwal, S.; Aggarwal, S. Convolutional Neural Network-Based EEG Signal Analysis: A Systematic Review. Arch. Comput. Methods Eng. 2023, 30, 3585–3615. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.Q.; Xie, S.Q. Continuous Motion Intention Prediction Using sEMG for Upper-Limb Rehabilitation: A Systematic Review of Model-Based and Model-Free Approaches. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1466–1483. [Google Scholar] [CrossRef] [PubMed]
- Tchantchane, R.; Zhou, H.; Zhang, S.; Alici, G. A review of hand gesture recognition systems based on noninvasive wearable sensors. Adv. Intell. Syst. 2023, 5, 2300207. [Google Scholar] [CrossRef]
- Ozdemir, M.A.; Kisa, D.H.; Guren, O.; Akan, A. Dataset for multi-channel surface electromyography (sEMG) signals of hand gestures. Data Brief 2022, 41, 107921. [Google Scholar] [CrossRef] [PubMed]
- Strzecha, K.; Krakós, M.; Więcek, B.; Chudzik, P.; Tatar, K.; Lisowski, G.; Mosorov, V.; Sankowski, D. Processing of EMG Signals with High Impact of Power Line and Cardiac Interferences. Appl. Sci. 2021, 11, 4625. [Google Scholar] [CrossRef]
- Zhang, X.; Barkhaus, P.E.; Rymer, W.Z.; Zhou, P. Machine learning for supporting diagnosis of amyotrophic lateral sclerosis using surface electromyogram. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 22, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Suh, E.-S.; Mandal, S.; Harding, R.; Ramsay, M.; Kamalanathan, M.; Henderson, K.; O’Kane, K.; Douiri, A.; Hopkinson, N.S.; Polkey, M.I.; et al. Neural respiratory drive predicts clinical deterioration and safe discharge in exacerbations of COPD. Thorax 2015, 70, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Aziz, S.; Amjad, F.; Mohsin, M. Detection of dilated cardiomyopathy using pulse plethysmographic signal analysis. In Proceedings of the 2019 22nd International Multitopic Conference (INMIC), Islamabad, Pakistan, 29–30 November 2019; pp. 1–5. [Google Scholar]
- Conradsen, I.; Beniczky, S.; Wolf, P.; Jennum, P.; Sorensen, H.B. Evaluation of novel algorithm embedded in a wearable sEMG device for seizure detection. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2048–2051. [Google Scholar]
- Conradsen, I.; Beniczky, S.; Wolf, P.; Kjaer, T.W.; Sams, T.; Sorensen, H.B. Automatic multi-modal intelligent seizure acquisition (MISA) system for detection of motor seizures from electromyographic data and motion data. Comput. Methods Programs Biomed. 2012, 107, 97–110. [Google Scholar] [CrossRef]
- Sarcher, A.; Brochard, S.; Perrouin-Verbe, B.; Raison, M.; Letellier, G.; Leboeuf, F.; Gross, R. Detection of pronator muscle overactivity in children with unilateral spastic cerebral palsy: Development of a semi-automatic method using EMG data. Ann. Phys. Rehabil. Med. 2019, 62, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Vescio, B.; Nisticò, R.; Augimeri, A.; Quattrone, A.; Crasà, M.; Quattrone, A. Development and Validation of a New Wearable Mobile Device for the Automated Detection of Resting Tremor in Parkinson’s Disease and Essential Tremor. Diagnostics 2021, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, C.; Pirovano, I.; Mira, R.M.; Rizzo, G.; Scano, A.; Mastropietro, A. Combined use of EMG and EEG techniques for neuromotor assessment in rehabilitative applications: A systematic review. Sensors 2021, 21, 7014. [Google Scholar] [CrossRef] [PubMed]
- Laganà, F.; De Carlo, D.; Calcagno, S.; Oliva, G.; Pullano, S.A.; Fiorillo, A.S. Modeling of Electrical Impedance Tomography for Carcinoma Detection. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022. [Google Scholar]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern diagnostic imaging technique applications and risk factors in the medical field: A review. BioMed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Kadja, G.T.; Culsum, N.T.; Mardiana, S.; Azhari, N.J.; Fajar, A.T.; Irkham. Recent advances in the enhanced sensing performance of zeolite-based materials. Mater. Today Commun. 2022, 33, 104331. [Google Scholar] [CrossRef]
- Cheng, L.; Li, J.; Guo, A.; Zhang, J. Recent advances in flexible noninvasive electrodes for surface electromyography acquisition. npj Flex. Electron. 2023, 7, 39. [Google Scholar] [CrossRef]
- Scott, D.N.; Frank, M.J. Adaptive control of synaptic plasticity integrates micro-and macroscopic network function. Neuropsychopharmacology 2023, 48, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive–Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Sosnik, R. Neuroengineering. In Springer Handbook of Computational Intelligence; Springer: Berlin/Heidelberg, Germany, 2015; pp. 727–769. [Google Scholar]
- Mazzucato, L. Neural mechanisms underlying the temporal organization of naturalistic animal behavior. eLife 2022, 11, e76577. [Google Scholar] [CrossRef]
- Rampichini, S.; Vieira, T.M.; Castiglioni, P.; Merati, G. Complexity analysis of surface electromyography for assessing the myoelectric manifestation of muscle fatigue: A review. Entropy 2020, 22, 529. [Google Scholar] [CrossRef]
- Boyer, M.; Bouyer, L.; Roy, J.-S.; Campeau-Lecours, A. Reducing noise, artifacts and interference in single-channel EMG signals: A review. Sensors 2023, 23, 2927. [Google Scholar] [CrossRef] [PubMed]
- La Foresta, F.; Morabito, F.C.; Azzerboni, B.; Ipsale, M. PCA and ICA for the extraction of EEG components in cerebral death assessment. In Proceedings of the 2005 IEEE International Joint Conference on Neural Networks, Montreal, QC, Canada, 31 July–4 August 2005; Volume 4, pp. 2532–2537. [Google Scholar]
- Calcagno, S.; La Foresta, F.; Versaci, M. Independent component analysis and discrete wavelet transform for artifact removal in biomedical signal processing. Am. J. Appl. Sci. 2014, 11, 57–68. [Google Scholar] [CrossRef]
- Chatterjee, S.; Thakur, R.S.; Yadav, R.N.; Gupta, L.; Raghuvanshi, D.K. Review of noise removal techniques in ECG signals. IET Signal Process. 2020, 14, 569–590. [Google Scholar] [CrossRef]
- Ehrmann, G.; Blachowicz, T.; Homburg, S.V.; Ehrmann, A. Measuring biosignals with single circuit boards. Bioengineering 2022, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Blachowicz, T.; Wójcik, D.; Surma, M.; Magnuski, M.; Ehrmann, G.; Ehrmann, A. Textile fabrics as electromagnetic shielding materials—A review of preparation and performance. Fibers 2023, 11, 29. [Google Scholar] [CrossRef]
- Li, W. Big Data precision marketing approach under IoT cloud platform information mining. Comput. Intell. Neurosci. 2022, 2022, 4828108. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef]
- Rodríguez-Tapia, B.; Soto, I.; Martínez, D.M.; Arballo, N.C. Myoelectric Interfaces and Related Applications: Current State of EMG Signal Processing—A Systematic Review. IEEE Access 2020, 8, 7792–7805. [Google Scholar] [CrossRef]
- Del Toro, S.F.; Wei, Y.; Olmeda, E.; Ren, L.; Guowu, W.; Díaz, V. Validation of a Low-Cost Electromyography (EMG) System via a Commercial and Accurate EMG Device: Pilot Study. Sensors 2019, 19, 5214. [Google Scholar] [CrossRef]
- Laganà, F.; Cacciola, M.; Calcagno, S.; De Carlo, D.; Megali, G.; Versaci, M.; Morabito, F.C. Evaluating Soft Computing Techniques for Path Loss Estimation in Urban Environments. Front. Artif. Intell. Appl. 2009, 204, 323–331. [Google Scholar]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, S.C.; Edo, G.I.; Jikah, A.N.; Emakpor, O.L.; Akpoghelie, P.O.; Agbo, J.J. Recent advances in the role of mass spectrometry in the analysis of food: A review. J. Food Meas. Charact. 2024, 18, 4272–4287. [Google Scholar] [CrossRef]
- Pratticò, D.; Calcagno, S.; Gattuso, F.; Laganà, F.; Oliva, G.; Pullano, S.A.; La Foresta, F. A Soft Computing Approach for Sensory Analysis with Thermographic Techniques for Structural Monitoring of Bronze Statues. In Proceedings of the NMP 2024, Reggio Calabria, Italy, 22–24 May 2024. in press. [Google Scholar]
- Liu, L.; Si, Y.W. 1D convolutional neural networks for chart pattern classification in financial time series. J. Supercomput. 2022, 78, 14191–14214. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ali, W.; Abdullah, T.A.A.; Malebary, S.J. Classifying Cardiac Arrhythmia from ECG Signal Using 1D CNN Deep Learning Model. Mathematics 2023, 11, 562. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Islam, S.; Islam, A.M.; Shatabda, S. An ensemble 1D-CNN-LSTM-GRU model with data augmentation for speech emotion recognition. Expert Syst. Appl. 2023, 218, 119633. [Google Scholar] [CrossRef]
- Zhao, X.; Solé-Casals, J.; Li, B.; Huang, Z.; Wang, A.; Cao, J.; Zhao, Q. Classification of Epileptic IEEG Signals by CNN and Data Augmentation. In Proceedings of the ICASSP 2020—2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain, 4–8 May 2020; pp. 926–930. [Google Scholar]
- Jia, H.; Huang, Z.; Caiafa, C.F.; Duan, F.; Zhang, Y.; Sun, Z.; Solé-Casals, J. Assessing the Potential of Data Augmentation in EEG Functional Connectivity for Early Detection of Alzheimer’s Disease. Cogn. Comput. 2024, 16, 229–242. [Google Scholar] [CrossRef]
- Coskun, M.; Yildirim, O.; Demir, Y.; Acharya, U.R. Efficient deep neural network model for classification of grasp types using sEMG signals. J. Ambient. Intell. Humaniz. Comput. 2022, 13, 4437–4450. [Google Scholar] [CrossRef]
- Baygin, M.; Barua, P.D.; Dogan, S.; Tuncer, T.; Key, S.; Acharya, U.R.; Cheong, K.H. A hand-modeled feature extraction-based learning network to detect grasps using sEMG signal. Sensors 2022, 22, 2007. [Google Scholar] [CrossRef]
- Bilotta, G.; Calcagno, S.; Bonfa, S. Wildfires: An application of remote sensing and OBIA. WSEAS Trans. Environ. Dev. 2021, 17, 282–296. [Google Scholar] [CrossRef]
- Chen, L.; Fu, J.; Wu, Y.; Li, H.; Zheng, B. Hand Gesture Recognition Using Compact CNN via Surface Electromyography Signals. Sensors 2020, 20, 672. [Google Scholar] [CrossRef]
- Bianco, M.G.; Quattrone, A.; Sarica, A.; Vescio, B.; Buonocore, J.; Vaccaro, M.G.; Aracri, F.; Calomino, C.; Gramigna, V.; Quattrone, A. Cortical atrophy distinguishes idiopathic normal-pressure hydrocephalus from progressive supranuclear palsy: A machine learning approach. Park. Relat. Disord. 2022, 103, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; He, K.; Qian, M.; Gouda, M.A. TFN-FICFM: sEMG-Based Gesture Recognition Using Temporal Fusion Network and Fuzzy Integral-based Classifier Fusion. J. Bionic Eng. 2024, 21, 1878–1891. [Google Scholar] [CrossRef]
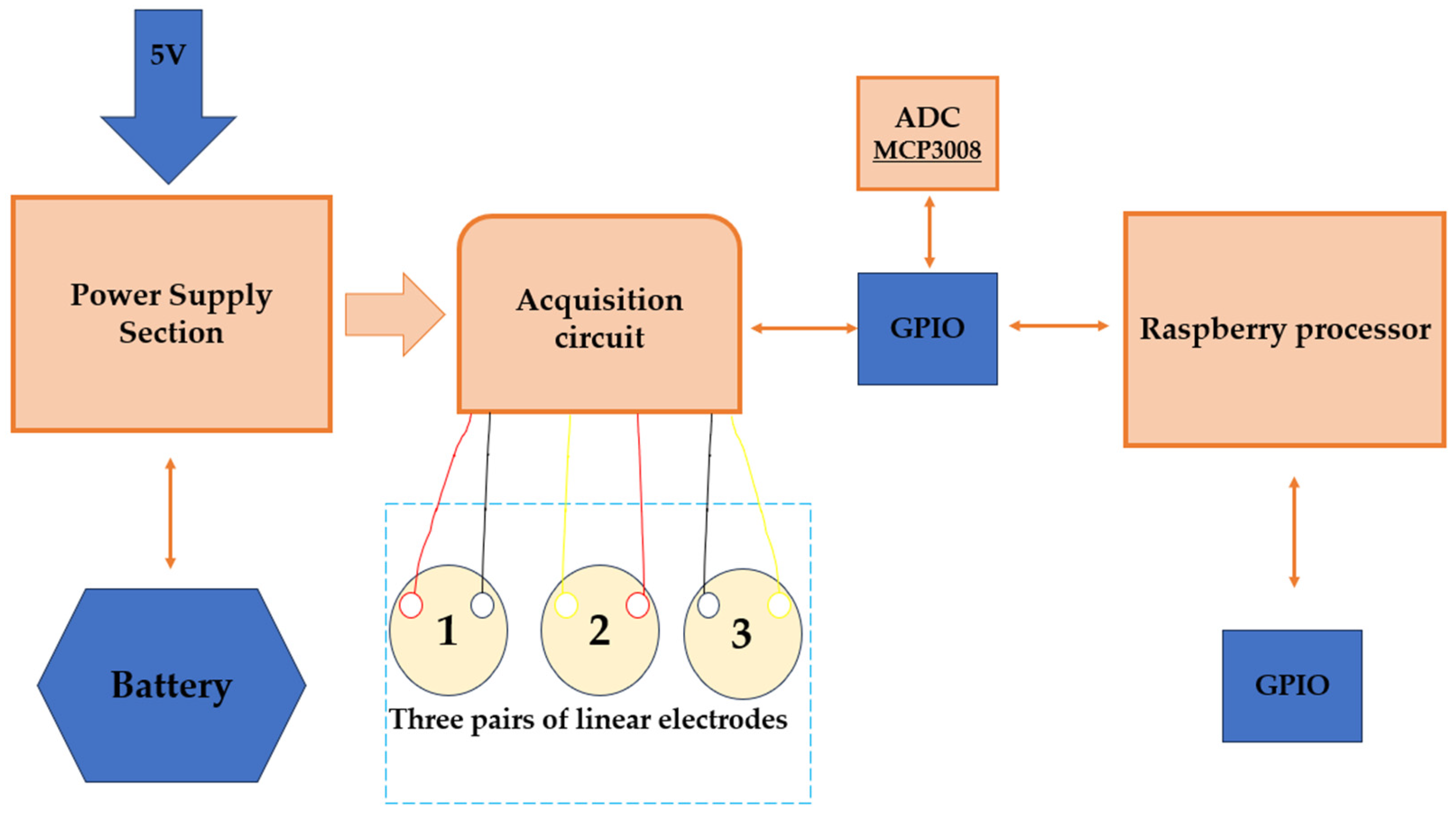
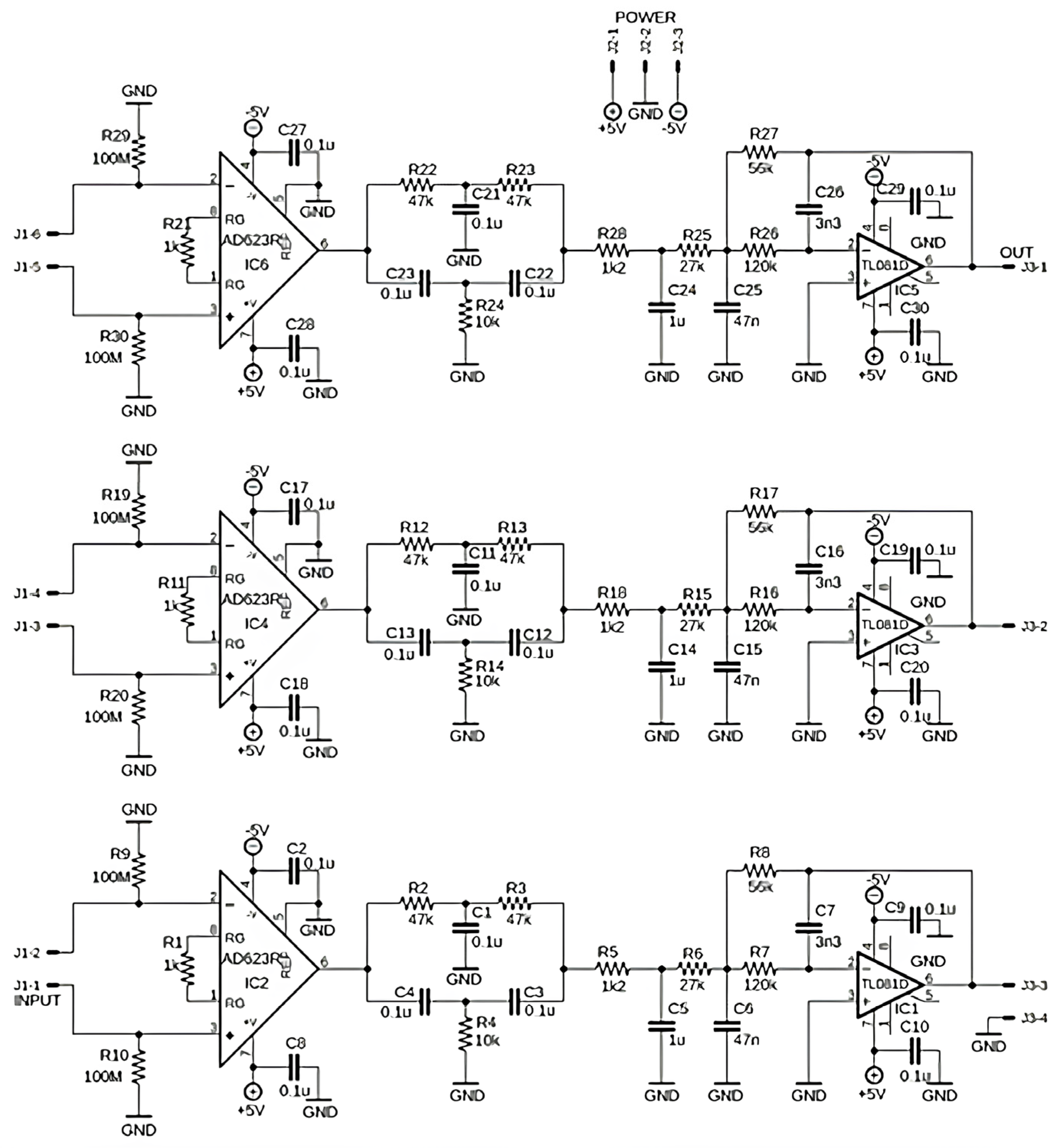
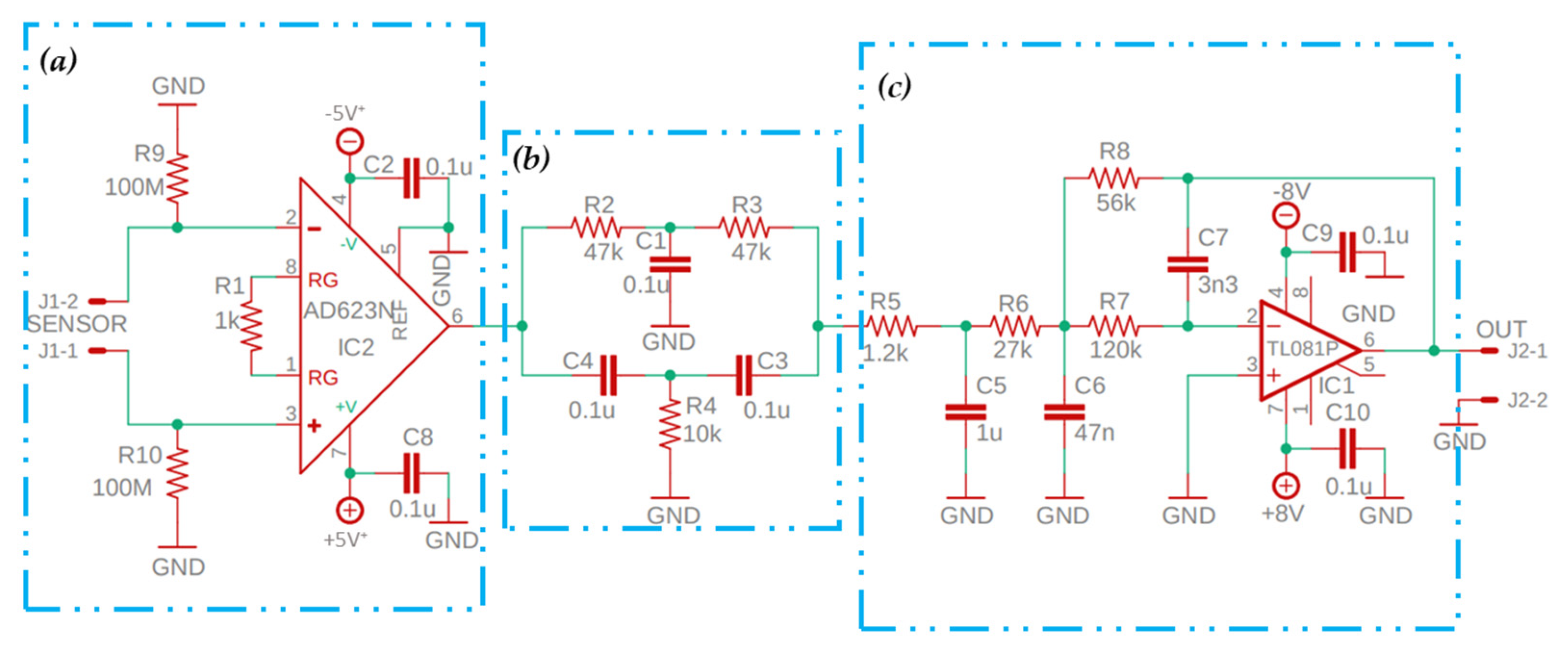
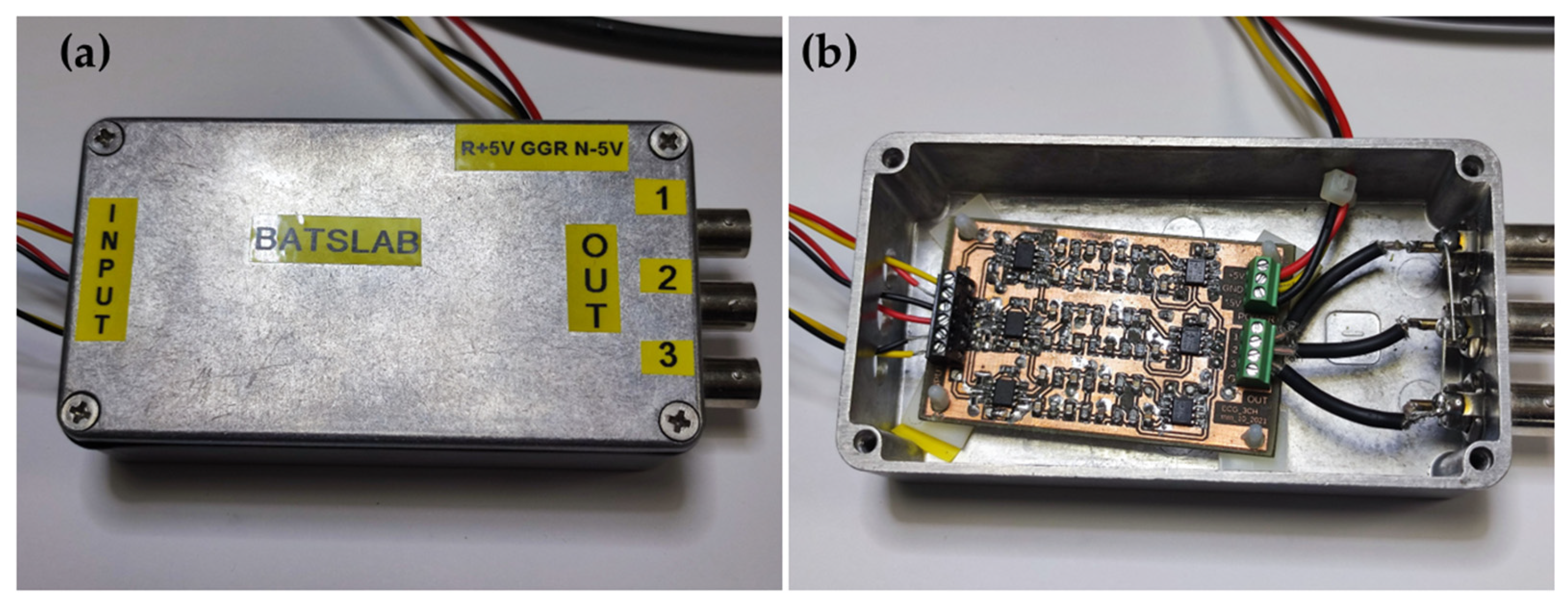
| Application | Electrodes | Algorithm | Accuracy | Advantages and Disadvantages |
|---|---|---|---|---|
| Detect neuromyopathy disorders | 9 mm silver/silver chloride electrodes | DWT WPT SVM | 8% False detection rate | Advantages Higher sensitivity and specificity Relatively simple processing |
| Diagnoses diseases | Surface electrode array Gel surface electrodes Ag/AgCl bipolar electrode | LDA Cubic SVM AR DWT ANN PCA SVM | 83–100% | Advantages Low cost and less time-consuming |
| Disease prognosis | Surface electrode array | Wilcoxon signed-rank tests LR ROC RMS RMSD | ≈74.7% | Disadvantages Unsure ability in distinguishing disorders Cross-talk overestimation of amplitude |
| Layer Number | Layer Name | Input Shape | Output Shape | Kernel Size | Stride |
|---|---|---|---|---|---|
| 1 | Conv1D | (batch_size, 20,000, 6) | (batch_size, 19,996, 64) | 5 | 1 |
| 2 | BatchNormalization | (batch_size, 19,996, 64) | (batch_size, 19,996, 64) | - 1 | - |
| 3 | MaxPooling1D | (batch_size, 19,996, 64) | (batch_size, 9998, 64) | 2 | 2 |
| 4 | Dropout | (batch_size, 9998, 64) | (batch_size, 9998, 64) | - | - |
| 5 | Conv1D | (batch_size, 9998, 64) | (batch_size, 9994, 128) | 5 | 1 |
| 6 | BatchNormalization | (batch_size, 9994, 128) | (batch_size, 4997, 128) | - | - |
| 7 | MaxPooling1D | (batch_size, 9994, 128 | (batch_size, 4997, 128) | 2 | 2 |
| 8 | Dropout | (batch_size, 4997, 128) | (batch_size, 4997, 128) | - | - |
| 9 | Conv1D | (batch_size, 4997, 128) | (batch_size, 4993, 256) | 5 | 1 |
| 10 | BatchNormalization | (batch_size, 4993, 256) | (batch_size, 2496, 256) | - | - |
| 11 | MaxPooling1D | (batch_size, 4993, 256) | (batch_size, 2496, 256) | 2 | 2 |
| 12 | Dropout | (batch_size, 2496, 256) | (batch_size, 2496, 256) | - | - |
| 13 | Conv1D | (batch_size, 2496, 256) | (batch_size, 2492, 512) | 5 | 1 |
| 14 | BatchNormalization | (batch_size, 2492, 512) | (batch_size, 2492, 512) | - | - |
| 15 | GlobalAveragePooling1D | (batch_size, 2492, 512) | (batch_size, 512) | - | - |
| 16 | Dropout | (batch_size, 512) | (batch_size, 512) | - | - |
| 17 | Dense | (batch_size, 512) | (batch_size, 256) | - | - |
| 18 | Dropout | (batch_size, 256) | (batch_size, 256) | - | - |
| 19 | Dense | (batch_size, 256) | (batch_size, 1) | - | - |
| Training | Test | |
|---|---|---|
| Accuracy | 96.732% | 94.234% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laganà, F.; Pratticò, D.; Angiulli, G.; Oliva, G.; Pullano, S.A.; Versaci, M.; La Foresta, F. Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques. Signals 2024, 5, 476-493. https://doi.org/10.3390/signals5030025
Laganà F, Pratticò D, Angiulli G, Oliva G, Pullano SA, Versaci M, La Foresta F. Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques. Signals. 2024; 5(3):476-493. https://doi.org/10.3390/signals5030025
Chicago/Turabian StyleLaganà, Filippo, Danilo Pratticò, Giovanni Angiulli, Giuseppe Oliva, Salvatore A. Pullano, Mario Versaci, and Fabio La Foresta. 2024. "Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques" Signals 5, no. 3: 476-493. https://doi.org/10.3390/signals5030025
APA StyleLaganà, F., Pratticò, D., Angiulli, G., Oliva, G., Pullano, S. A., Versaci, M., & La Foresta, F. (2024). Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques. Signals, 5(3), 476-493. https://doi.org/10.3390/signals5030025











