Simulation-Based Study on Round Window Atresia by Using a Straight Cochlea Model with Compressible Perilymph
Abstract
:1. Introduction
2. Acoustics of Cochlea Based on Even and Odd-Mode Analysis
2.1. Fluid Equations for Compressible Media
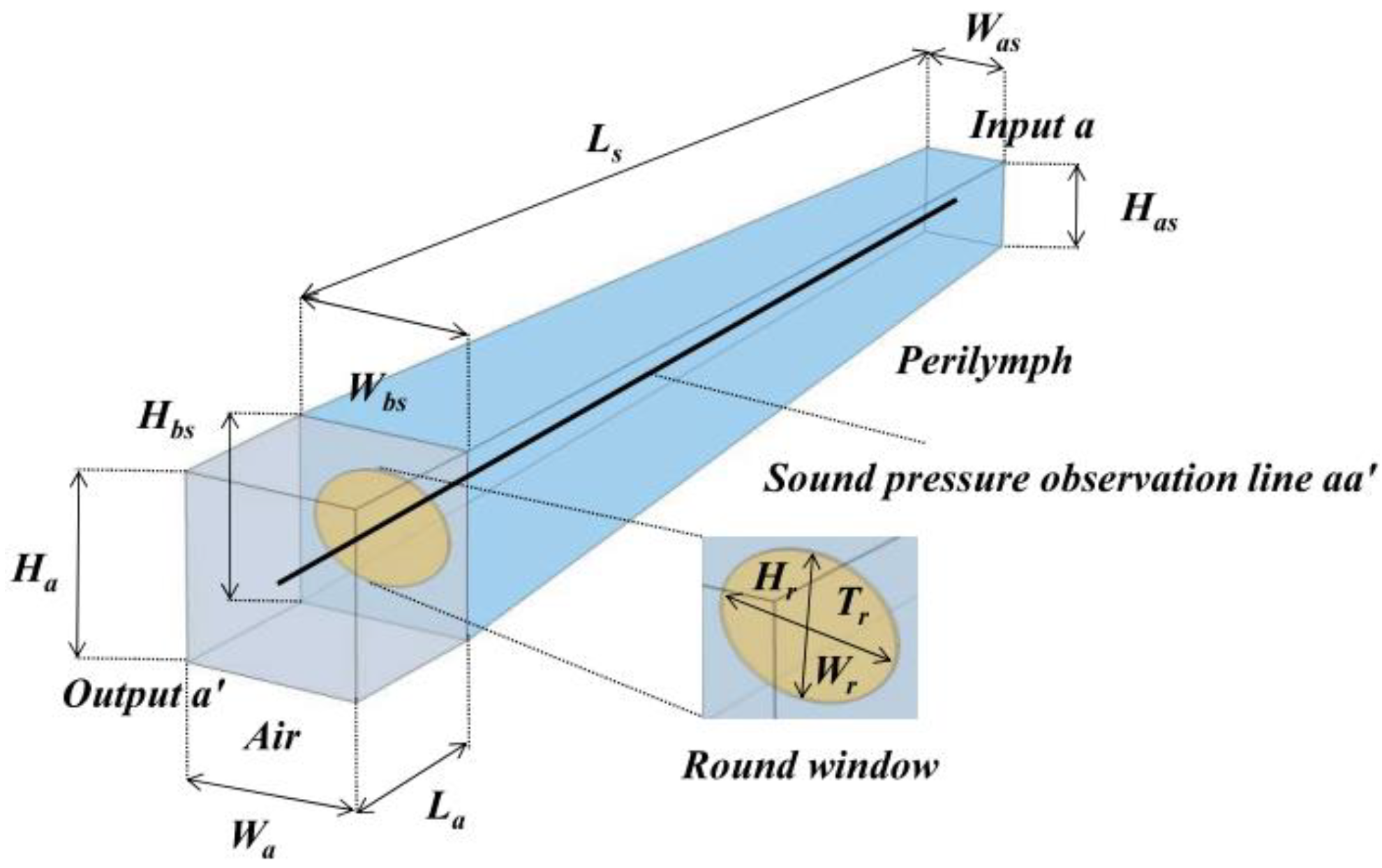
2.2. Modeling of the Cochlea
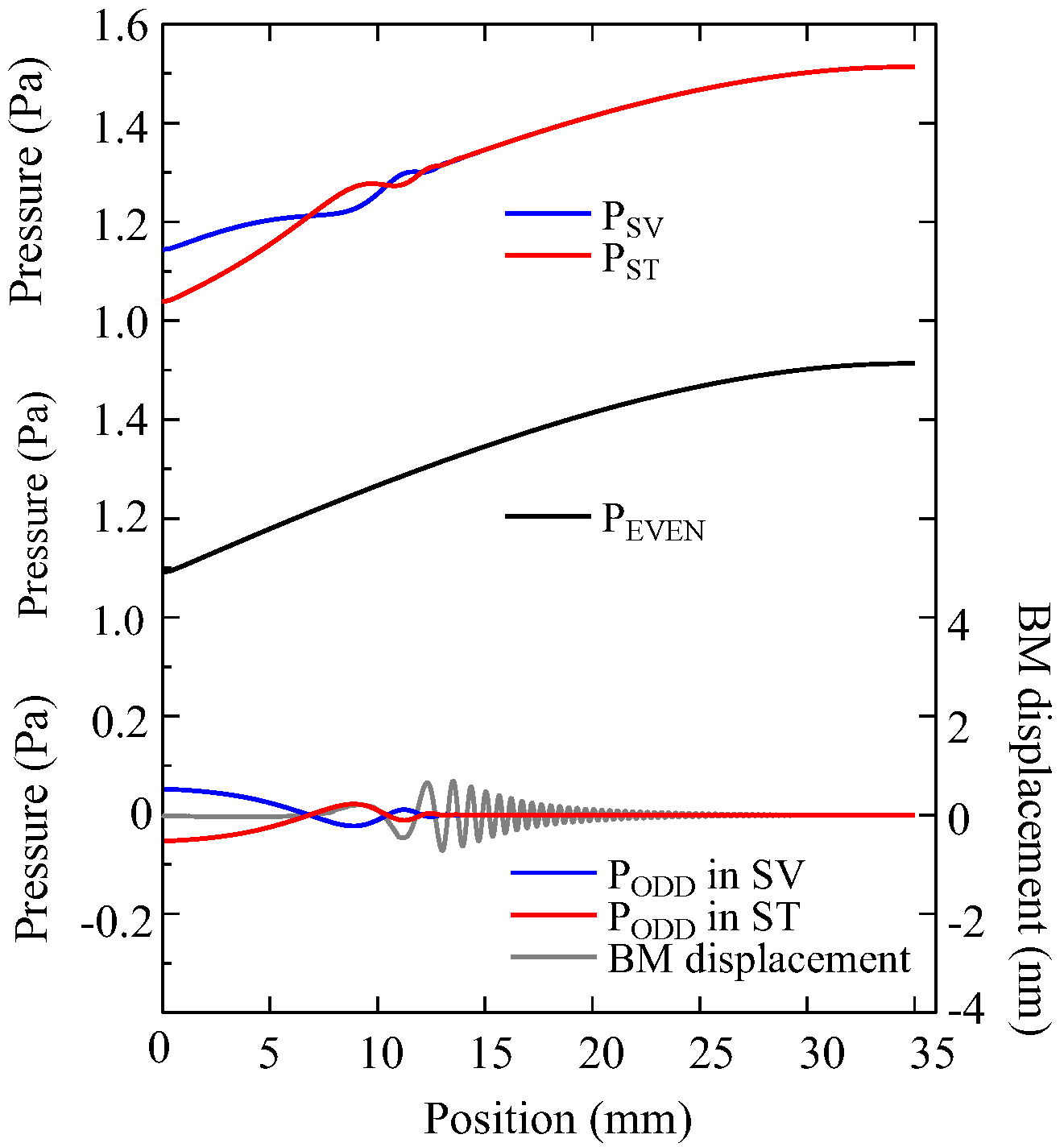
2.3. Sound Waves in Cochlea and Generation of Traveling Waves
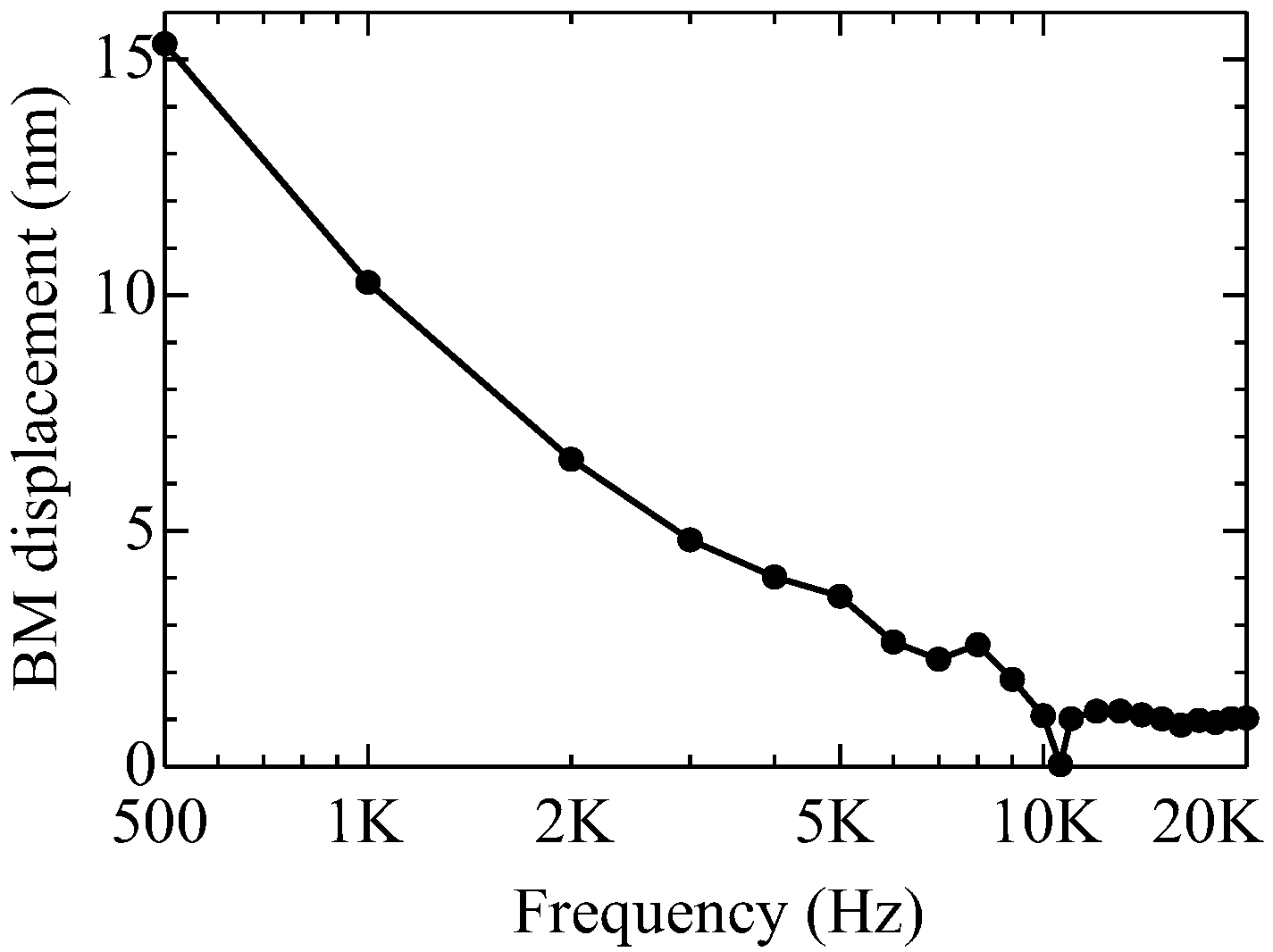
2.4. Validity of Even and Odd Mode Approaches in Other Frequencies
3. Hearing Loss by Round Window Atresia
3.1. Round Window Atresia
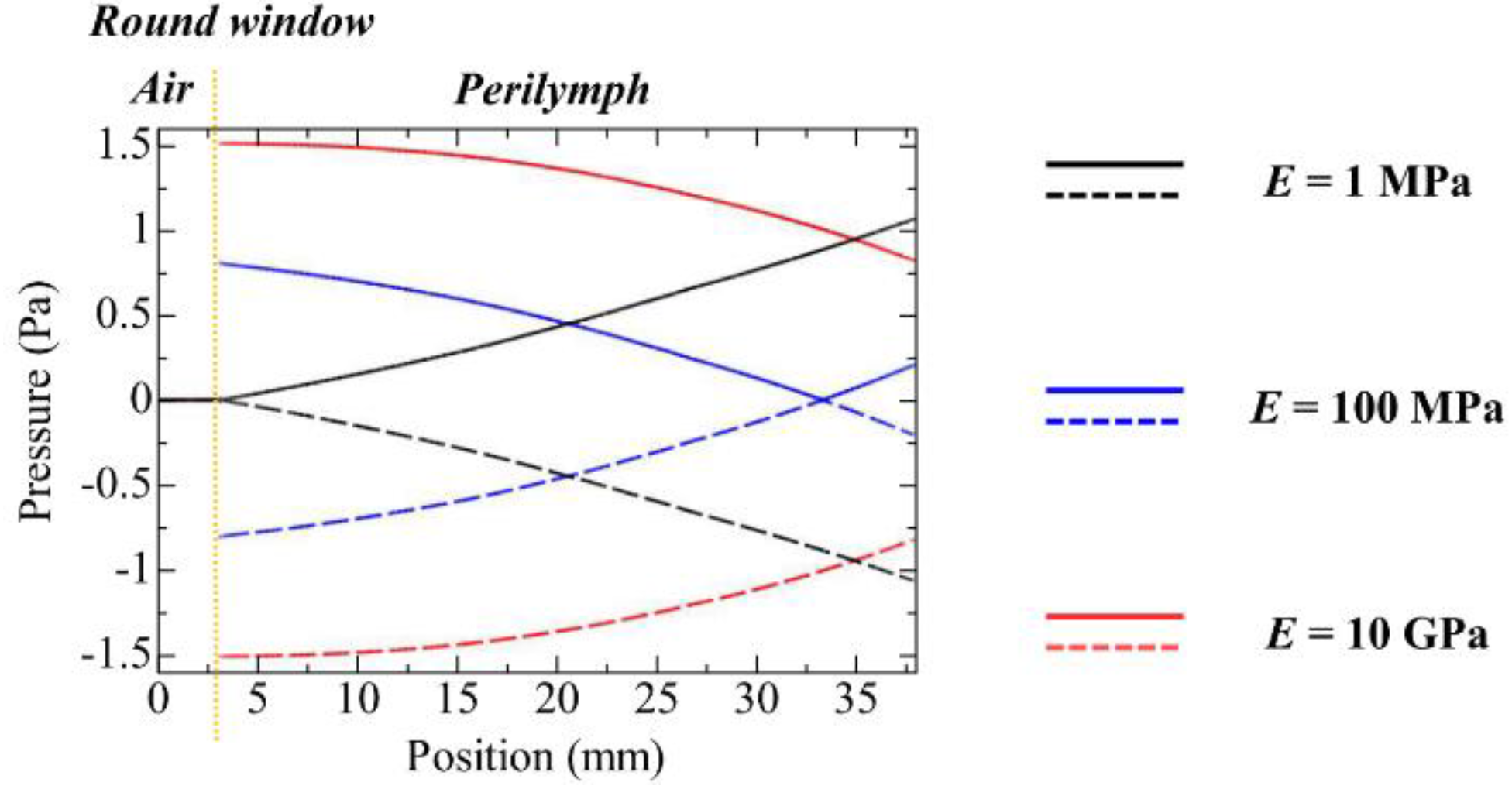
3.2. Reflection Properties of the Round Window with Round Window Atresia
- -
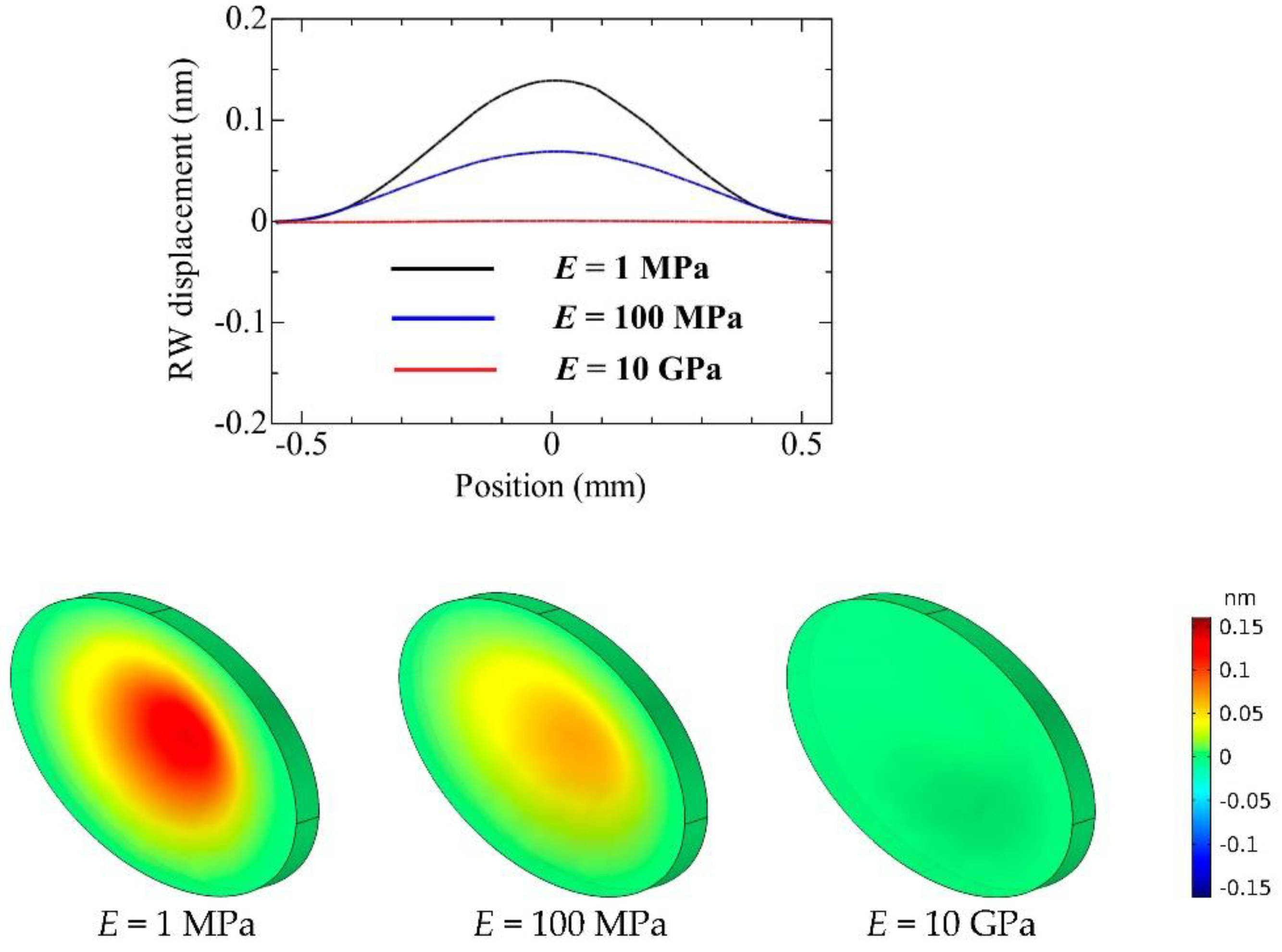
- E = 1 MPa (normal mobility—healthy ear);
- -
- E = 100 MPa (lack of mobility to some extent—mild round window atresia);
- -
- E = 10 GPa (complete lack of mobility—severe round window atresia).
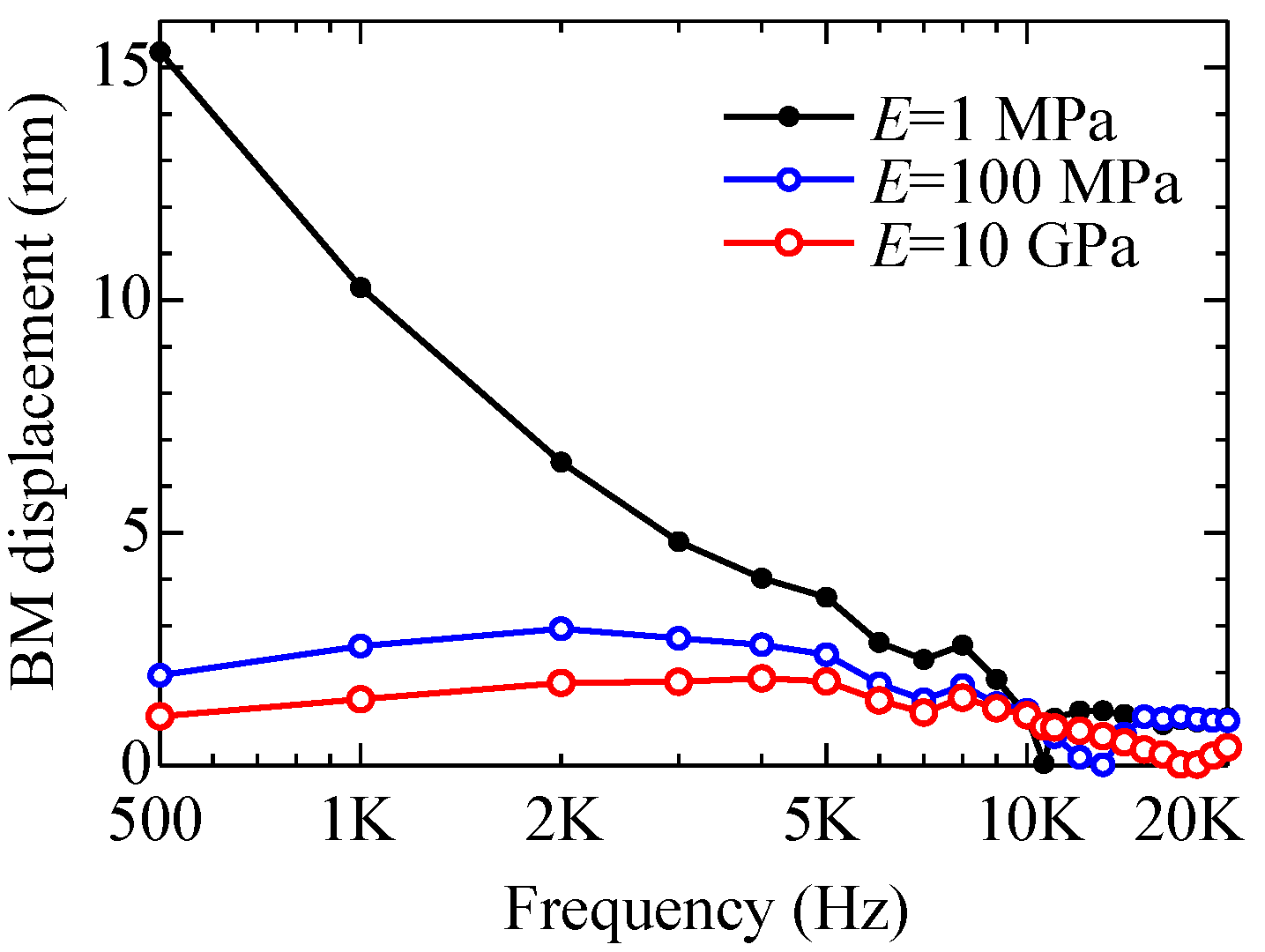
3.3. Demonstration of Round Window Atresia
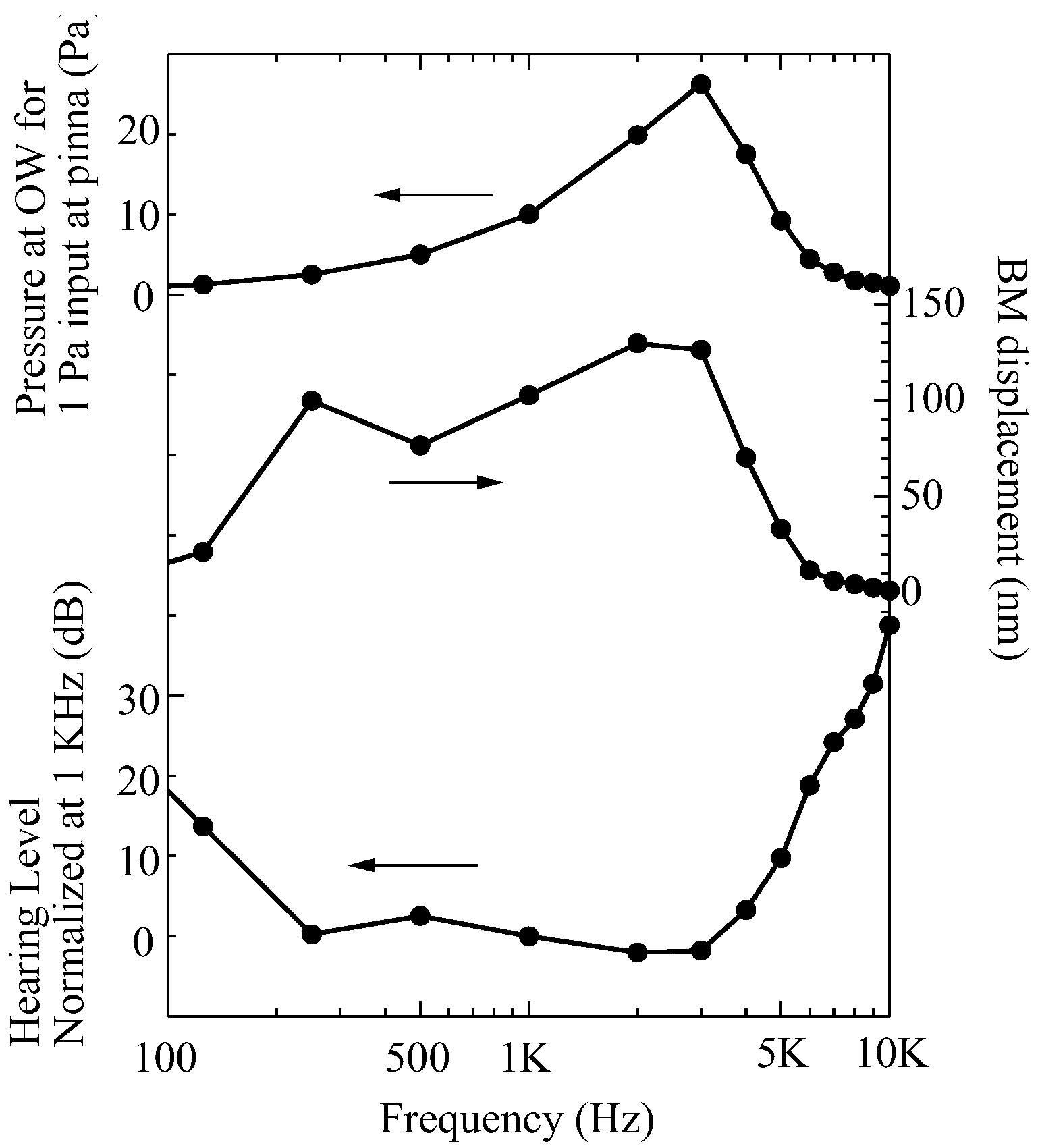
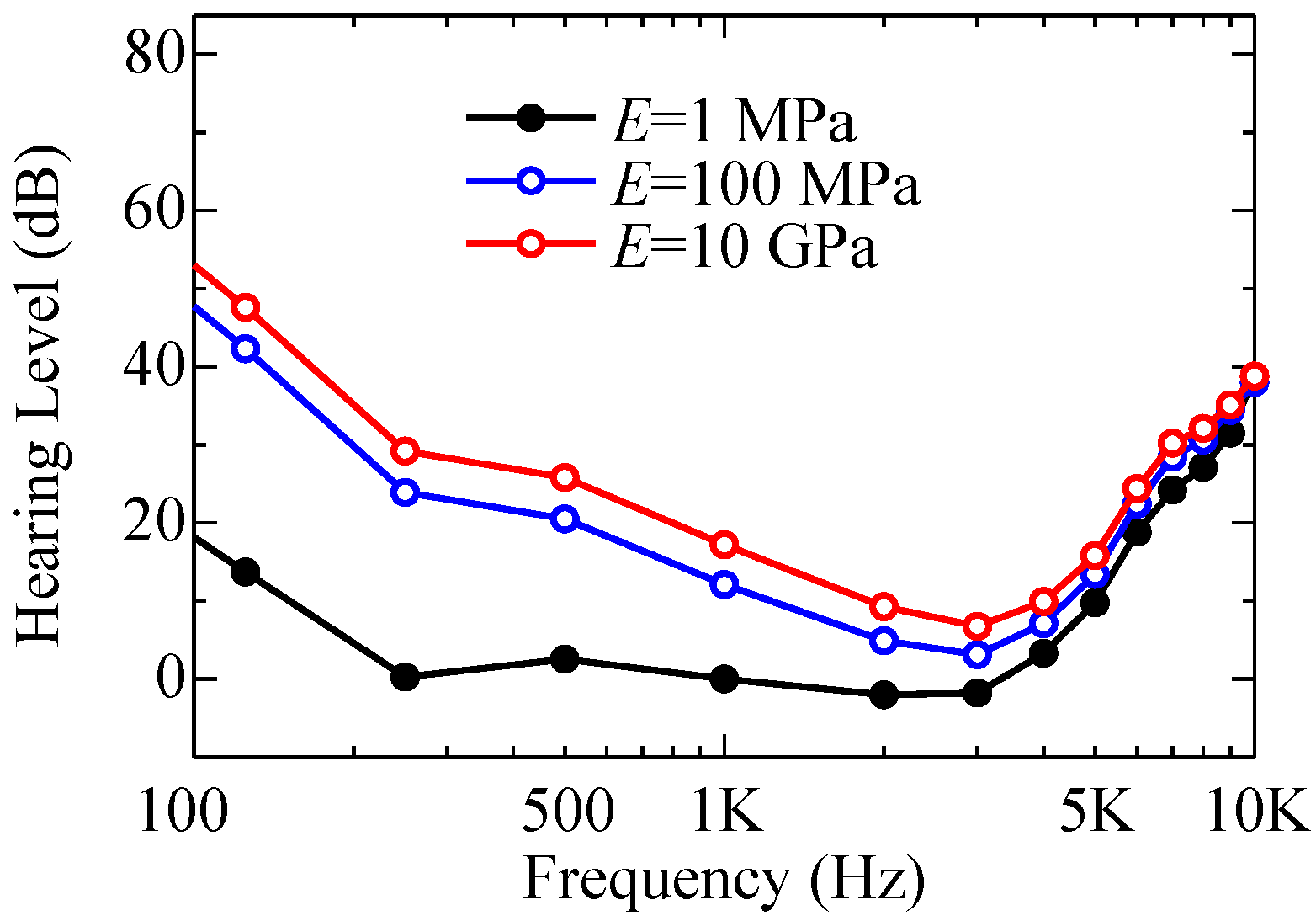
3.4. Hearing with Round Window Atresia
4. Conclusions
- (1)
- The sound waves traveling in the cochlea were classified into the even and odd symmetric modes and were expressed by the sum of these modes;
- (2)
- The odd mode excited the displacement of the basilar membrane and generated the Békésy’s traveling wave on the membrane;
- (3)
- The even mode generated a standing wave in the cochlea due to a fixed-end reflection at the cochlea apex;
- (4)
- The acoustic properties of the cochlea were determined by the contributions of the even and odd modes.
- (5)
- When the Young’s modulus E of the round window membrane is normal (e.g., a healthy ear with E = 1 MPa), the round window membrane provides a free-end reflection condition against the sound waves traveling in the scala tympani heading to the round window;
- (6)
- When the Young’s modulus E of the round window membrane is higher (e.g., an ear with severe round window atresia E = 10 GPa), the round window membrane provides a fixed-end reflection condition against the sound waves traveling in the scala tympani heading to the round window;
- (7)
- It is reported from the clinical medicine perspective that patients who have round window atresia tend to have their hearing ability degraded by 10 dB to 20 dB below 4000 Hz. Our simulation results show good agreement with the reported symptoms, and this ensures that our approach to round window atresia is correct.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, S.; Howell, P. Signals and Systems for Speech and Hearing; BRILL: Leiden, The Netherlands, 2011; p. 163. [Google Scholar] [CrossRef]
- Békésy, V. Experiments in Hearing; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Greenwood, D.D. A cochlear frequency-position function for several species–29 years later. J. Acoust. Soc. Am. 1990, 87, 2592–2605. [Google Scholar] [CrossRef]
- Ohmori, H. Gating properties of the mechanoelectrical transducer channel in the dissociated vestibular hair cell of the chick. J. Physiol. 1987, 387, 589–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Shen, W.; He, D.Z.; Long, K.B.; Madison, L.D.; Dallos, P. Prestin is the motor protein of cochlear outer hair cells. Nature 2000, 405, 149–155. [Google Scholar] [CrossRef]
- Brownell, W.E.; Bader, C.R.; Bertrand, D.; Ribaupierre, Y.D. Evoked mechanical responses of isolated cochlear outer hair cells. Science 1985, 227, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Kachar, B.; Brownell, W.E.; Altschuler, R.; Fex, J. Electrokinetic shape changes of cochlear outer hair cells. Nature 1986, 322, 365–368. [Google Scholar] [CrossRef]
- Liberman, M.C.; Gao, J.; He, D.Z.; Wu, X.; Jia, S.; Zuo, J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002, 419, 300–304. [Google Scholar] [CrossRef]
- Pickles, J.O. An Introduction to the Physiology of Hearing; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Dallos, P.; Popper, A.N.; Fay, R.R. The Cochlea; Springer: New York, NY, USA, 1996. [Google Scholar]
- Peterson, B.P.; Bogert, L.C. A dynamic theory of the cochlea. J. Acoust. Soc. Am. 1950, 22, 369–381. [Google Scholar]
- Zwislocki, J.J. Theory of the acoustic action of the Cochlea. J. Acoust. Soc. Am. 1950, 22, 778–784. [Google Scholar] [CrossRef]
- Hawkins, H.L.; McMullen, T.A.; Popper, A.N.; Fay, R.R. Auditory Computation; Springer: New York, NY, USA, 1995. [Google Scholar]
- Wada, H.; Takasaka, T.; Ikeda, K.; Ohyama, K.; Koike, T. Recent Developments in Auditory Mechanics; World Scientific: Singapore, 2000. [Google Scholar]
- Kim, N.; Homma, K.; Puria, S. Inertial bone conduction: Symmetric and anti–symmetric components. J. Assoc. Res. Otolaryngol. 2011, 12, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Kwacz, M.; Marek, P.; Borkowski, P.; Mrowka, M. A three–dimensional finite element model of round window membrane vibration before and after stapedotomy surgery. Biomech. Modeling Mechanobiol. 2013, 12, 1243–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, J.; Kim, G. A novel frequency selectivity approach based on travelling wave propagation in mechanoluminescence basilar membrane for artificial cochlea. Sci. Rep. 2018, 8, 12023. [Google Scholar] [CrossRef] [PubMed]
- Givelberg, E.; Bunn, J. A comprehensive three–dimensional model of the cochlea. J. Comput. Phys. 2003, 191, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, X.; Ta, N.; Rao, Z.; Tian, J. Finite element modeling of the human cochlea using fluid-structure interaction method. J. Mech. Med. Biol. 2015, 15, 1550039. [Google Scholar] [CrossRef]
- Tang, Z.; Shen, Q.; Xu, C.; Hou, X.; Wang, Q.; Liu, Z.; Li, S. Research on the characteristics of dynamic behavior of basilar membrane in spiral cochlea. J. Vibroeng. 2017, 19, 3809–3821. [Google Scholar]
- Yao, W.; Zhong, J.; Icon, O.; Duan, M. Three-dimensional finite–element analysis of the cochlear hypoplasia. Acta Otolaryngol. 2018, 138, 961–965. [Google Scholar] [CrossRef]
- Yao, W.; Liang, J.; Ren, L.; Ma, J.; Zhao, Z.; Wang, J.; Xie, Y.; Dai, P.; Zhang, T. Revealing the actions of the human cochlear basilar membrane at low frequency. Commun. Nonlinear Sci. Numer. Simul. 2022, 104, 106043. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, J.; Ta, N.; Rao, Z. Finite element analysis of round-window stimulation of the cochlea in patients with stapedial otosclerosis. J. Acoust. Soc. Am. 2019, 146, 4122–4130. [Google Scholar] [CrossRef]
- Xue, L.; Liu, H.; Yang, J.; Liu, S.; Zhao, Y.; Huang, X. Research on coupling effects of actuator and round window membrane on reverse stimulation of human cochlea. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 447–458. [Google Scholar] [CrossRef]
- Hong, W.; Horii, Y. Contribution of even/odd sound wave modes in human cochlear model on excitation of traveling waves and determination of cochlear input impedance. Acoustics 2022, 4, 168–182. [Google Scholar] [CrossRef]
- Würfel, W.; Lanfermann, H.; Lenarz, T.; Majdani, O. Cochlear length determination using cone beam computed tomography in a clinical setting. Hear. Res. 2014, 316, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Gaurkar, S.; Deshmukh, P.T.; Khatri, M.; Kalambe, S.; Lakhotia, P.; Chandravanshi, D.; Disawal, A. Applied anatomy of round window and adjacent structures of tympanum related to cochlear implantation. Braz. J. Otorhinolaryngol. 2019, 85, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, W.; Zhao, Y.; Yang, J.; Yang, S.; Huang, X.; Liu, W. Effect of stimulation sites on the performance of electromagnetic middle ear implant: A finite element analysis. Comput. Biol. Med. 2020, 124, 103918. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Sakamoto, C.; Sakashita, T.; Hayashi, K.; Kanzaki, S.; Ogawa, K. Effects of a perilymphatic fistula on the passive vibration response of the basilar membrane. Hear. Res. 2012, 283, 117–125. [Google Scholar] [CrossRef]
- Singla, A.; Sahni, D.; Gupta, A.K.; Loukas, M.; Aggarwal, A. Surgical anatomy of round window and its implications for cochlear implantation. Clin. Anat. 2014, 27, 331–333. [Google Scholar] [CrossRef]
- Goycoolea, M.V.; Lundman, L. Round window membrane. Structure function and permeability. Microsc. Res. Tech. 1997, 36, 201–211. [Google Scholar] [CrossRef]
- Carpenter, A.M.; Muchow, D.; Goycoolea, M.V. Ultrastructural studies of the human round window membrane. Arch. Otolaryngol. Head Neck Surg. 1989, 115, 585–590. [Google Scholar] [CrossRef]
- Suzuki, Y.; Takeshima, H. Equal-loudness-level contours for pure tones. J. Acoust. Soc. Am. 2004, 116, 918–933. [Google Scholar] [CrossRef] [Green Version]
- Thongchom, C.; Saffari, P.R.; Refahati, N.; Saffari, P.R.; Pourbashash, H.; Sirimontree, S.; Keawsawasvong, S. An analytical study of sound transmission loss of functionally graded sandwich cylindrical nanoshell integrated with piezoelectric layers. Sci. Rep. 2022, 12, 3048. [Google Scholar] [CrossRef]
- Thongchom, C.; Jearsiripongkul, T.; Refahati, N.; Roudgar Saffari, P.; Sirimontree, S.; Keawsawasvong, S. Sound transmission loss of a honeycomb sandwich cylindrical shell with functionally graded porous layers. Buildings 2022, 12, 151. [Google Scholar] [CrossRef]
- Su, W.Y.; Marion, M.S.; Hinojosa, R.; Matz, G.J. Anatomical measurements of the cochlear aqueduct, round window membrane, round window niche, and facial recess. Laryngoscope 1982, 92, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, A.; Arnold, W. Non-syndromal round window atresia: An autosomal dominant genetic disorder with variable penetrance? Eur. Arch. Otorhinolaryngol. 2007, 264, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Kubo, K.; Matsumoto, M.; Tamae, A.; Ohashi, M.; Umeno, Y.; Komune, S.; Nakagawa, T. The hearing level in a patient with otosclerosis may not recover due to ossification of the round window. Otol. Fukuoka 2018, 64, 1–6. [Google Scholar]
- Wong, W.K.; Salkeld, L.; Flint, D. Isolated Congenital Round Window Atresia: Report of 2 Cases. Ear Nose Throat J. 2020, 99, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.J.; Farrior, J.B. Stapedectomy and round window closure. Laryngoscope 1987, 97, 10–12. [Google Scholar] [CrossRef]
- Linder, T.E.; Ma, F.; Huber, A. Round Window Atresia and its Effect on Sound Transmission. Otol. Neurotol. 2003, 24, 259–263. [Google Scholar] [CrossRef]
- Mehrgardt, S.; Mellert, V. Transformation characteristics of the external human ear. J. Acoust. Soc. Am. 1977, 61, 1567–1576. [Google Scholar] [CrossRef]
- Ruggero, M.A.; Temchin, A.N. Middle-ear transmission in humans: Wide-band, not frequency-tuned? Acoust. Res. Lett. Online 2003, 4, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Mansour, S.; Nicolas, K.; Ahmad, H.H. Round window otosclerosis: Radiologic classification and clinical correlations. Otol. Neurotol. 2011, 32, 384–392. [Google Scholar] [CrossRef]



| FEM Mesh Settings (typical cochlea model) | |
|---|---|
| Maximum mesh size | 1000 μm |
| Minimum mesh size | 10 μm |
| Mesh generation | automatic |
| Number of mesh elements | 1,033,262 |
| FEM Settings for Time Domain Analysis | |
| Time step | 0.01 ms |
| Time range | 0 ms – 40 ms |
| Computation time (depend on convergence) | 48 h – 72 h |
| FEM Material Settings | |
| Compressible perilymph | |
| Viscosity | 0.7027 mPa·s |
| Density | 994.6 kg/m3 |
| Sound velocity | 1520 m/s |
| Basilar membrane | |
| Density | 1200 kg/m3 |
| Young’s modulus | 1 MPa |
| Poisson’s ratio | 0.49 |
| Round window membrane | |
| Density | 1200 kg/m3 |
| Young’s modulus | 1 MPa |
| Poisson’s ratio | 0.49 |
| PC specification | |
| CPU | Corei9–7980XE |
| Clock | 2.6 GHz |
| Memory | 128 GB |
| OS | Win 10 Pro 64bit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, W.; Horii, Y. Simulation-Based Study on Round Window Atresia by Using a Straight Cochlea Model with Compressible Perilymph. Acoustics 2022, 4, 345-361. https://doi.org/10.3390/acoustics4020021
Hong W, Horii Y. Simulation-Based Study on Round Window Atresia by Using a Straight Cochlea Model with Compressible Perilymph. Acoustics. 2022; 4(2):345-361. https://doi.org/10.3390/acoustics4020021
Chicago/Turabian StyleHong, Wenjia, and Yasushi Horii. 2022. "Simulation-Based Study on Round Window Atresia by Using a Straight Cochlea Model with Compressible Perilymph" Acoustics 4, no. 2: 345-361. https://doi.org/10.3390/acoustics4020021
APA StyleHong, W., & Horii, Y. (2022). Simulation-Based Study on Round Window Atresia by Using a Straight Cochlea Model with Compressible Perilymph. Acoustics, 4(2), 345-361. https://doi.org/10.3390/acoustics4020021






