Abstract
Non-IgE immune-mediated gastrointestinal disorders constitute a heterogeneous group of enigmatic conditions that are on the rise. This category encompasses entities like food protein-induced enterocolitis syndrome (FPIES), food protein-induced allergic proctocolitis (FPIAP), and food protein-induced enteropathy (FPE). These are immune-mediated reactions to certain foods without the involvement of allergen-specific IgE in their pathogenesis. Eosinophilic esophagitis (EoE) is also included in this group, acknowledged for its mixed IgE and non-IgE-mediated characteristics. The diagnostic landscape is fraught with challenges, given the poorly understood nature of these disorders and their propensity to manifest with varying and overlapping clinical presentations, typically emerging in infancy with common potential triggers such as cow’s milk and soy. Presently, confirmatory testing for most of these conditions is limited and invasive, emphasizing the pivotal role of a thorough history and physical examination in reaching a diagnosis. Notably, there are limited guidelines for diagnosis and management for most of these disorders. This article elucidates the key distinctions among these disorders, provides an overview of existing diagnostic and therapeutic approaches, and addresses existing knowledge and research gaps. The considerable impact on the quality of life of non-IgE immune-mediated allergic disorders of the gastrointestinal tract, which can result in debilitating complications such as nutritional deficiencies, mental health disorders, and eating disorders, underscores the urgency for comprehensive exploration and management strategies.
Keywords:
food allergy; non-IgE-mediated; FPIES; FPE; FPIAP; proctocolitis; eosinophilic esophagitis; elimination diet 1. Introduction
Non-IgE-mediated allergic gastrointestinal (GI) disorders pose a captivating and intricate challenge at the intersection of gastroenterology, allergy, and immunology. In contrast to the well-established paradigm of IgE-mediated food allergy, non-IgE-mediated allergic GI disorders unfold a complex narrative where immune reactions in the GI tract transcend the traditional boundaries of immunoglobulin E binding and mast cell activation.
In this multifaceted group, disorders such as food protein-induced enterocolitis syndrome (FPIES), food protein-induced allergic proctocolitis (FPIAP), food protein-induced enteropathy (FPE), and eosinophilic GI diseases (EGID) challenge conventional diagnostic and therapeutic approaches. The manifestations of these disorders often elude immediate recognition, given their association with sometimes delayed reactions rather than the rapid onset typically observed in IgE-mediated allergies. As we navigate through the intricacies of these conditions, the goal is to unravel the underlying mechanisms, identify key diagnostic methods, and enhance our comprehension of the diverse clinical presentations exhibited by affected individuals. The majority of these conditions, historically deemed uncommon, have exhibited a growing prevalence over recent decades.
The clinical presentations of these disorders demonstrate considerable overlap [1]. Beyond the shared clinical manifestations, each condition shows significant variability in its presentation, often contributing to diagnostic delays. Common mechanisms entail activation of the innate immune system, T-cell involvement, cellular modification of the intestinal lumen marked by the infiltration of inflammatory cells, and histologic changes. Furthermore, the associated cytokine responses signify food-specific, T-cell-mediated involvement [2]. Complicating matters, there are presently no specific diagnostic biomarkers identified for these disorders, necessitating reliance on clinical history, invasive procedures like biopsies, or resource-intensive food challenges for accurate diagnosis. A common thread among these conditions is the pivotal role of trigger elimination diets as the single most crucial element in their treatment. Refer to Table 1 for a concise comparison of these four (4) conditions.

Table 1.
Comparison of Acute FPIES versus FPIAP versus FPE versus EoE.
This review thoroughly explores non-IgE-mediated allergic GI disorders, aiming to illuminate the complex interplay between the GI system and the immune response, which has implications for clinical understanding and patient care.
2. Discussion of the Disease
2.1. Food Protein-Induced Enterocolitis Syndrome (FPIES)
FPIES is a potentially severe form of non-IgE-mediated GI disorder, with its characteristic feature being chronic emesis, usually associated with other GI symptoms and failure to thrive [7]. It can be either acute or chronic FPIES [8]. Acute FPIES classically presents in infancy. For diagnosis, the major criterion and three or more minor criteria must be met [9]. Major criteria include protracted emesis within 1 to 4 h after ingestion of the allergen, frequently followed by lethargy, pallor, and diarrhea, without classical IgE-mediated allergic skin or respiratory symptoms. Minor criteria include a second episode of repetitive vomiting after ingesting the same suspect food, vomiting occurring 1–4 h after eating a different food, extreme lethargy or marked pallor during a suspected reaction, the need for emergency department visits, intravenous fluid support, or experiencing diarrhea within 24 h of ingestion [10,11]. In severe cases, symptoms can progress to hypothermia, dehydration, methemoglobinemia, acidemia, and shock brought on by low blood pressure and poor perfusion, mimicking sepsis [9].
In comparison, chronic FPIES occurs with regular, often daily, ingestion of the allergen, causing chronic symptoms such as emesis-mimicking reflux, diarrhea, and failure to thrive. When the allergen is eliminated from the diet in chronic FPIES, the symptoms resolve but can recur as acute FPIES when the allergen is reintroduced. The most common allergens in acute FPIES include cow’s milk, soy, eggs, fish, and cereals [11,12]. However, with recent guidelines endorsing the early introduction of peanuts in early infancy, there has been an increase in peanut-induced FPIES [13].
While acute FPIES typically manifests in early infancy, there is a growing acknowledgment of adult-onset acute FPIES, particularly triggered by seafood [14,15,16]. Interestingly, a recent report highlights the rare incidence of symptomatic FPIES in fetal and neonatal cases attributed to intrauterine sensitization [17]. Estimations indicate that the prevalence of FPIES in most Western countries ranges between 0.01% and 0.7%, encompassing approximately 1 million patients in the United States [3].
The pathophysiology of FPIES remains inadequately understood, but the prevailing hypothesis speculates that an immune reaction directed against food proteins triggers gut inflammation. This inflammatory process results in heightened intestinal permeability, causing a fluid shift that manifests in clinical symptoms. FPIES can induce inflammation in the colon and, to varying degrees, the ileum, as confirmed by endoscopic, colonoscopic, and biopsy examinations [18,19]. Clinical symptoms result from mechanisms involving antigen-specific T cells and inflammatory cytokines like TNF-α and IFN-γ. Additionally, elevated expression of HLA-DR in dendritic cells has been observed [20]. Serum humoral response is not associated with FPIES, but heightened serum levels of IL-8 and tryptase are noted during active FPIES, suggesting potential involvement of neutrophils and mast cells [21]. Studies show the activation of innate immune cells, including monocytes, neutrophils, natural killer cells, and eosinophils, in whole blood after positive Oral Food Challenges (OFCs) in FPIES [22]. However, research is needed to understand the precise underlying mechanisms and food specificity of these processes (Figure 1).
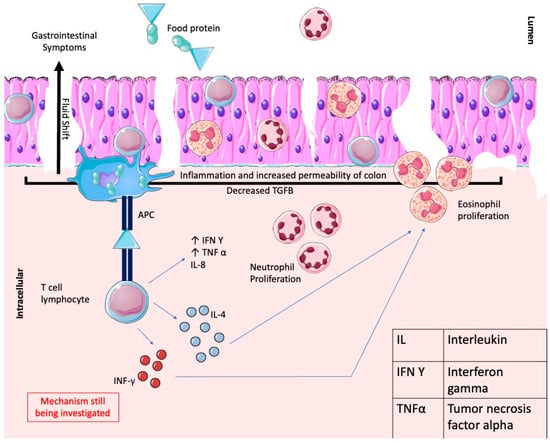
Figure 1.
Shows the pathophysiology of FPIES, whereby the food protein molecule acts as an antigen, which is then presented to the T cell lymphocyte by antigen-presenting cells (APCs). This leads to the release of IL-4, causing eosinophilic proliferation, and IL-8, causing neutrophilic proliferation. Decreased expression of TGF-β in the epithelial cells and increased levels of TNF-α and IFN-γ lead to inflammation and increased permeability, allowing fluid to shift into the lumen. Parts of the figure were drawn using Servier Medical Art, licensed under a Creative Commons Attribution 4.0 Unported License: https://creativecommons.org/licenses/by/4.0/ (accessed on 1 January 2024).
In 2017, the International Consensus Guidelines for the Diagnosis and Management of FPIES were published [9]. The diagnosis primarily relies on a detailed clinical history indicating characteristic signs and symptoms, demonstrating improvement upon removing the suspected trigger food. For most patients, a comprehensive history is sufficient for an acute diagnosis. In uncertain cases, open OFCs serve as the gold standard for confirmation. It is important to note that FPIES lacks a specific diagnostic procedure or laboratory test. Food-specific IgE levels are negative in most patients, although in atypical cases, there may be positive IgE [23]. Infants with a convincing FPIES history do not require challenges for confirmation, and as there is significant risk associated with FPIES challenges, it is not advised to challenge each patient to confirm the diagnosis [9].
The management of acute FPIES is tailored to the severity of the condition and discussed individually with caregivers. Mild-to-moderate cases may resolve with oral rehydration, including breastfeeding. Severe cases, which can lead to hypovolemic shock in about 15% of patients, focus on stabilizing hemodynamics with aggressive fluid resuscitation and intravenous fluids [23]. The use of intravenous methylprednisolone for presumed inflammation lacks robust evidence but is still commonly used in those patients who are critically ill with FPIES. Supplemental measures like oxygen therapy, mechanical ventilation, vasopressors, bicarbonate, and methylene blue may also be necessary for severe reactions [24]. While epinephrine autoinjectors are not routine for FPIES, they may be considered for those with concurrent IgE-mediated allergies or those with a history of severe hypotension from an FPIES reaction who cannot easily reach emergency medical services for IV fluid resuscitation. As a symptomatic approach, ondansetron can be considered adjunctive therapy for emesis in acute cases [25,26,27]. Its effective application in addressing vomiting, abdominal pain, and lethargy during FPIES challenges suggests the potential involvement of neuroimmune mechanisms. This medication may also impede the immunological progression of the reaction [26]. Many allergists provide patients with ondansetron for use as outpatients in case of an FPIES reaction [27,28].
Long-term management involves eliminating trigger foods, developing dietary plans, addressing symptoms during exposure, and monitoring for resolution. FPIES action plans (the FPIES Foundation) are also available for patients to have on hand, and allergists often provide letters for patients to have on hand to bring to the emergency room in case of a reaction.
Despite the potential severity of the reactions, FPIES demonstrates an excellent prognosis, with no reported fatalities, and typically resolves in most cases by the age of 3–5 years [29].
2.2. Food Protein-Induced Allergic Proctocolitis (FPIAP)
FPIAP is characterized by proctocolitis in early infancy [30]. A recent 2020 study reports the cumulative incidence of FPIAP in infants to be approximately 17% [4]. This condition usually presents by 6 months, with peak presentation between 2–8 weeks of age. It most commonly occurs in breastfed infants but can also occur in those who are formula-fed. The proctocolitis will manifest as bloody, watery stools without significant emesis or failure to thrive, and symptoms will resolve within a few days of removing the offending allergen. There have been rare, reported cases in childhood and adulthood with similar clinical manifestations, but these persons had no history of FPIAP in infancy [31,32].
A defect in oral tolerance is thought to lead to FPIAP through multiple pathways [30]. Studies suggest the presence of non-IgE-associated mechanisms with crucial roles for food antigen sensitization in the development of allergic colitis [33]. Immune alterations, notably reduced TGF-β1+ factor and increased TNF-α, have been observed in affected children, which is thought to compromise the epithelial barrier and lead to fluid shift into the lumen and subsequent symptoms (Figure 2) [34]. The Gastrointestinal Microbiome and Allergic Proctocolitis study also highlighted microbiome differences, with higher Enterobacteriaceae and lower Clostridiales levels in infants with FPIAP during symptomatic periods [35]. Despite these findings, the pathogenesis of FPIAP remains poorly understood due to limited routine GI endoscopy and biopsies [36].
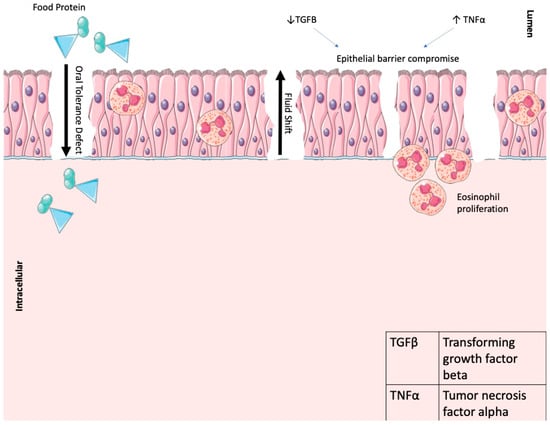
Figure 2.
Shows the pathophysiology of FPIAP, whereby the food protein molecule acts as an antigen due to a defect in oral food tolerance leading to eosinophilic-mediated tissue damage. Decreased expression of TGF-β in the epithelial cells and increased levels of TNF-α lead to inflammation and increased permeability, allowing fluid to shift into the lumen. Parts of the figure were drawn using Servier Medical Art, licensed under a Creative Commons Attribution 4.0 Unported License: https://creativecommons.org/licenses/by/4.0/ (accessed on 1 January 2024).
Around 60% of instances of FPIAP manifest in breastfed infants, wherein the immune reaction stems from the mother’s consumption of the food allergen, typically cow’s milk, transmitted in an immunologically identifiable form to the breast milk. For formula-fed infants, the response is linked to cow’s milk or, less frequently, soy [37,38,39]. Infants with FPIAP present with blood and mucus in stools, rarely with painful defecation or colicky symptoms, but are usually well appearing with no evidence of failure to thrive, a key distinguishing feature of this condition compared to FPE.
The diagnosis of FPIAP in affected infants is based clinically on history and response to an allergen-free diet. Diagnosis is challenging due to the lack of defined criteria and involves excluding other medical conditions [40]. Colonic biopsies can be helpful in confirming allergic colitis, although not routinely recommended, but can demonstrate eosinophilic infiltration of the colonic epithelium, lamina propria, and muscularis, particularly in the sigmoid and rectal colon [40,41,42].
Current strategies for managing FPIAP in infants involve eliminating triggering antigens, particularly cow’s milk proteins, from the maternal diet in breast-fed infants and substitution with extensively hydrolyzed formulas in formula-fed infants [33]. Symptoms generally resolve completely with dietary restrictions [35]. As much as 20% of infants fed breast milk undergo spontaneous resolution of bleeding without any need for adjustments to the maternal diet [40]. Therefore, watchful waiting for an otherwise healthy infant is another alternative approach for those patients with mild symptoms.
Studies by Vassilopoulou et al. and Feketea et al. highlight the influence of maternal diet during pregnancy and breastfeeding on the development and duration of FPIAP. Both studies recommend dietary changes for pregnant mothers of FPIAP infants, advising them to increase the consumption of yogurt and olive oil during pregnancy and lactation while avoiding the consumption of grilled foods and foods with high salt content during lactation. Mothers of children with FPIAP can implement these adjustments with the aim of potentially reducing the duration of FPIAP in future infants [43]. However, these data stem from observational retrospective studies showing an association supporting these dietary changes. We cannot rule out a cofounder in this data and these data have not been supported by any prospective randomized trials.
An additional risk factor for the development of FPIAP is patients who are exclusively breastfed with supplementation of small amounts of formula within the first few days of life. This cow’s milk formula intake likely provides the sensitization required for these patients to develop the immunological reaction against the cow’s milk protein in the mother’s breast milk [44]. This has been demonstrated in numerous studies and may also explain the higher incidence of FPIAP in the United States as opposed to other countries, as formula supplementation in early infancy is more common in the United States [45].
Standardized protocols for managing FPIAP are lacking. Mennini et al. proposed a possible protocol for the diagnosis and management of FPIAP based on symptomatology, duration of symptoms, treatment varying based on breastfed vs. formula-fed infants, and response to treatment [33]. This protocol emphasizes that bloody stools in infancy for less than 4 weeks can be self-limiting and can be re-evaluated after 2–4 weeks to see if they are persistent before making dietary changes. These guidelines also highlight that symptoms should improve within 3 days and that infants can be rechallenged with the offending allergen to confirm the diagnosis and see if symptoms recur [33].
The prognosis is favorable, as the condition usually resolves within 1 year of life [46]. This is different from FPE, which often lasts until 2–3 years of life. However, children with FPIAP are twice as likely to develop IgE-mediated food allergies, perhaps owing to the need for an antigen elimination diet during the critical period in the first year of life [47]. Common practice is to reintroduce the offending allergen into the infant’s diet at 1 year of life, but due to the high risk of sensitization with avoidance, more research needs to be performed to see if earlier introduction can be done to prevent this potential IgE sensitization [48]. Of note, IgE allergy testing has no role prior to the reintroduction of dairy in patients with FPIAP [46].
2.3. Food Protein-Induced Enteropathy (FPE)
Food protein-induced enteropathy (FPE) is a non-IgE-mediated food hypersensitivity of the GI tract that is commonly diagnosed during infancy and often self-resolves by early childhood. In contrast to the other conditions, FPE has observed a decline in incidence over the past decade [5].
The pathophysiology of FPE, like other non-IgE-mediated allergic disorders of the GI tract, is not well understood but is hypothesized to be caused by jejunal mucosal damage involving eosinophil-mediated and T cell lymphocytic damage to villous architecture (Figure 3) [5,36]. Increased INF-γ and IL-4, commonly seen in intestinal injuries, have also been observed [49]. The most common food-specific protein allergens known to cause FPE are cow’s milk—often introduced to infants via formula—and soy [46]. The most common manifestations that occur within a few weeks after the ingestion of the specific allergen are diarrhea and malabsorption [50]. There are no laboratory tests that have been proven to definitively diagnose patients with suspected FPE, thus the diagnosis of FPE is one of mostly clinical suspicion and medical history, including the following characteristic manifestations: (1) being 9 months of age or younger at the initial diagnosis, (2) symptomatic GI manifestations, including failure to thrive and vomiting after repetitive ingestion of food-protein, (3) a biopsy of the small bowel showing inflammation, villous injury, and crypts hyperplasia, (4) the elimination of food protein resulting in resolution of symptoms [36]. Other physical manifestations of the disease may include abdominal distension and edema [5]. Of note, an endoscopy and small bowel biopsy are necessary components for a definitive diagnosis of FPE, unlike similar differentials of FPIES and FPIAP, which do not necessitate a biopsy [5].
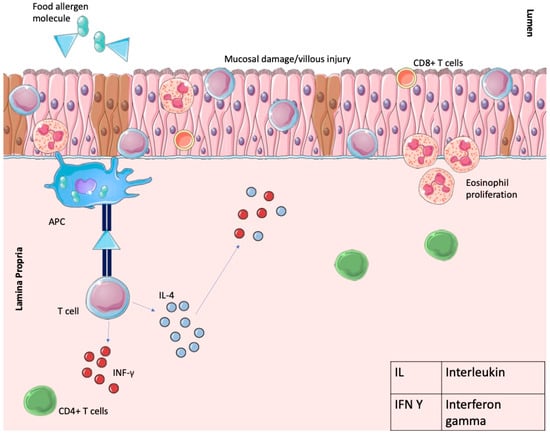
Figure 3.
Shows the pathophysiology of FPE, whereby the food protein molecule acts as an antigen, which is then presented to the T cell lymphocyte by antigen-presenting cells (APCs). This leads to the release of IL-4 and subsequent eosinophilic-mediated tissue damage and T cell-mediated damage of intestinal villi. Increased levels of INF-γ and IL-4 are shown, which are also involved in intestinal injury. Parts of the figure were drawn using Servier Medical Art, licensed under a Creative Commons Attribution 4.0 Unported License: https://creativecommons.org/licenses/by/4.0/. Accessed on 1 January 2024.
To aid in further diagnosis and subsequent resolution of symptoms, an elimination diet followed by an at-home oral food challenge is recommended. After removing the suspected food protein from the diet, symptoms will typically begin to subside after 1–4 weeks, and GI mucosal repair will progressively occur over the course of several months [46]. After 4–8 weeks of dietary elimination, the food protein can be gradually reintroduced [46].
There is a very good prognosis for infants diagnosed with FPE, as cases tend to resolve on their own within a few years of diagnosis, most commonly by 2 years of age, without long-term sequelae [5]. Further investigation into the pathophysiology of food protein-induced enteropathy is warranted to better understand the underlying mechanisms of the disease and to create definitive diagnostic criteria.
2.4. Eosinophilic GI Diseases
Eosinophilic gastrointestinal disorders (EGID) encompass a group of allergic conditions characterized by eosinophilic infiltration in various sections of the gastrointestinal tract. These include eosinophilic gastritis (EoG), eosinophilic esophagitis (EoE), eosinophilic enteritis (EoN), and eosinophilic colitis (EoC), where chronic eosinophilic inflammation subsequently leads to gut dysfunction and structural changes [51]. The epidemiology of these conditions is rapidly changing, and various treatment modalities are being investigated with the aim of targeting the mediators of these conditions [52].
Eosinophilic esophagitis (EoE) is the most common form of EGID. It is a chronic disorder whose pathogenesis is influenced by genetic, environmental, and host immune system factors, leading to hypersensitivity reactions to specific allergens [53]. EoE has a prevalence of approximately 10–57 in 100,000 and shows a strong heritability pattern, with a relative risk ratio of up to 64-fold amongst brothers [6]. It also shows a strong male predominance [54]. In a systematic review by Arias et al., the prevalence was found to be higher in adults at 43% compared to 30% in the pediatric population [55].
Due to its higher incidence overall, in addition to a higher incidence in older children who are more likely to undergo endoscopy, biopsy, and further testing, we have much greater data on the pathophysiology of EoE as opposed to FPIES, FPIAP, and FPE. Genes such as TSLP (thymic stromal lymphopoietin), CAPN14 (calpain 14), and LRRC32 (leucine-rich repeat containing 32), amongst many others, have been found to be involved in the mechanism of EoE [56,57]. Furthermore, genes under epigenetic regulation, such as CCL26 (encoding eotaxin-3, a potent eosinophil chemoattractant and activating factor induced by IL-3) and CAPN14 (encoding CAPN14, an intracellular calcium-dependent cysteine protease with the potential to compromise the integrity of the esophageal epithelial barrier), are also involved in EoE [58].
The initiation of EoE involves adaptive T helper type 2 (Th2) cell-mediated responses triggered by food protein antigens, leading to the activation of interleukin (IL)-4, IL-5, and IL-13 [59]. Il-4 is involved in eosinophil translocation, while IL-5 promotes eosinophil growth and activation [60]. IL-13 activates eotaxin 3 and calpain 14, which results in an influx of eosinophils into the tissue and a disturbance in the epithelial barrier, respectively (Figure 4) [61,62]. These mechanisms, in addition to IgE-mediated responses, contribute to cellular damage, eventually leading to esophageal lesions, dysmotility, secondary remodeling, and fibrosis. It must be emphasized that research over the past few decades has shown that the underlying mechanism of EoE is mainly allergic.
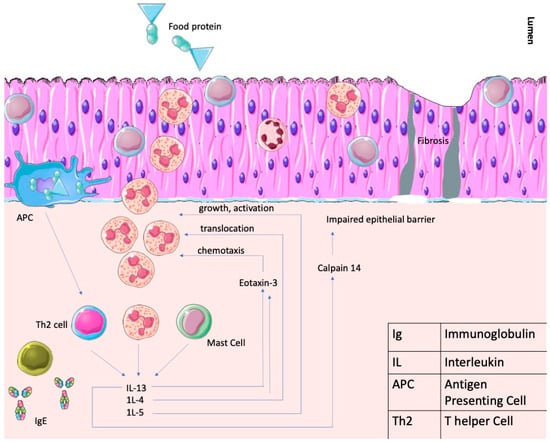
Figure 4.
Shows the pathophysiology of EoE, whereby the food protein molecule acts as an antigen, which is then presented to the T cell lymphocyte by antigen-presenting cells (APCs). A Th2-mediated response occurs whereby IL-13, IL-4, and IL-15 all lead to eosinophilic-mediated tissue injury and, eventually, fibrotic changes. There is also a partial IgE mediated response not shown in this figure. Parts of the figure were drawn using Servier Medical Art, licensed under a Creative Commons Attribution 4.0 Unported License: https://creativecommons.org/licenses/by/4.0/. Accessed on 1 January 2024.
EoE is characterized as a clinical-pathological disorder presenting with esophageal symptoms, accompanied by eosinophil-predominant inflammation involving 15 or more eosinophils per high-power field (≥15 eos/hpf) in a minimum of two esophageal tissue biopsies, each obtained from different levels, following the exclusion of other disorders [63]. Notably, recent consensus guidelines have revised the diagnostic criteria for EoE by eliminating the requirement of failed high-dose proton pump inhibitor (PPI) therapy [64]. Current recommendations advise referring individuals suspected of having EoE to a gastroenterologist before initiating PPI therapy [64].
The clinical manifestations of EoE can exhibit variations depending on the age at which symptoms first manifest. In infants, presentations may include food refusal, failure to thrive, and symptoms associated with gastroesophageal reflux, while school-aged children commonly exhibit gastroesophageal reflux symptoms such as dysphagia, vomiting, heartburn, and feeding aversion. In contrast, adolescents and adults typically present with dysphagia, chest pain, altered eating habits like slower chewing or increased water consumption with meals, and episodes of food impaction [65]. Notably, an intriguing finding in a study by Hiremath et al. (2015) reveals that approximately 50% of patients undergoing endoscopy after suspected food impaction are subsequently diagnosed with EoE [66].
The gold standard for EoE diagnosis is endoscopic biopsy findings that demonstrate increased intraepithelial esophageal eosinophil counts without concomitant eosinophilic infiltration in the stomach or duodenum [67,68]. Other histologic features of EoE include superficial layering of the eosinophils, eosinophilic microabscesses (clusters of > 4 eosinophils), basal zone hyperplasia, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis [65].
On endoscopy, several gross mucosal abnormalities have been identified, including longitudinal furrowing, friability, edema, longitudinal shearing, raised white specks, whitish exudates, “crêpe paper mucosa”, narrow caliber esophagus, Schatzki ring, felinization, and transient or fixed rings [67]. A less invasive method that can be useful is barium esophagography, which is more sensitive than endoscopy for detecting more subtle esophageal strictures and diffuse small-caliber esophagus [69]. The cytosponge is also under development as a less invasive method for diagnosing or following response to treatment in EoE, whereby a mesh contained in a capsule attached to a string is swallowed and then retracted for analysis [70]. Endoluminal Functional Lumen Imaging Probe (EndoFLIP) is one of the newer modalities used during endoscopy to assess the overall distensibility of the esophagus by assessing parameters such as dimension, movement, and pressure. This is very useful in diagnosing and assessing fibrostenotic disease [71,72].
It should be noted that although there is a strong IgE-mediated allergic component involved in the mechanism of EoE, skin prick testing, serum-specific IgE testing, and patch testing have not been shown to be reliable in diagnosis or treatment with regard to guiding food elimination [73].
It is imperative to treat the potential primary cause of esophageal eosinophilia and evaluate whether this induces remission. For example, it has been shown that patients receiving oral immunotherapy for the treatment of IgE-mediated food allergies are also at risk for EoE, but discontinuation of the immunotherapeutic antigen usually results in remission [74]. This population of patients is suspected to be even larger, as many patients on food oral immunotherapy stop therapy for GI complaints but do not undergo endoscopy for etiology diagnosis. Proton pump inhibitors (PPIs) are also a common first-line treatment, as many patients with EoE have underlying gastrointestinal reflux. PPI’s were approximately 41% effective in inducing a histologic response in patients with EoE [75]. However, the effective dose is 1 mg/kg twice daily, which comes with its own risks at such a high dose. Therefore, it is recommended to re-scope after three months of therapy to determine if weaning is possible [76].
The cornerstone of treating EoE remains the elimination diet. There are three main approaches of dietary management. Empiric food elimination based on the most common allergic triggers (i.e., milk, wheat, egg, soy, nuts, and seafood) can be done via the six (6) food elimination diet, which eliminates top allergens in the US (milk, soy, egg, wheat, nuts, shellfish/fish), the four (4) food elimination diet (eliminates milk, soy, egg, and wheat), or the two (2) food elimination diet (eliminates milk and wheat). The allergy test-directed elimination diet, based on a positive allergy skin prick test and/or patch test, involves the elimination of most common allergic triggers as well as suspected allergens based on test results. Lastly, a strictly elemental (amino acid) formula-based diet removes all foods.
In a comparison of these diets by Henderson et al. (2012), the elemental diet showed 96% histologic remission but is, overall, the most challenging and impractical approach. In the empiric elimination diet, the overall effectiveness was also impressive at 81%. The allergy test-direct elimination diet did not show superiority to the empiric elimination diet, which is why it has fallen out of favor [77].
Topical steroids achieve successful remission in up to 80% of patients, although 10% of patients are refractory to this treatment [75,78,79]. Esophageal dilation, mostly performed on adult patients, can be considered a treatment option for symptomatic patients with fibro-stenotic disease if medical or dietary therapy has failed [80]. A systematic review and meta-analysis of 27 studies by Moawad et al. (2017) showed clinical improvement in 95% of EoE patients, with a median duration of improvement lasting 12 months after mechanical dilation [80]. However, it has not been shown to treat underlying inflammation [80].
Results from studies of biologics used in other allergic conditions, such as dupilumab (anti-IL-4), cendakimab (anti-IL-13), and anti-IL-5 therapies, showed a histologic reduction in mean esophageal eosinophil counts in patients with EoE [81,82,83,84,85,86,87]. Following a successful Phase 3 clinical trial, the Food and Drug Administration approved dupilumab for the treatment of EoE in adults and children over 12 years of age in May 2022 [88]. In this randomized, placebo-controlled trial, dupilumab administered as a once-weekly subcutaneous injection led to histologic remission (defined as eosinophil count <6/hpf) in 60% of patients (versus 5% on placebo; p < 0.001) and was associated with significant alleviation of dysphagia severity [82]. Notably, injection every 2 weeks was also associated with histologic remission, but there was a more significant improvement in dysphagia with more frequent dosing. This demonstrates that the inflammation in EoE is likely more than just histologic [82]. Recent unpublished data have also allowed the approval for dupilumab in the United States to be recently lowered to 1 year of age. Though the cost of dupilumab is obviously significantly higher than PPI, swallowed steroids, and a dietary elimination diet, it is the preferred treatment option for many patients with EoE due to the low incidence of adverse events, high efficacy, and improved quality of life. The adverse events of dupilumab for the treatment of EoE include injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections [89].
2.5. Mimickers of Non-IgE-Mediated Allergic GI Disorders
While this review focuses on non-IgE-mediated allergic GI disorders, multiple GI disorders exist that are not allergic but mimic an allergic disorder. For example, celiac disease (CD), triggered by gluten consumption, unfolds as an abnormal immune response directed against gluten peptides, activating T cells and prompting the release of inflammatory cytokines. The consequence is a targeted immune attack on the small intestine, leading to mucosal inflammation, villous atrophy, compromised nutrient absorption, and extra-GI manifestations [90]. Serum antibody testing can identify those patients at risk for celiac disease, though confirmatory testing is performed through an endoscopic biopsy. Celiac disease is an autoimmune disease associated with other autoimmune phenomena and is treated with strict gluten elimination, which resolves the patient’s symptoms.
Of worthy mention is food intolerance, one of the most prevalent forms of adverse food reactions [91], which is a separate entity from IgE- and non-IgE-mediated allergic GI disorders. Food intolerance encompasses non-immune mediated responses within the GI tract induced by various factors, including fermentable oligo-, di-, and mono-saccharides and polyols (FODMAPs), food additives and chemicals, toxins, enzyme deficiencies, and non-celiac gluten sensitivity, among other contributors [92]. There is no formalized testing shown to be effective in diagnosing triggers for food intolerance, also known as food sensitivity, and it is not quite clear if other systemic symptoms can be associated with food intolerance as well.
Lactose intolerance, classified under the umbrella of food intolerance, is a prevalent condition that is better studied and specific only to lactose, with bloating, abdominal distension, and diarrhea acutely after consumption of lactose. This disease affects approximately 65% of the global population, with discernible variability across different ethnic groups [93]. The primary etiological factor often observed is lactase non-persistence, characterized by a decline in lactase levels with advancing age [94]. Secondary lactase deficiency can be transient, such as from infections, or chronic, where disease causes damage to the small intestine. Congenital lactase deficiency, akin to other congenital disaccharide deficiencies, is a rare occurrence inherited in an autosomal recessive pattern. Irrespective of the underlying etiology, the absence of >50% lactase in the gut results in an augmented osmotic load and lactose fermentation, culminating in a spectrum of undesirable symptoms [95]. Symptoms resolve with lactose elimination, and it can be formally diagnosed by a hydrogen breath test. Similar to lactose intolerance, sucrase-isomaltase deficiency and glucose-galactose malabsorption, both rare autosomal recessive disaccharide deficiencies, manifest with GI symptoms and are amenable to therapeutic interventions involving elimination diets [96].
3. Conclusions
Despite our deepened understanding of non-IgE-mediated allergic GI disorders over the past few decades, there remains a considerable gap in our comprehension of the underlying pathophysiology of these conditions. This explains the lack of protocols and guidelines, and perhaps the absence of non-invasive biomarkers and other diagnostic testing, all of which would lead to less underdiagnosis and more opportunities to improve the quality of life of those afflicted with these conditions. While elimination diets serve as the fundamental aspect of treatment, numerous challenges may co-exist in implementing such dietary interventions, such as food insecurity, inaccessibility to allergen-free foods, and the potential risks of the development of food aversions and other eating disorders. Finally, a multidisciplinary approach is crucial for better outcomes.
Author Contributions
Conceptualization: V.A.P. and M.D.G.; Literature Review: V.A.P., N.M., K.M.B.C., F.S., T.S. and J.K.; Figures and Tables: V.A.P. and F.S.; Writing: Review and Editing: V.A.P., N.M., M.D.G., K.M.B.C., F.S., T.S. and J.K.; Supervision: V.A.P., M.D.G. and G.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
1. Melissa D. Gans: In the last 12 months, Gans has the following relevant financial activities outside the submitted work: consultant for Opinion Leader Group and Sumitomo Pharma; royalties from Elsevier; and research with DBV Technologies. 2. Gary I. Kleiner: Kleiner’s disclosures include Principal Investigator activities with Regeneron, Novartis and DBV Technology pharma trials. 3. Valishti Artee Pundit, Nadia Makkoukdji, Farrah Stone, Travis Satnarine, and Jessica Kuhn have no disclosures.
References
- Labrosse, R.; Graham, F.; Caubet, J.C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sicherer, S.; Berin, M.C.; Agyemang, A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021, 10, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Food protein-induced enterocolitis syndrome epidemiology. Ann. Allergy Asthma Immunol. 2021, 126, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.M.; Virkud, Y.V.; Seay, H.; Hickey, A.; Ndahayo, R.; Rosow, R.; Southwick, C.; Elkort, M.; Gupta, B.; Kramer, E.; et al. Prospective Assessment of Pediatrician-Diagnosed Food Protein-Induced Allergic Proctocolitis by Gross or Occult Blood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1692–1699.e1691. [Google Scholar] [CrossRef]
- Savilahti, E. Food-Induced Malabsorption Syndromes. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J. Eosinophilic Esophagitis: Incidence and Prevalence. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Bianchi, A.; D’Auria, E.; Cardinale, F.; Votto, M.; Martelli, A.; Tosca, M.; Chiappini, E.; Brambilla, I.; et al. Update on Food protein-induced enterocolitis syndrome (FPIES). Acta Biomed. 2021, 92, e2021518. [Google Scholar]
- Panel, N.I.-S.E.; Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar]
- Nowak-Wegrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar]
- Mehr, S.; Frith, K.; Campbell, D.E. Epidemiology of food protein-induced enterocolitis syndrome. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 208–216. [Google Scholar] [CrossRef]
- Powell, G.K. Milk- and soy-induced enterocolitis of infancy: Clinical features and standardization of challenge. J. Pediatr. 1978, 93, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.K. Enterocolitis in low-birth-weight infants associated with milk and soy protein intolerance. J. Pediatr. 1976, 5, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Rotella, K.; Lee, A.S.E.; Lopes, J.P.; Sicherer, S.H.; Kattan, J.D.; Baker, M.G. Food protein-induced enterocolitis syndrome (FPIES) to peanut: Characteristics and long-term outcomes of a large cohort. J. Allergy Clin. Immunol. Pract. 2023, 12, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Nowak-Wegrzyn, A.; Vadas, P. FPIES in adults. Ann. Allergy Asthma Immunol. 2018, 121, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Gonzalez-Delgado, P.; Vadas, P. Food protein-induced enterocolitis syndrome: Not just in children. Ann. Allergy Asthma Immunol. 2021, 127, 291–292. [Google Scholar] [CrossRef]
- Fernandes, B.N.; Boyle, R.J.; Gore, C.; Simpson, A.; Custovic, A. Food protein-induced enterocolitis syndrome can occur in adults. J. Allergy Clin. Immunol. 2012, 130, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Bosa, L.; Martelossi, S.; Tardini, G.; Midrio, P.; Lago, P. Early onset food protein-induced enterocolitis syndrome in two breastfed newborns masquerading as surgical diseases: Case reports and literature review. J. Matern. Fetal Neonatal Med. 2021, 34, 390–394. [Google Scholar] [CrossRef]
- Goldman, H.; Proujansky, R. Allergic proctitis and gastroenteritis in children. Clinical and mucosal biopsy features in 53 cases. Am. J. Surg. Pathol. 1986, 10, 75–86. [Google Scholar] [CrossRef]
- Halpin, T.C.; Byrne, W.J.; Ament, M.E. Colitis, persistent diarrhea, and soy protein intolerance. J. Pediatr. 1977, 91, 404–407. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Caparros, E.; Moreno, M.V.; Clemente, F.; Flores, E.; Velasquez, L.; Rubio, G.; Fernandez, J. Clinical and immunological characteristics of a pediatric population with food protein-induced enterocolitis syndrome (FPIES) to fish. Pediatr. Allergy Immunol. 2016, 27, 269–275. [Google Scholar] [CrossRef]
- Leonard, S.A.; Pecora, V.; Fiocchi, A.G.; Nowak-Wegrzyn, A. Food protein-induced enterocolitis syndrome: A review of the new guidelines. World Allergy Organ. J. 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.C.; Bencharitiwong, R.; Ross, A.; Sampson, H.A.; Berin, M.C.; Nowak-Wegrzyn, A. Humoral and cellular responses to casein in patients with food protein-induced enterocolitis to cow’s milk. J. Allergy Clin. Immunol. 2017, 139, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H. Food protein-induced enterocolitis syndrome: Case presentations and management lessons. J. Allergy Clin. Immunol. 2005, 115, 149–156. [Google Scholar] [CrossRef]
- Murray, K.F.; Christie, D.L. Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J. Pediatr. 1993, 122, 90–92. [Google Scholar] [CrossRef]
- Onesimo, R.; Dello Iacono, I.; Giorgio, V.; Limongelli, M.G.; Miceli Sopo, S. Can food protein induced enterocolitis syndrome shift to immediate gastrointestinal hypersensitivity? A report of two cases. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 61–63. [Google Scholar] [PubMed]
- Holbrook, T.; Keet, C.A.; Frischmeyer-Guerrerio, P.A.; Wood, R.A. Use of ondansetron for food protein-induced enterocolitis syndrome. J. Allergy Clin. Immunol. 2013, 132, 1219–1220. [Google Scholar] [CrossRef] [PubMed]
- Michelet, M.; Schluckebier, D.; Petit, L.M.; Caubet, J.C. Food protein-induced enterocolitis syndrome—A review of the literature with focus on clinical management. J. Asthma Allergy 2017, 10, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Cianferoni, A.; Bird, J.A.; Fiocchi, A.; Caubet, J.C.; Medical Advisory Board of the International FPIES Association. Managing food protein-induced enterocolitis syndrome during the coronavirus disease 2019 pandemic: Expert recommendations. Ann. Allergy Asthma Immunol. 2020, 125, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Jarocka-Cyrta, E.; Moschione Castro, A. Food Protein-Induced Enterocolitis Syndrome. J. Investig. Allergol. Clin. Immunol. 2017, 27, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chehade, M.; Mayer, L. Oral tolerance and its relation to food hypersensitivities. J. Allergy Clin. Immunol. 2005, 115, 3–12; quiz 13. [Google Scholar] [CrossRef]
- Ravelli, A.; Villanacci, V.; Chiappa, S.; Bolognini, S.; Manenti, S.; Fuoti, M. Dietary protein-induced proctocolitis in childhood. Am. J. Gastroenterol. 2008, 103, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Morfino, G.; D’Alcamo, A.; Di Paola, V.; Iacono, G.; Soresi, M.; Scerrino, G.; Maresi, E.; Gulotta, G.; et al. Oligo-antigenic diet in the treatment of chronic anal fissures. Evidence for a relationship between food hypersensitivity and anal fissures. Am. J. Gastroenterol. 2013, 108, 825–832. [Google Scholar] [CrossRef]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Di Nardo, G. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ. J. 2020, 13, 100471. [Google Scholar] [CrossRef]
- Perez-Machado, M.A.; Ashwood, P.; Thomson, M.A.; Latcham, F.; Sim, R.; Walker-Smith, J.A.; Murch, S.H. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 2003, 33, 2307–2315. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Dahan, E.; Seay, H.L.; Itzkovits, D.; Vlamakis, H.; Xavier, R.; Shreffler, W.G.; Yuan, Q.; Yassour, M. Longitudinal disease-associated gut microbiome differences in infants with food protein-induced allergic proctocolitis. Microbiome 2022, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.C.; Szajewska, H.; Shamir, R.; Nowak-Wegrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef]
- Arvola, T.; Ruuska, T.; Keranen, J.; Hyoty, H.; Salminen, S.; Isolauri, E. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Pediatrics 2006, 117, e760–e768. [Google Scholar] [CrossRef]
- Lake, A.M.; Whitington, P.F.; Hamilton, S.R. Dietary protein-induced colitis in breast-fed infants. J. Pediatr. 1982, 101, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.M. Food-induced eosinophilic proctocolitis. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S58–S60. [Google Scholar] [CrossRef]
- Maloney, J.; Nowak-Wegrzyn, A. Educational clinical case series for pediatric allergy and immunology: Allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE-mediated cow’s milk allergy. Pediatr. Allergy Immunol. 2007, 18, 360–367. [Google Scholar]
- Xanthakos, S.A.; Schwimmer, J.B.; Melin-Aldana, H.; Rothenberg, M.E.; Witte, D.P.; Cohen, M.B. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: A prospective cohort study. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Odze, R.D.; Bines, J.; Leichtner, A.M.; Goldman, H.; Antonioli, D.A. Allergic proctocolitis in infants: A prospective clinicopathologic biopsy study. Hum. Pathol. 1993, 24, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Zekakos Xypolias, D.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front. Nutr. 2022, 9, 843437. [Google Scholar] [CrossRef] [PubMed]
- Host, A.; Husby, S.; Osterballe, O. A prospective study of cow’s milk allergy in exclusively breast-fed infants. Incidence, pathogenetic role of early inadvertent exposure to cow’s milk formula, and characterization of bovine milk protein in human milk. Acta Paediatr. Scand. 1988, 77, 663–670. [Google Scholar] [CrossRef]
- Wolf, J.H. Historical Research: The Origin of ‘Formula’: State of the Science, 1890s. J. Hum. Lact. 2020, 36, 410–413. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.M.; Virkud, Y.V.; Phadke, N.A.; Su, K.W.; Seay, H.; Atkins, M.R.; Keet, C.; Shreffler, W.G.; Yuan, Q. Increased IgE-Mediated Food Allergy With Food Protein-Induced Allergic Proctocolitis. Pediatrics 2020, 146, e20200202. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Hildebrand, K.J.; Chan, E.S. Non-IgE-mediated food allergy: Evaluation and management. Paediatr. Child. Health 2021, 26, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, L.; Vaarala, O.; Karttunen, R.; Tuure, T.; Korpela, R.; Kokkonen, J. Increased IFN-gamma secretion from duodenal biopsy samples in delayed-type cow’s milk allergy. Pediatr. Allergy Immunol. 2005, 16, 439–444. [Google Scholar] [CrossRef]
- Connors, L.; O’Keefe, A.; Rosenfield, L.; Kim, H. Non-IgE-mediated food hypersensitivity. Allergy Asthma Clin. Immunol. 2018, 14, 56. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gonsalves, N.; Abonia, J.P.; Alexander, J.A.; Arva, N.C.; Atkins, D.; Attwood, S.E.; Auth, M.K.H.; Bailey, D.D.; Biederman, L.; et al. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin. Gastroenterol. Hepatol. 2022, 20, 2474–2484.e2473. [Google Scholar] [CrossRef]
- Marasco, G.; Visaggi, P.; Vassallo, M.; Fiocca, M.; Cremon, C.; Barbaro, M.R.; De Bortoli, N.; Bellini, M.; Stanghellini, V.; Savarino, E.V.; et al. Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 15165. [Google Scholar] [CrossRef] [PubMed]
- Lehman, H.K.; Lam, W. Eosinophilic Esophagitis. Immunol. Allergy Clin. N. Am. 2021, 41, 587–598. [Google Scholar] [CrossRef]
- Sperry, S.L.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Influence of race and gender on the presentation of eosinophilic esophagitis. Am. J. Gastroenterol. 2012, 107, 215–221. [Google Scholar] [CrossRef]
- Arias, Á.; Pérez-Martínez, I.; Tenías, J.M.; Lucendo, A.J. Systematic review with meta-analysis: The incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther. 2016, 43, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Mukkada, V.; Eichinger, C.S.; Schofield, H.; Todorova, L.; Falk, G.W. Natural history of eosinophilic esophagitis: A systematic review of epidemiology and disease course. Dis. Esophagus 2018, 31, doy015. [Google Scholar] [CrossRef]
- Noti, M.; Wojno, E.D.; Kim, B.S.; Siracusa, M.C.; Giacomin, P.R.; Nair, M.G.; Benitez, A.J.; Ruymann, K.R.; Muir, A.B.; Hill, D.A.; et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat. Med. 2013, 19, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Wang, N.; Stringer, K.F.; Mishra, A.; Fulkerson, P.C.; Abonia, J.P.; Jameson, S.C.; Kirby, C.; Konikoff, M.R.; Collins, M.H.; et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Investig. 2006, 116, 536–547. [Google Scholar] [CrossRef]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef]
- Underwood, B.; Troutman, T.D.; Schwartz, J.T. Breaking down the complex pathophysiology of eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 2023, 130, 28–39. [Google Scholar] [CrossRef]
- Hogan, S.P.; Mishra, A.; Brandt, E.B.; Foster, P.S.; Rothenberg, M.E. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc. Natl. Acad. Sci. USA 2000, 97, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Litosh, V.A.; Rochman, M.; Rymer, J.K.; Porollo, A.; Kottyan, L.C.; Rothenberg, M.E. Calpain-14 and its association with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2017, 139, 1762–1771.e1767. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e313. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e1010. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Liotti, L.; Saretta, F.; Castagnoli, R.; Caminiti, L.; et al. Pediatric eosinophilic esophagitis: A review for the clinician. Ital. J. Pediatr. 2021, 47, 230. [Google Scholar] [CrossRef]
- Hiremath, G.S.; Hameed, F.; Pacheco, A.; Olive, A.; Davis, C.M.; Shulman, R.J. Esophageal Food Impaction and Eosinophilic Esophagitis: A Retrospective Study, Systematic Review, and Meta-Analysis. Dig. Dis. Sci. 2015, 60, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E.; et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007, 133, 1342–1363. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N.P.; Aceves, S.S. Diagnosis and treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2020, 145, 1–7. [Google Scholar] [CrossRef]
- Menard-Katcher, C.; Swerdlow, M.P.; Mehta, P.; Furuta, G.T.; Fenton, L.Z. Contribution of Esophagram to the Evaluation of Complicated Pediatric Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 541–546. [Google Scholar] [CrossRef]
- Katzka, D.A.; Smyrk, T.C.; Alexander, J.A.; Geno, D.M.; Beitia, R.A.; Chang, A.O.; Shaheen, N.J.; Fitzgerald, R.C.; Dellon, E.S. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am. J. Gastroenterol. 2017, 112, 1538–1544. [Google Scholar] [CrossRef]
- Carlson, D.A.; Lin, Z.; Hirano, I.; Gonsalves, N.; Zalewski, A.; Pandolfino, J.E. Evaluation of esophageal distensibility in eosinophilic esophagitis: An update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol. Motil. 2016, 28, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.; Gupta, S.K. Promising Modalities to Identify and Monitor Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Pitsios, C.; Vassilopoulou, E.; Pantavou, K.; Terreehorst, I.; Nowak-Wegzryn, A.; Cianferoni, A.; Tsigkrelis, G.P.; Papachristodoulou, M.; Bonovas, S.; Nikolopoulos, G.K. Allergy-Test-Based Elimination Diets for the Treatment of Eosinophilic Esophagitis: A Systematic Review of Their Efficacy. J. Clin. Med. 2022, 11, 5631. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.; Aceves, S.S. Allergic components of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2018, 142, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.A.; Sharaf, R.N.; Furuta, G.T.; Aceves, S.S.; Greenhawt, M.; Spergel, J.M.; Falck-Ytter, Y.T.; Dellon, E.S.; Chachu, K.A.; Day, L.; et al. Technical Review on the Management of Eosinophilic Esophagitis: A Report From the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology 2020, 158, 1789–1810.e1715. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Mougey, E.B.; Dellon, E.S.; Gutierrez-Junquera, C.; Fernandez-Fernandez, S.; Venkatesh, R.D.; Gupta, S.K. Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions. J. Asthma Allergy 2022, 15, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.J.; Abonia, J.P.; King, E.C.; Putnam, P.E.; Collins, M.H.; Franciosi, J.P.; Rothenberg, M.E. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol. 2012, 129, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011, 128, 3–20.e26; quiz 21–22. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Chan, E.S.; Rank, M.A.; Sharaf, R.N.; Stollman, N.H.; Stukus, D.R.; Wang, K.; Greenhawt, M.; Falck-Ytter, Y.T.; Chachu, K.A.; et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020, 158, 1776–1786. [Google Scholar] [CrossRef]
- Moawad, F.J.; Molina-Infante, J.; Lucendo, A.J.; Cantrell, S.E.; Tmanova, L.; Douglas, K.M. Systematic review with meta-analysis: Endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017, 46, 96–105. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019, 156, 592–603.e510. [Google Scholar] [CrossRef]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Stein, M.L.; Collins, M.H.; Villanueva, J.M.; Kushner, J.P.; Putnam, P.E.; Buckmeier, B.K.; Filipovich, A.H.; Assa’ad, A.H.; Rothenberg, M.E. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J. Allergy Clin. Immunol. 2006, 118, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Conus, S.; Grzonka, P.; Kita, H.; Kephart, G.; Bussmann, C.; Beglinger, C.; Smith, D.A.; Patel, J.; Byrne, M.; et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut 2010, 59, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Assa’ad, A.H.; Gupta, S.K.; Collins, M.H.; Thomson, M.; Heath, A.T.; Smith, D.A.; Perschy, T.L.; Jurgensen, C.H.; Ortega, H.G.; Aceves, S.S. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011, 141, 1593–1604. [Google Scholar] [CrossRef]
- Markowitz, J.E.; Jobe, L.; Miller, M.; Frost, C.; Laney, Z.; Eke, R. Safety and Efficacy of Reslizumab for Children and Adolescents With Eosinophilic Esophagitis Treated for 9 Years. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 893–897. [Google Scholar] [CrossRef]
- Alkhowaiter, S. Eosinophilic esophagitis. Saudi Med. J. 2023, 44, 640–646. [Google Scholar] [CrossRef]
- Available online: https://www.ajmc.com/view/fda-approves-dupilumab-as-first-therapy-for-eosinophilic-esophagitis (accessed on 1 January 2024).
- Dupixent Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf (accessed on 1 January 2024).
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C. The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-persistence and Lactose Intolerance. Curr. Gastroenterol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.B.; Waud, J.P.; Roberts, A.G.; Campbell, A.K. Systemic lactose intolerance: A new perspective on an old problem. Postgrad. Med. J. 2005, 81, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed]
- Tomar, B.S. Lactose Intolerance and Other Disaccharidase Deficiency. Indian J. Pediatr. 2014, 81, 876–880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).