Case Report: Modulation of Effective Connectivity in Brain Networks after Prosthodontic Tooth Loss Repair
Abstract
:1. Introduction
2. Material and Methods
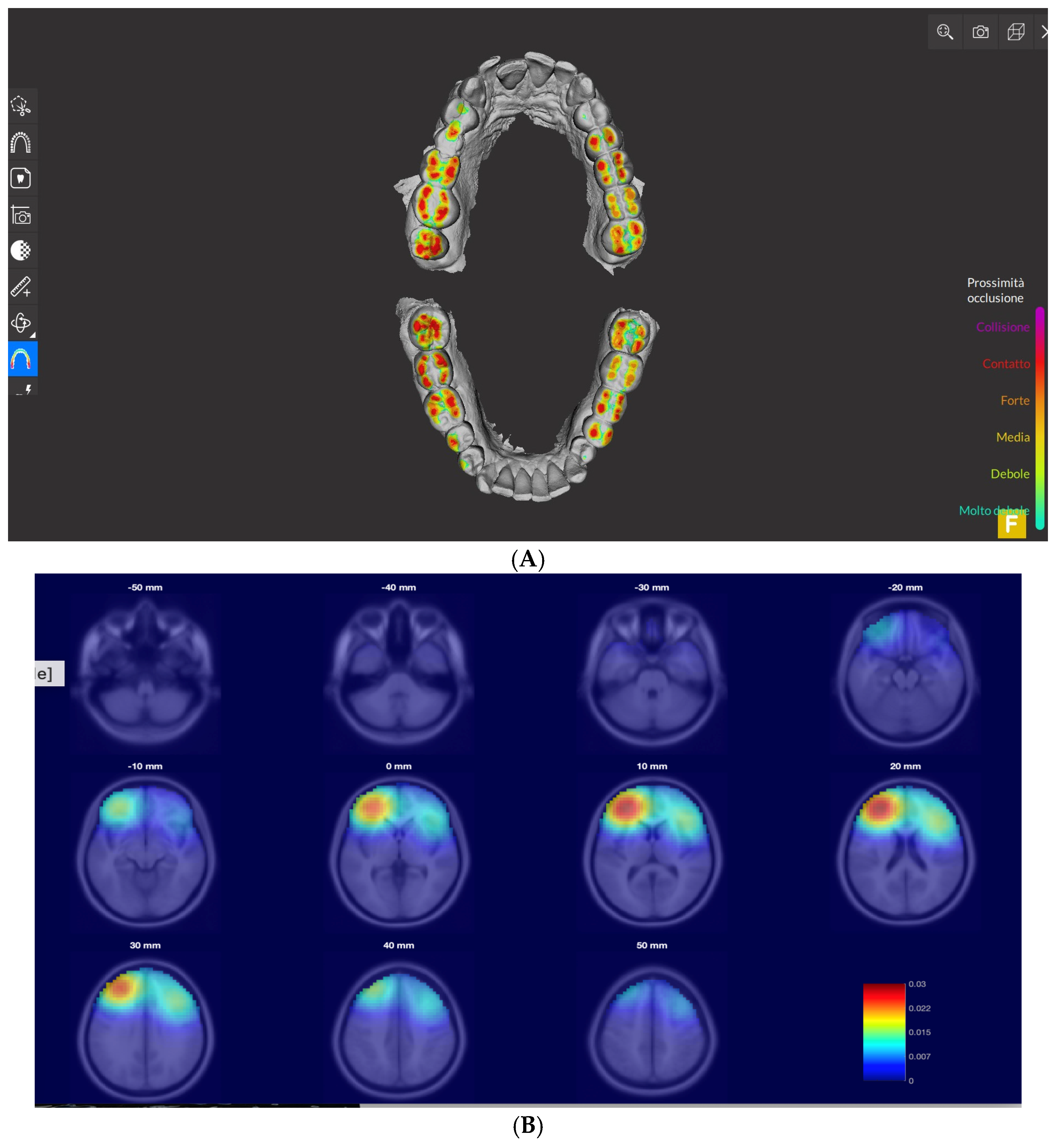
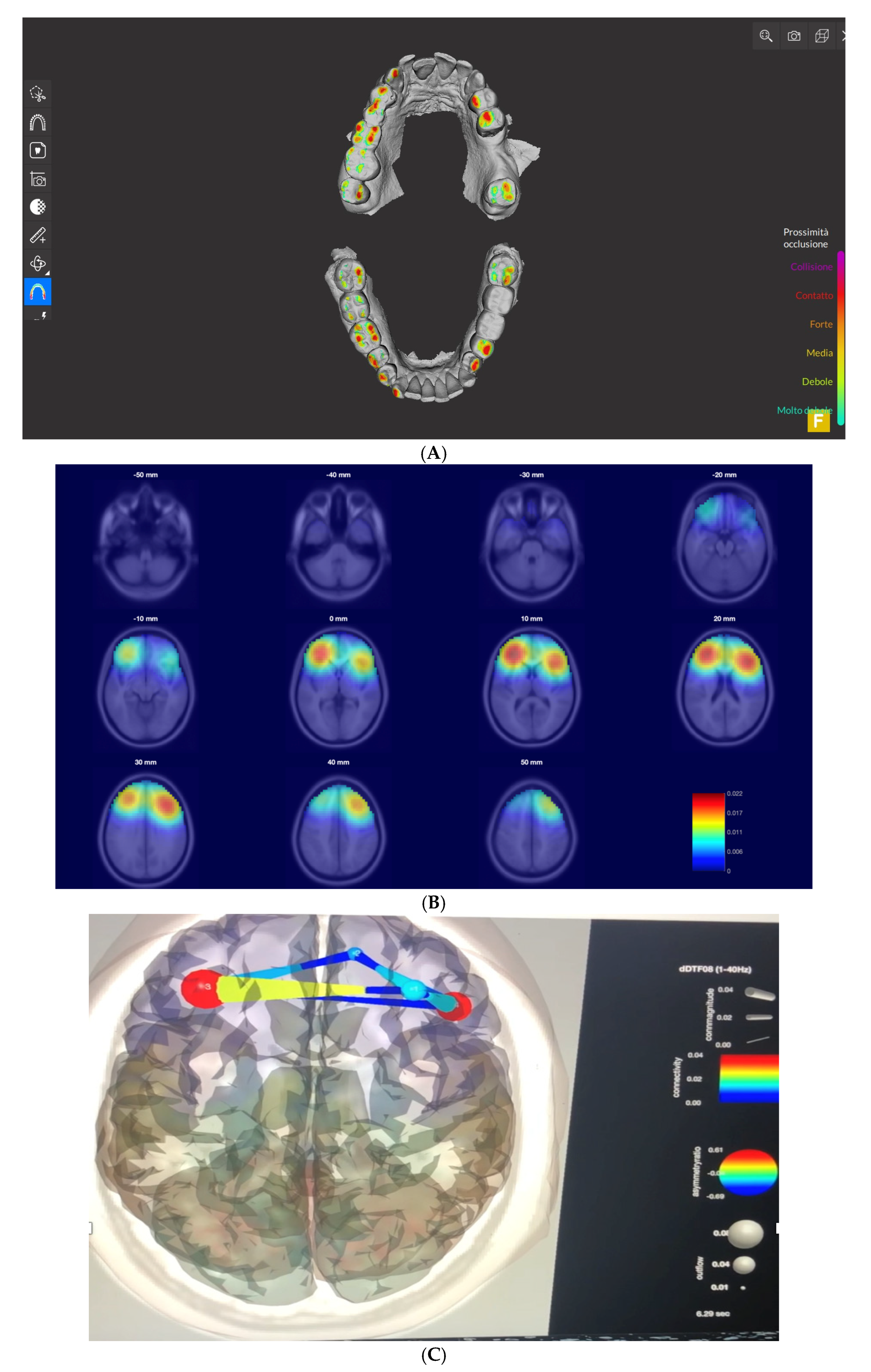
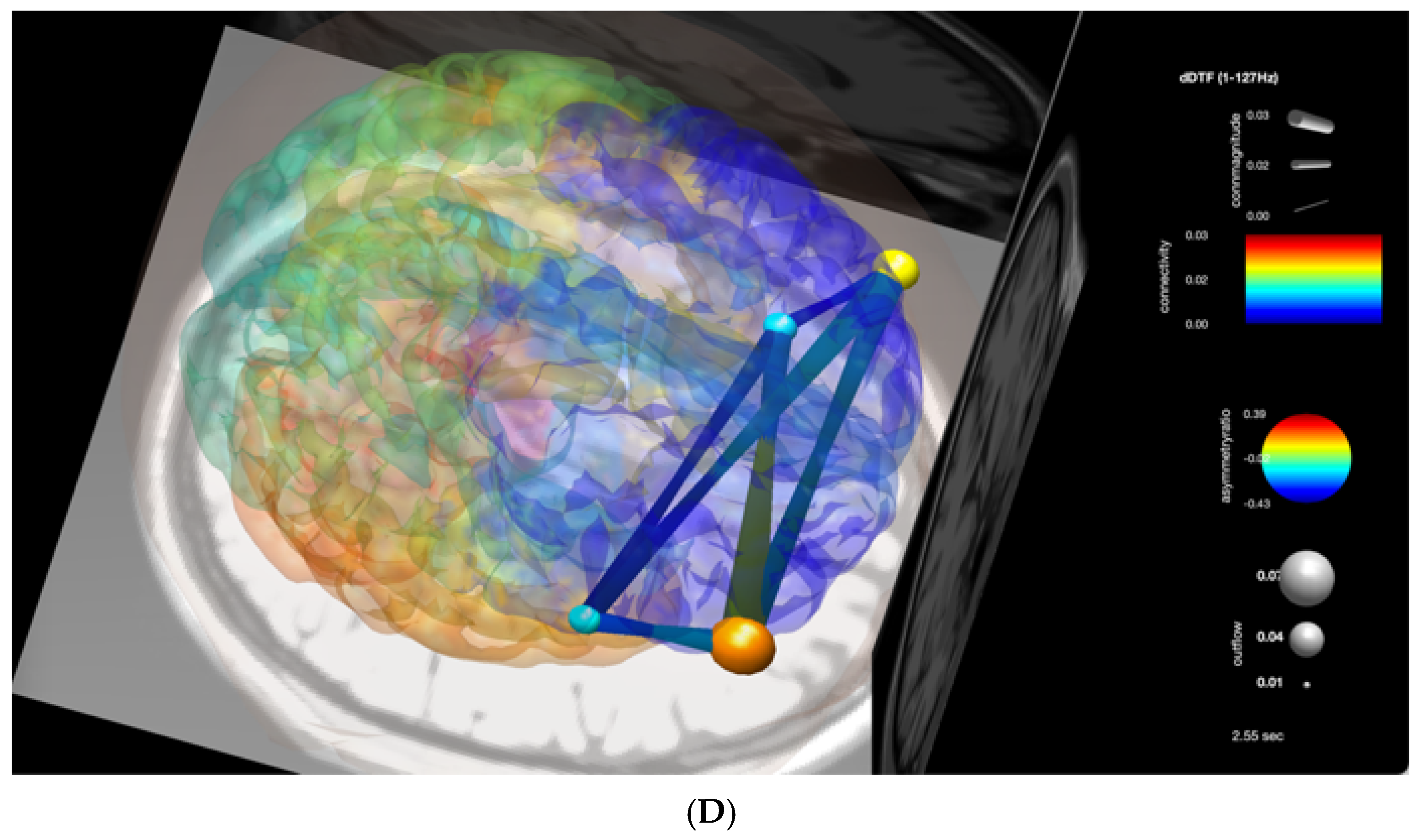
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dye, B.A. The Global Burden of Oral Disease: Research and Public Health Significance. J. Dent. Res. 2017, 96, 361–363. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Avivi-Arber, L. Neuroplasticity and the edentulous patient- toward a paradigm shift in oral rehabilitation. Int. J. Prosthodont. 2015, 28, 115. [Google Scholar] [CrossRef] [PubMed]
- Cerutti-Kopplin, D.; Feine, J.; Padilha, D.M.; De Souza, R.F.; Ahmadi, M.; Rompré, P.; Booij, L.; Emami, E. Tooth loss increases the risk of diminished cognitive function: A systematic review and meta-analysis. JDR Clin. Transl. Res. 2016, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Zamora, C.; Pons-Fuster Lopez, L.E.; Tvarijonaviciute, A. Oral Health Status in Older People with Dementia: A Case-Control Study. J. Clin. Med. 2021, 10, 477. [Google Scholar] [CrossRef]
- Avivi-Arber, L.; Lee JCSood, M.; Lakschevitz, F.; Fung, M.; Barashi-Gozal, M.; Giogauer, M.; Sessle, B.J. Long-term neuroplasticity of the face primary motor cortex and adjacent somatosensory cortex induced by tooth loss can be reversed following dental implant replacement in rats. J. Comp. Neurol. 2015, 523, 2372–2389. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Bullmore, E. Fundamentals of Brain Network Analysis; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780124079083/9780124081185. [Google Scholar]
- Luraschi, J.; Korgaonkar, M.S.; Whittle, T.; Schimmel, M.; Müller, F.; Klineber, I.g. Neuroplasticity in the adaptation to prosthodontic treatment. J. Oral Facial Pain Headache 2013, 27, 206–216. [Google Scholar] [CrossRef]
- Avivi-Arber, L.; Lee, J.C.; Sessle, B.J. Dental occlusal changes induce motor cortex neuroplasticity. J. Dent. Res. 2015, 94, 1757–1764. [Google Scholar] [CrossRef]
- Friston, K. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Jones, D.W. International dental standard. Br. Dent. J. 2007, 203, 361–369. [Google Scholar] [CrossRef]
- Krigolson, O.E.; Williams, C.C.; Norton, A.; Hassall, C.D.; Colino, F.L. Choosing MUSE: Validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraschini, M.; Demuru, M.; Crobe, A.; Marrosu, F.; Cornelis, J.; Stam, C.J.; Hillebrand, A. The effect of epoch length on estimated EEG functional connectivity and brain network organization. J. Neural Eng. 2016, 13, 036015. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Mullen, T.; Kothe, C.; Akalin Acar, Z.; Bigdely-Shamlo, N.; Vankov, A.; Makeig, S. EEGLAB, SIFT, NFT, BCILAB, and ERICA: New tools for advanced EEG Processing. Comput. Intell. Neurosci. 2011, 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Marek, S.; Dosenbach, N.U.F. The frontoparietal network: Function, electro-physiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Jin, Z.; Li, K.; Liu, G. Neuroplasticity of edentulous patients with implant-supported full dentures. Eur. J. Oral Sci. 2008, 118, 387–393. [Google Scholar] [CrossRef]
- Sessle, B.J. Sensation from the face. In Encyclopedia of Neuroscience; Squire, L., Ed.; Academic Press: Oxford, UK, 2009; Volume 8, pp. 585–592. [Google Scholar]
- Kaczmarek, L. Expression of c-fos and other genes encoding transcription factors in long-term potentiation. Behav. Neural Biol. 1992, 57, 263–266. [Google Scholar] [CrossRef]
- Jaworski, J.; Kalita, K.; Kanapska, E. c-Fos and neuronal plasticity: The aftermath of Kaczmarek’s theory. Acta Neurobiol. Exp. 2018, 78, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Yamamoto, T.; Kubo, K.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef]
- Xu, S.; Huang, X.; Gong, Y.; Sun, J. Association between tooth loss rate and risk of mild cognitive impairment in older adults: A population-based longitudinal study. J. Aging 2021, 13, 21599–21609. [Google Scholar] [CrossRef]
- Gomaa, N.; Tenenbaum, H.; Glogauer, M.; Quiñonez, C. The Biology of Social Adversity Applied to Oral Health. J. Dent. Res. 2019, 98, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of Artifact Subspace Reconstruction for Automatic EEG Artifact Removal. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Moussa, R.; Alghazaly, A.; Althagafi, A.; Eshky, R.; Borzangy, S. Effectiveness of Virtual Reality and Interactive Simulators on Dental Education Outcomes: Systematic Review. Eur. J. Dent. 2021, 16, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Dolega-Dolegowski, D.K.; Dolega-Dolegowska, M.; Pregowska, A.; Hajto-Bryk, J.; Trojak, M.; Chmiel, J.; Walecki, P.; Fudalej, P.S. Application of holography and augmented reality based technology to visualize the internal structure of the dental root—A proof of concept. Head Face Med. 2022, 18, 12. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muroni, A.; Barbar, D.; Fraschini, M.; Monticone, M.; Defazio, G.; Marrosu, F. Case Report: Modulation of Effective Connectivity in Brain Networks after Prosthodontic Tooth Loss Repair. Signals 2022, 3, 550-558. https://doi.org/10.3390/signals3030033
Muroni A, Barbar D, Fraschini M, Monticone M, Defazio G, Marrosu F. Case Report: Modulation of Effective Connectivity in Brain Networks after Prosthodontic Tooth Loss Repair. Signals. 2022; 3(3):550-558. https://doi.org/10.3390/signals3030033
Chicago/Turabian StyleMuroni, Antonella, Daniel Barbar, Matteo Fraschini, Marco Monticone, Giovanni Defazio, and Francesco Marrosu. 2022. "Case Report: Modulation of Effective Connectivity in Brain Networks after Prosthodontic Tooth Loss Repair" Signals 3, no. 3: 550-558. https://doi.org/10.3390/signals3030033
APA StyleMuroni, A., Barbar, D., Fraschini, M., Monticone, M., Defazio, G., & Marrosu, F. (2022). Case Report: Modulation of Effective Connectivity in Brain Networks after Prosthodontic Tooth Loss Repair. Signals, 3(3), 550-558. https://doi.org/10.3390/signals3030033






