Abstract
In Europe, transjugular intrahepatic portosystemic shunt (TIPS) is considered the primary treatment for gastric varix (GV) bleeding to reduce portal hypertension. However, in Asian countries, balloon-occluded retrograde transvenous obliteration (BRTO) and its variants plug/coil assisted transvenous retrograde obliteration (PARTO/CARTO) are the preferred approaches. The purpose of this study is to report a European single-center experience in the use of PARTO/CARTO techniques for the treatment of GVs in patients with portal hypertension, focusing on the effectiveness and safety of the procedure. All the procedures involving the PARTO/CARTO techniques performed from 2019 to 2023 were retrospectively evaluated. Technical success was defined as the complete obliteration of both the GVs and the gastro-renal (GR) shunt on review of the Computed Tomography (CT) scan performed 3 days after the procedure. The obliteration rate was also evaluated through performing CT scans 1 and 12 months after the procedure, and through endoscopic follow-up at 3 and 6 months. Additionally, major and minor complications were reported. The study involved seven patients, with a technical success of 100%. During follow-up, there were no episodes of variceal rebleeding or upper gastrointestinal bleeding. Two patients developed ascites, which resolved with medical therapy. One patient exhibited focal portal thrombosis, which was successfully treated with heparin. PARTO/CARTO techniques demonstrated high technical and clinical success rates, offering advantages over traditional BRTO. The use of coils and plugs simplifies the procedure, eliminates sclerosing agents, and prevents complications associated with balloon guiding catheters.
1. Introduction
Gastric varices (GVs) bleed less frequently than esophageal varices (EVs), while having higher risks of morbidity and mortality [1]. The treatment for GVs includes medical therapy, endoscopic therapy, surgery, and endovascular interventions. However, clear guidelines for the management of bleeding GVs are lacking [1]. The endoscopic approach is not always suitable to treat GV bleeding, due to the larger size and complex anatomy of GVs, which contribute to a lower long-term success rate in managing GVs compared to EVs [2]. Transjugular intrahepatic portosystemic shunt (TIPS) also aims to redirect portal flow into the systemic circulation, thereby reducing hepatic blood flow [2]. However, this can potentially lead to liver failure and exacerbate hepatic encephalopathy. Moreover, GVs typically manifest at lower portal pressures than EVs [2]. This raises a question regarding the effectiveness of comprehensive portal pressure reduction using TIPS in the management of GVs [2].
These limitations have prompted the advancement of minimally invasive procedures, such as balloon-occluded retrograde transvenous obliteration (BRTO) [1]. BRTO involves the insertion of a balloon-guiding catheter (BCG) into an outflow shunt, e.g., gastric-renal or gastric-inferior vena cava, through either the femoral or the internal jugular vein. The blood flow is then blocked, and a sclerosing agent is injected endovascularly directly into the varices [3]. Due to the obstruction of a large gastro-renal (GR) shunt, BRTO can potentially result in an elevation of the portal pressure gradient. However, it also increases hepatic blood flow, possibly leading to improved liver function in patients with compromised reserves, reducing the incidence of hepatic encephalopathy [4].
PARTO/CARTO represent a recent advancement of the BRTO procedure, as they use coils and plugs instead of BGC to achieve a simpler and faster permanent occlusion of the shunt. This ensures shorter recovery times and better procedure tolerability for the patient [4].
Another advantage of these procedures is the absence of need for the use of sclerosing agents such as ethanolamine oleate, substances with potential adverse effects. Both PARTO and CARTO utilize Gelfoam as an embolization material, which ensures higher safety in terms of complications related to non-target embolization [4].
In Europe, TIPS is considered the primary treatment for GV bleeding to reduce portal hypertension. However, in Asian countries, BRTO and its variants (PARTO/CARTO) are the preferred approaches [5].
The purpose of this study was to report a European single-center experience in the use of PARTO/CARTO techniques for the treatment of GVs in patients with portal hypertension, and to underline the effectiveness and safety of the procedure. Moreover, our experience could lead other European interventional radiologists to become aware and perform these procedures in suitable patients.
2. Results
The study involved seven patients diagnosed with both liver cirrhosis and GVs. The procedures were performed by three physicians, with individual experience ranging from 10 to more than 20 years.
The patient sample included four males (57%) and three females (43%), with an average age of 58.7 years (range: 41–92 years). The causes of liver cirrhosis were identified as alcoholic in four cases (57%), hepatitis C virus (HCV) in two cases (29%), and nonalcoholic steatohepatitis (NASH) in one case (14%). The average model for end-stage liver disease (MELD) score was 11.4 (from a range of 5–15).
Among these patients, we identified five (71%) presenting gastroesophageal varix (GOV) 1, extending down to cardia or lesser curve) and 2 (29%) IGV1 (isolated gastric varices of the fundus) using esophagogastroduodenoscopy and CT scans; five (71%) had a history of previous hemorrhage, and one (14%) presented with acute massive hematemesis, while one (14%) had no history of hemorrhage (Table 1).

Table 1.
Patient characteristics.
Except for the patient experiencing massive hematemesis (Figure 2), all others were hemodynamically stable. This patient initially presented with severe hypotension and required hemodynamic stabilization before the procedure. However, the hemodynamic condition normalized between the end of the procedure and the following hours.
Prior to the procedures, each patient underwent a contrast-enhanced computed tomography (CT) scan to map the anatomy of the varices and facilitate planning for subsequent interventions.
Among the participants, four (57%) underwent CARTO via the transfemoral approach, while three (43%) received PARTO with the transjugular approach; of these, in one (14%) patient a transfemoral approach was firstly used, which was then switched to transjugular due to difficulties in catheterizing the GR shunt and advancing the guiding sheath.
The average duration of the procedure, from venous access to the end of the procedure, was 117 min (with a range of 48 min–165 min). The technical success rate was 100%: successful coil and plug occlusion of the efferent shunt was achieved, along with complete Gelfoam embolization and thrombosis of the gastric varices, as showed by a CT scan 3 days after the procedure.
Clinical success was achieved in all seven patients, resulting in a complete resolution of variceal bleeding, as confirmed by both clinical indicators (hemodynamic stability, no need for blood transfusion, and the absence of hematemesis or melena) and endoscopic and radiological evidence (disappearance of shunt observed in CT scans and varices at endoscopy). In four (57%) patients, the shunt remained patent at 20 min after precise coils/plug deployment (three for CARTO and one for PARTO, respectively) due to severe thrombocytopenia of these patients; therefore, few drops of NBCA + Lipiodol (1:1) were administered to close the shunt.
No immediate major complications related to the procedure or patient deaths were reported.
Follow-up CT scans at 1 month confirmed the disappearance of targeted varices in all patients. All patients had a 1-year follow-up CT scan, which confirmed the resolution of varices. Additionally, six patients underwent periodic endoscopic follow-up, showing the complete resolution of intraluminal gastric varices at 6 months and 1 year.
Safety
None of the patients experienced variceal rebleeding and symptoms correlated to it, such as hemodynamic instability, decreasing hemoglobin, hematemesis, anemia, or melena.
Among the five patients with known esophageal varices, two exhibited endoscopic evidence of worsened esophageal varices compared to their pre-CARTO evaluation. However, to date, none of the patients have experienced esophageal variceal bleeding.
Additionally, two patients developed new ascites/hydrothorax or experienced worsened ascites the following days after CARTO/PARTO. In both cases, ascites completely resolved after medical therapy. The only patient (92 years old) treated acutely for massive hematemesis with hemodynamic instability developed significant ascites approximately one year after the procedure. This late complication was controlled through medical therapy and paracentesis.
In one patient treated with CARTO for primary prophylaxis, without a history of hematemesis, the 3-day follow-up CT scan revealed signs of partial and focal portal thrombosis, which was successfully treated with heparin therapy and was completely resolved at the 1-month CT scan.
A patient treated for secondary prophylaxis, who presented with significant ascites during the pre-procedural phase, underwent a successful transplantation approximately 7 months after the CARTO procedure. One year after the procedure, all treated patients are alive.
3. Materials and Methods
3.1. Methods
Institutional review board approval for this study was waived due to its retrospective nature. All the procedures involving the PARTO/CARTO technique performed between December 2019 and December 2023 were retrospectively evaluated and the following data were collected (Figure 1): patient features (age, sex), causes of liver cirrhosis, average model for end-stage liver disease (MELD) score [6], Child–Pugh class, GOV type according to Sarin’s classification, hemodynamic condition, vascular access, and procedural time.
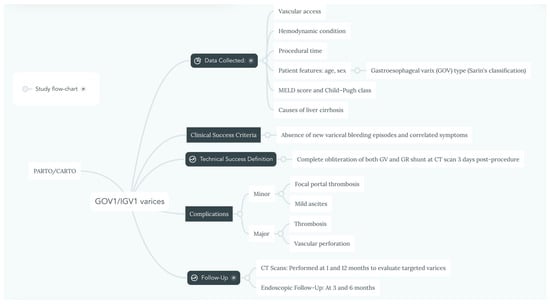
Figure 1.
Flow-chart summarizing study’s materials and methods.
Technical success was defined as complete obliteration of both GVs and the GR shunt on the review of the CT scan performed 3 days after the procedure.
Follow-up CT scans at 1 and 12 months were performed to evaluate the targeted varices in all patients, as well as endoscopic follow-up at 3 and 6 months. Clinical success was expressed by the absence of new episodes of variceal bleeding and correlated symptoms, such as hemodynamic instability, hematemesis, anemia, or melena.
Additionally, procedure-related major complications (i.e., vascular perforation or thrombosis) and minor complications (focal portal thrombosis or mild ascites) were evaluated.
3.2. Technique
3.2.1. PARTO
The procedure was performed under local anesthesia using a transjugular approach in all patients. The left renal vein and GR shunt were accessed using a 4 Fr Cobra glide catheter (Terumo, Tokyo, Japan) and a 180 cm 0.035-inch guidewire (Terumo, Tokyo, Japan) (Figure 2). To deploy the vascular plug, a 7 Fr guiding sheath (Flexor Shuttle-Sheet; Cook, IL, USA) was used in all patients (Figure 2A). Once the guiding sheath was positioned securely in the GR shunt, a vascular plug and a 4 Fr glide catheter were introduced coaxially (Figure 2B). The Amplatzer Vascular Plug II and IV (AGA Medical, Plymouth, MN, USA) were used for all patients, with the size of the plug chosen to be approximately 15–25% larger than the narrowest part of the GR shunt.
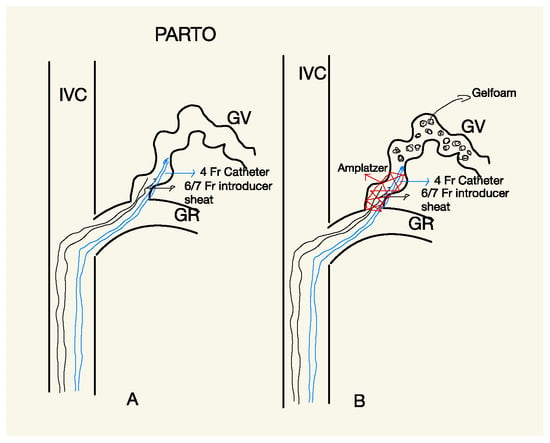
Figure 2.
Scheme explaining PARTO procedure; to deploy the vascular plug, a 7 Fr guiding sheath was used in all patients (A). Once the guiding sheath was positioned securely in the GR shunt, a vascular plug and a 4 Fr glide catheter were introduced coaxially (B).
Retrograde phlebography was performed through a 4 Fr glide catheter placed above the vascular plug occluding the GR shunt. After confirming the varices anatomy and GR shunt occlusion, retrograde embolization of the GV was conducted using gelatin sponge (Gelfoam) particles measuring 1 to 5 mm mixed with iodinated contrast media (Figure 3e). In two patients, the inferior phrenic vein was embolized using detachable coils before administering the gelatin sponge, to avoid non-target embolization.
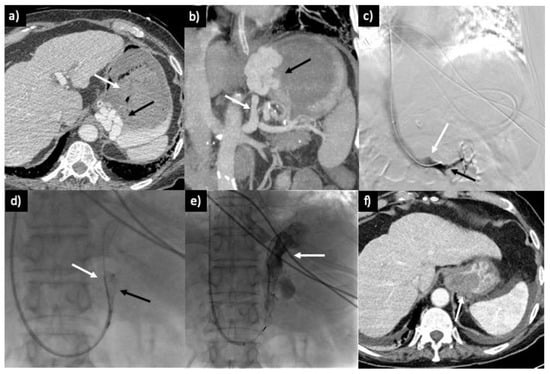
Figure 3.
A 92-year-old male patient presenting with severe hematemesis and unstable hemodynamic conditions. (a) The CT scan reveals extensive gastric distention due to blood accumulation (white arrow) and substantial gastric varices in the cardial and lesser curvature region (black arrow). (b) Portal-phase CT scan with coronal maximum intensity projection (MIP) reconstruction, displaying GV (black arrow) associated with a large GR shunt (white arrow). (c) Intraprocedural phlebography of the left renal vein (black arrow) showing the confluence of the non-opacified GR shunt (white arrow). (d) GR shunt catheterization and deployment of the Amplatzer plug (black arrow) in conjunction with a 4 Fr glide catheter (white catheter), introduced through a 7 Fr guiding sheath from the right jugular approach. (e) Administration of Gelfoam and contrast media (white arrow) through the 4 Fr angiographic catheter. (f) CT scan performed 3 days after the procedure, revealing complete thrombosis and moderate perisplenic fluid.
Following the complete filling of the GV with Gelfoam, the 4 Fr angiographic catheter was gently withdrawn, and the plug was detached and left in place to permanently occlude the shunt.
3.2.2. CARTO
All CARTO procedures were conducted under local anesthesia. In all patients, a bilateral transfemoral approach was chosen; through one access, a 6 Fr 90 cm long introducer (Flexor Shuttle-Sheet; Cook, IL, USA) was positioned, with a 5 Fr cobra glide catheter introduced coaxially. The long introducer was left in all cases; either in the left renal vein or in the inferior cave vein (IVC) near the confluence of the renal vein. This was carried out to provide greater stability to the system and avoid the more complex catheterization maneuvers required to place it in the GR shunt, as is necessary in the PARTO procedure. A 4 or 5 Fr glide catheter was positioned distally in the GR shunt (Figure 4A).
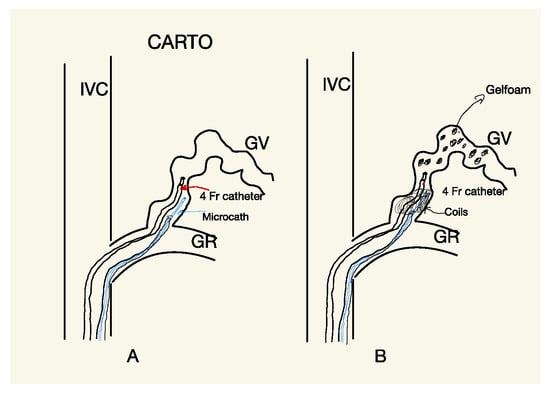
Figure 4.
Scheme explaining CARTO procedure; (A) a 5 Fr Berestain-type angiographic catheter and a Lantern microcatheter (Penumbra, Alameda, CA, USA) were introduced coaxially and positioned at the level of the GR shunt, proximally to the distal end of the glide catheter. The Lantern microcatheter was used to deliver different types of detachable coils for the permanent embolization of the GR shunt. (B) Following the shunt occlusion, the glide catheter was used to retrogradely administer Gelfoam particles (1 to 5 mm) mixed with iodinated contrast media.
From the second femoral access, a 5 Fr Berestain-type angiographic catheter and a Lantern microcatheter (Penumbra, Alameda, CA, USA) were also introduced coaxially and positioned at the level of the GR shunt, proximally to the distal end of the glide catheter (Figure 5c). The Lantern microcatheter was used to deliver different types of detachable coils for the permanent embolization of the GR shunt (Figure 5d).
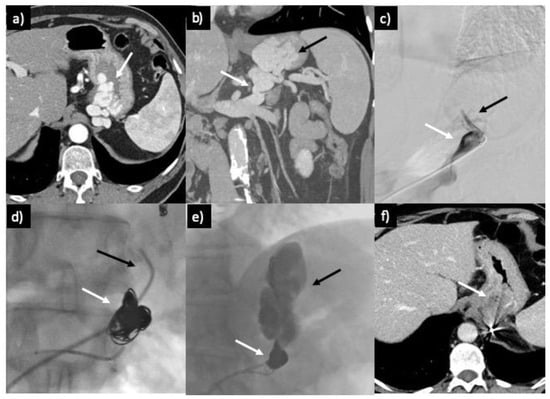
Figure 5.
(a) Axial CT scan shows unruptured massive gastric varix (GV) (white arrow) in a 57-year-old male patient. (b) Portal-phase CT scan with coronal MIP reconstruction providing a panoramic view of GV (black arrow) and GR shunt (white arrow). (c) Venography shows the placement of the guiding sheath in the proximal GR shunt with partial opacification of the left superior phrenic vein (black arrow). (d) Embolization of the GR shunt with coil cast (white arrow), with a 5 Fr glide catheter (black arrow) positioned above it for the administration of Gelfoam. (e) Retrograde filling of the GV (black arrow) by Gelfoam, with coils preventing its dislodgement. (f) CT scan performed 3 days after the procedure, revealing complete thrombosis of GV.
Following the shunt occlusion, the glide catheter was used to retrogradely administer Gelfoam particles (1 to 5 mm) mixed with iodinated contrast media (Figure 5e), filling the gastric varices and the GR shunt completely.
After observing the complete filling of the varices and the absence of reflux in the left renal vein, the glide catheter was gently withdrawn, taking care to not dislodge the coils cast.
In three patients with severe thrombocytopenia (i.e., <50 × 103/μL), the shunt remained patent at 20 min after coil positioning (Figure 6a), despite their correct deployment. Therefore, few drops of N-butyl 2-cyanoacrylate (NBCA) + Lipiodol (1:1) were administered through a microcatheter (Progreat 2.7 Fr; Terumo) introduced coaxially within the 4 Fr catheter (Figure 6b,c), with the distal end bent posteriorly toward the coil cast. This resulted in immediate shunt occlusion (Figure 6d).
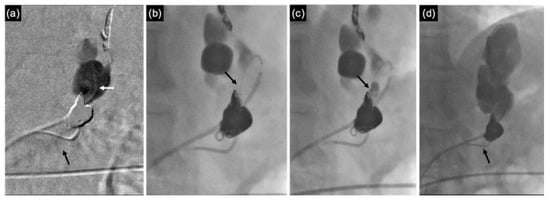
Figure 6.
(a) Phlebography performed using 4 Fr angiographic catheter (white arrow) revealing the persistent patency of the GR shunt (black arrow), despite the presence of a dense coil cast, in a patient with thrombocytopenia. (b) A microcatheter (Progreat 2.7 Fr; Terumo) is coaxially introduced within the 4 Fr catheter, with its distal end bent backward (black arrow). (c) Administration of a mixture of NBCA and Lipiodol (1:1) is performed directly into the coil cast (black arrow), filling the empty spaces. (d) The GR shunt is completely occluded, with no evidence of contrast wash-out observed towards the left renal vein (black arrow).
A similar approach was performed for a PARTO procedure in a patient with severe thrombocytopenia (43 × 103/μL), where, despite the correct deployment of the plug, the shunt remained patent. A few drops of NBCA + Lipiodol (1:1) were administered through a microcatheter (Progreat 2.7 Fr) introduced via the 5 Fr catheter with the tip bent towards the plug, occluding its meshwork through polymerization. Subsequent venography confirmed complete occlusion of the shunt and retrograde filling of the GV.
4. Discussion
This study highlights the favorable treatment outcomes achieved through modified BRTO techniques, specifically PARTO and CARTO, in patients with GVs. These techniques were applied for both primary and secondary prophylaxis, as well as in urgent clinical situations involving acute bleeding. Notably, PARTO proved effective in promptly controlling variceal bleeding within approximately one hour, even in a highly frail elderly patient with precarious hemodynamic conditions and massive hematemesis, without encountering significant complications (Figure 3).
Our study achieved a remarkable 100% technical and clinical success rate, aligning with recent research on modified BRTO procedures. For PARTO, technical success rates range from 94.7% to 100%, with clinical success rates ranging from 90.6% to 100%. Improvement in liver function after PARTO has been observed in up to 67% of patients. This improvement in liver function is greater in the first six months compared with the baseline [7,8,9]. Patients in Child–Pugh group B or C show greater improvement in liver function than those in Child–Pugh group A [9]. Rebleeding rates are comparable to BRTO and were reported to be 11% per year. In a recent retrospective study, Park et al. reported the exacerbation of esophageal varices (EVs) in 53% of patients undergoing PARTO, including 16 of 26 patients who developed post-PARTO EVs who underwent endoscopic varicose vein ligation [10].
In terms of CARTO, the technical and clinical fulfillment rates are up to 100%, as stated by Lee et al. [11], with no variceal rebleeding mentioned with an average follow-up of around 12 ± 5 months (Figure 7). Similar technical (100%) and clinical (97.2%) success rates have been reported in a retrospective examination of 36 patients by Yamamoto et al. [12].
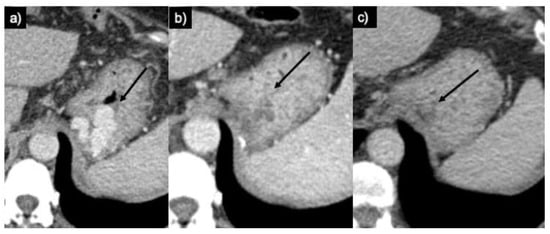
Figure 7.
(a) Axial CT scan in portal phase images revealing GV in the cardial–lesser curvature region (arrow). (b) A CT scan performed one month later illustrates the complete thrombosis of submucosal gastric varices (arrow). (c) One-year CT follow-up confirms the complete thrombosis of the varices and their reduction in size (arrow).
We accomplished permanent GR shunt occlusion using plugs and coils instead of the traditional balloon-occluded gastro-renal shunt (BGC) method. This approach allowed us to complete the procedure in a single session, offering a more comfortable and less invasive approach for the patient, with a lower incidence of complications than that associated with BGC. Our experience confirms the efficacy of CARTO/PARTO techniques in achieving permanent GR shunt and GV occlusion, in contrast to BRTO, in which late GR shunt recanalization was described [7,8].
In our experience, NBCA + Lipiodol (1:1) proved to be extremely useful and strategic in achieving the definitive closure of the shunt. In patients with thrombocytopenia, shunt occlusion is often delayed, sometimes taking several hours. The use of NBCA ensures the immediate closure of the plug’s mesh and empty spaces within the coil cast, significantly expediting the procedure. In our case study, we did not observe any signs of systemic embolization of NBCA + lipiodol. The Amplatzer device has a very tight mesh that retains the embolic material, promoting its polymerization. Regarding CARTO, to avoid systemic embolization, it is necessary to achieve a dense coils’ cast to minimize empty spaces.
One-year follow-up CT examinations confirmed the disappearance of varices in all patients, consistent with findings reported in prior studies [8,9]. Although patients may initially show residual perfusion of GVs at early CT follow-up, subsequent controls usually reveal complete disappearance of residual GVs: the initial follow-up CT scan indicates that the residual GV is still receiving blood flow from the short gastric veins. However, it is likely that the remaining intramural GV will resolve as venous flow gradually diminishes following complete occlusion of the GR shunt, which serves as the primary outflow pathway.
Moreover, one noteworthy advantage of CARTO/PARTO techniques is the ability to perform GV embolization using Gelfoam rather than sclerosing agents, such as ethanolamine oleate employed in traditional BRTO. This approach reduces potential side effects linked to sclerosing agents, including hemolysis, acute renal failure, pulmonary edema, cardiogenic shock, disseminated intravascular coagulation (DIC), and anaphylactic reactions.
Among modified BRTO procedures, the one with the most significant evidence in the literature is certainly the PARTO [8,9,10]. This technique should be considered in all patients eligible for BRTO, especially in circumstances where the hospital facility is not well equipped for continuous postoperative patient monitoring or in the case of non-compliant patients. CARTO also has adequate support in the medical literature and is essentially equivalent to PARTO in terms of both effectiveness and safety [11]. In our daily practice, we have developed a particular preference for CARTO technique, due to easier shunt catheterization maneuvers. Specifically, advancing the guiding catheter (GC) into the shunt site is not needed with two femoral accesses. Using this approach, the shunt can be easily reached with two diagnostic catheters, like Cobra glide catheter (5 Fr) and Ber-type catheter (4 Fr). These catheters are, respectively, used to administer embolization materials and accommodate the microcatheter required for coils deployment.
As mentioned above, the most challenging technical aspect of the PARTO procedure is the advancement of the GC to an appropriate position in the GR to deploy vascular plug. The venous vascular anatomy of the inferior vena cava and left renal vein district is variable, and in some cases, it is characterized by angled vascular segments that make catheterization maneuvers complex, particularly when using 6Fr and 7Fr GCs.
Given the limited number of patients included in this study, it is not possible to draw definitive conclusions regarding the safety of permanent occlusion of the GR shunt. As demonstrated in previous BRTO studies [10,13,14,15,16], increased portal venous pressure and associated clinical conditions related to portal hypertension (i.e., worsened esophageal varices or ascites development) may occur. In our experience, among the five patients with existing minimal esophageal varices, two experienced mild worsening of their varices. None of these patients required endoscopic treatments or experienced bleeding related to the worsening of esophageal varices. In one patient, we observed the development of significant late-onset ascites (1 year), which was successfully managed with paracentesis and medical therapy.
Regarding the PARTO procedure, previous studies described new or aggravated esophageal varices (EVs) in 22% to 33% of cases within 3 to 9 months post-intervention, which is similar to our results. The incidence of EV exacerbation post-CARTO is comparable at 23% [7,13,14,15,16]. Consequently, patients undergoing these procedures will necessitate ongoing endoscopic monitoring for EVs, with initial assessment recommended at one to three months post-intervention. Noteworthy risk factors for EV exacerbation include total bilirubin levels exceeding 1.6 mg/dL and a hepatic venous pressure gradient above 13 mmHg [16].
Another significant complication observed following BRTO, PARTO, and CARTO procedures is the development or worsening of ascites or hepatic hydrothorax, with incidence rates ranging from 0% to 44%, 11% to 58%, and 25%, respectively; these data are similar to our outcomes [7,16,17,18,19,20].
This study has some limitations: namely, its retrospective nature and the small cohort of patients. Moreover, a comparative analysis with different GV treatment modalities, including conventional BRTO, should be conducted.
5. Conclusions
In our experience, PARTO/CARTO techniques demonstrated high technical and clinical success rates with low incidences of short-term and long-term complications; our data are comparable with previous studies in literature.
Conversely to BRTO, the use of coils and plugs in CARTO/PARTO simplifies the procedure, eliminates the need of sclerosing agents, and prevents complications associated with BGC. Although concerns about permanent GR shunt occlusion remain, this study suggests that these techniques are effective and safe.
Author Contributions
Conceptualization, G.P. and F.A.; methodology, G.P. and F.A.; validation, E.N., A.S. and C.A.; formal analysis, E.N. and M.M.; investigation, S.N., L.R. and S.G.; resources, S.N., L.R. and S.G.; data curation, F.A. writing—original draft preparation, G.P. and F.A.; writing—review and editing, G.P., F.A., M.M. and A.T.; visualization, F.A.; supervision, G.P. and C.A.; project administration, G.P. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review board approval for this study was waived due to its retrospective nature.
Informed Consent Statement
Consent for publication was obtained for every individual person’s data included in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sarin, S.K.; Lahoti, D.; Saxena, S.P.; Murthy, N.S.; Makwana, U.K. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992, 16, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Paleti, S.; Nutalapatim, V.; Fathallah, J.; Jeepalyam, S.; Rustagi, T. Balloon-Occluded Retrograde Transvenous Obliteration (BRTO) Versus Transjugular Intrahepatic Portosystemic Shunt (TIPS) for Treatment of Gastric Varices Because of Portal Hypertension a Systematic Review and Me-ta-Analysis. Swathi. J. Clin. Gastroenterol. 2019, 54, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.T.; Schnell, G.A.; Walden, J.M. Bleeding from varices in the gastric fundus complicating sclerotherapy. Gastrointest. Endosc. 1989, 35, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Florentina, R.I.; Nikolaos, T.; Pyrsopoulos, M.J.; Ion, C.T.; Zhonghua, S. Liver Diseases a Multidisciplinary Textbook; Springer Nature Switzerland AG: Cham, Switzerland, 2020; ISBN 978-3-030-24431-6. [Google Scholar]
- Kim, M.Y.; Um, S.H.; Baik, S.K.; Seo, Y.S.; Park, S.Y.; Lee, J.I.; Lee, J.W.; Cheon, G.J.; Sohn, J.H.; Kim, T.Y.; et al. Clinical features and outcomes of gastric variceal bleeding: Retrospective Korean multicenter data. Clin. Mol. Hepatol. 2013, 19, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Emenena, I.; Emenena, B.; Kweki, A.G.; Aiwuyo, H.O.; Osarenkhoe, J.O.; Iloeje, U.N.; Ilerhunmwuwa, N.; Torere, B.E.; Akinti, O.; Akere, A.; et al. Model for End Stage Liver Disease (MELD) Score: A Tool for Prognosis and Prediction of Mortality in Patients with Decompensated Liver Cirrhosis. Cureus 2023, 15, e39267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, M.-Y.; Kim, M.-D.; Kim, T.; Shin, W.; Shin, M.; Kim, G.M.; Won, J.Y.; Park, S.I.; Lee, D.Y. Plug-Assisted Retrograde Transvenous Obliteration for the Treatment of Gastric Variceal Hemorrhage. Korean J. Radiol. 2016, 17, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Gwon, D.I.; Ko, G.-Y.; Yoon, H.-K.; Sung, K.-B.; Kim, J.H.; Shin, J.H.; Ko, H.K.; Song, H.-Y. Gastric Varices and Hepatic Encephalopathy: Treatment with Vascular Plug and Gelatin Sponge–assisted Retrograde Transvenous Obliteration—A Primary Report. Radiology 2013, 268, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gwon, D.I.; Kim, Y.H.; Ko, G.-Y.; Kim, J.W.; Ko, H.K.; Kim, J.H.; Shin, J.H.; Yoon, H.-K.; Sung, K.-B. Vascular Plug–Assisted Retrograde Transvenous Obliteration for the Treatment of Gastric Varices and Hepatic Encephalopathy: A Prospective Multicenter Study. J. Vasc. Interv. Radiol. 2015, 26, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Yoo, J.-J.; Kim, S.G.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Lee, J.M.; Shim, J.J.; Cheon, G.J.; et al. Change in Portal Pressure and Clinical Outcome in Cirrhotic Patients with Gastric Varices after Plug-Assisted Retrograde Transvenous Obliteration. Gut Liver 2020, 14, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Saab, S.; Gomes, A.S.; Busuttil, R.; McWilliams, J.; Durazo, F.; Han, S.-H.; Goldstein, L.; Tafti, B.A.; Moriarty, J.; et al. Coil-Assisted Retrograde Transvenous Obliteration (CARTO) for the Treatment of Portal Hypertensive Variceal Bleeding: Preliminary Results. Clin. Transl. Gastroenterol. 2014, 5, e61. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Jogo, A.; Kageyama, K.; Sohgawa, E.; Hamamoto, S.; Hamuro, M.; Kamino, T.; Miki, Y. Utility of Coil-Assisted Retrograde Transvenous Obliteration II (CARTO-II) for the Treatment of Gastric Varices. Cardiovasc. Interv. Radiol. 2020, 43, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E. Balloon-Occluded Retrograde Transvenous Obliteration of Gastric Varices: Concept, Basic Techniques, and Outcomes. Semin. Intervent. Radiol. 2012, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Waguri, N.; Osaki, A.; Watanabe, Y.; Matsubara, T.; Yamazaki, S.; Yokoyama, H.; Kimura, K.; Wakabayashi, T.; Mito, M.; Yakubo, S.; et al. Balloon-occluded retrograde transvenous obliteration for gastric varices improves hepatic functional reserve in long-term follow-up. JGH Open 2021, 5, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.A.; Wagner, C.; Al-Osaimi, A.; Bliebel, W.; Lippert, A.; Davies, M.G.; Sabri, S.S.; Turba, U.C.; Matsumoto, A.H.; Angle, J.; et al. The Effect of Balloon-Occluded Transvenous Obliteration of Gastric Varices and Gastrorenal Shunts on the Hepatic Synthetic Function: A Comparison Between Child-Pugh and Model for End-Stage Liver Disease Scores. Vasc. Endovasc. Surg. 2013, 47, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Sasaki, R.; Nishimura, T.; Matsuda, T.; Maeda, M.; Iwamoto, T.; Saeki, I.; Hidaka, I.; Takami, T.; Sakaida, I. Comparison of patients with hepatic encephalopathy and those with gastric varices before and after balloon-occluded retrograde transvenous obliteration. Hepatol. Res. 2018, 48, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.A.; Sabri, S.S. Balloon-occluded Retrograde Transvenous Obliteration (BRTO): Technical Results and Outcomes. Semin. Interv. Radiol. 2011, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Masood, I.; Moshksar, A.; Wong, B.; Khan, H.; Saleem, A. A comprehensive review of transvenous obliteration techniques in the management of gastric varices. Diagn. Interv. Radiol. 2023, 29, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, Y.H.; Kim, C.S.; Kang, U.R.; Kim, S.H.; Kim, J.H. Comparison of balloon-occluded retrograde transvenous obliteration (BRTO) using ethanolamine oleate (EO), BRTO using sodium tetradecyl sulfate (STS) foam and vascular plug-assisted retro-grade transvenous obliteration (PARTO). Cardiovasc. Intervent. Radiol. 2016, 39, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Taehwan, K.; Heechul, Y.; Chun, K.L.; Gun, B.K. Vascular Plug Assisted Retrograde Transvenous Obliteration (PARTO) for Gastric Varix Bleeding Patients in the Emergent Clinical Setting. Yonsei Med. J. 2016, 57, 973–979. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).