Abstract
Monitoring complications of colonoscopies after a positive faecal immunochemical test (FIT-colonoscopies) is crucial in FIT-based colorectal cancer (CRC) screening. We investigated the occurrence of bleeding and perforation post FIT-colonoscopies (2013–2019) in Flanders and the contributing factors. A retrospective case–control study was conducted, including bleeding/perforation cases within 14 days after index colonoscopy, and controls without such events. Bleeding rates dropped from 0.9–1.1% (pre-2017) to 0.3% (2017–2018) and further to 0.05% (2019), while perforation rates remained at 0.05–0.11% (2014–2019). Male gender, polypectomy, general anaesthesia, and recent antiplatelet/antithrombotic drug use increased bleeding odds. Incomplete colonoscopy, polypectomy, general anaesthesia, and recent antiplatelet/antithrombotic drug use raised perforation odds. The endoscopists (n = 16) with highest bleeding rates (top 5%) performed only 6% of total FIT-colonoscopies, yet their patients experienced 45.5% of bleeding events. Similarly, for the top 5% of perforation rates, endoscopists conducting only 4.5% of total FIT-colonoscopy had 49.0% of perforation events occur in their patients. This study sheds light on FIT-colonoscopy-related complications in Flanders, their rates and risk factors. These findings can be incorporated into CRC screening materials and guide interventions to mitigate complications. A central colonoscopy register is currently lacking in Belgium, highlighting the need for its establishment to facilitate recurrent monitoring and evaluation.
1. Introduction
Colonoscopy has been widely used since its introduction in the 1960s [1]. It has served a critical role in colorectal cancer (CRC) management, functioning both as a primary screening test and as a follow-up diagnostic procedure after an abnormal result from another primary screening test (e.g., a faecal occult blood test). Additionally, it can be used for therapeutic purposes, including the removal of CRC adenomas and precursor lesions [2]. Through enabling the early detection of CRC and the removal of precancerous lesions, colonoscopy significantly contributes to reduce both CRC mortality and incidence [1,2].
Despite its evident benefits, one of the major concerns about colonoscopy is its complications, including bleeding and perforation. Previous studies have reported varying rates of colonoscopy-related bleeding complications, ranging between 0.04% and 6.1% [3,4,5,6,7,8,9,10,11,12,13,14], and perforation rates between 0.02% and 0.27% [3,4,5,7,8,9,10,12,13,14,15,16,17]. These risks increase significantly (up to four- to six-fold) in colonoscopies involving polypectomy [3,12,18].
While the overall risk of colonoscopy-associated complications remains generally low, it should not be underestimated, especially in the context of population-based CRC screening. In the CRC screening programmes using faecal immunochemical test (FIT) as the primary screening tool, such as in the Flemish CRC screening programme, it is notable that only half of the individuals undergoing colonoscopy after a positive FIT are diagnosed with CRC (~4%) or precursor lesions (advanced adenomas and advanced serrated polyps, ~50%). This means the other half of the FIT-positive population comprises completely healthy individuals without any colorectal abnormalities [19]. Hence, it is crucial to gain an accurate understanding of the occurrence of colonoscopy-associated complications and find effective ways to minimize the risk of colonoscopy-related complications.
In Flanders and across Belgium as a whole, a centralized registration system for colonoscopy is currently lacking, resulting in the absence of systematic recording for colonoscopy-related complications. The communication regarding potential colonoscopy complications to the target CRC screening population in Flanders has mainly relied on data from foreign countries, potentially introducing inaccuracies, due to substantial variations in complication rates across different countries and regions [20].
The current study aimed to investigate the rates and characteristics of bleeding and perforation complications following colonoscopies performed after a positive FIT result within the Flemish CRC screening programme and to identify the factors contributing to their occurrence. The insights gained from this study can be integrated into CRC screening materials, assisting the target population in making informed decisions about their participation. Furthermore, these findings can guide policy makers and healthcare professionals in developing and adopting targeted strategies to reduce these complications.
2. Results
2.1. Characteristics of the Study Population
In total, our study included 69,723 FIT-colonoscopies. The characteristics of the study population are shown in Table 1. There were more men than women (58.3% vs. 41.7%). Almost all cases (98.8%) underwent a complete colonoscopy. The majority of endoscopists were gastroenterologists (97.9%), and a substantial portion (82.8%) performed between 20 and 99 FIT-colonoscopies annually. Nearly 60% of the colonoscopies involved polypectomy and 81.7% were conducted under general anaesthesia or conscious sedation. Among the study subjects, 5.9% used systemic corticosteroids, while 35.4% used antiplatelet/antithrombotic medications within seven days before to two days after the index colonoscopy.

Table 1.
Characteristics of the study population—individuals undergoing at least one colonoscopy after a positive FIT result in Flanders (2013–2019).
2.2. Rates of Bleeding and Perforation over Time
The number of colonoscopies and rates of colonoscopy-related bleeding and perforation during the study period (2013–2019) are shown in Figure 1 and Supplementary Table S1. The rate of bleeding ranged between 0.93% and 1.08% during 2013–2016 and dropped significantly to around 0.3% during 2017–2018. The occurrence of only one bleeding event among 1970 FIT-colonoscopies (rate of 0.05%) suggests a further decline in the bleeding rate in 2019. The rate of perforation remained relatively stable, fluctuating between 0.05% and 0.11% during the study period (in 2013, the rate of perforation was 0.24, markedly higher than subsequent years, but the difference lacked statistical significance due to a small sample size in that year).
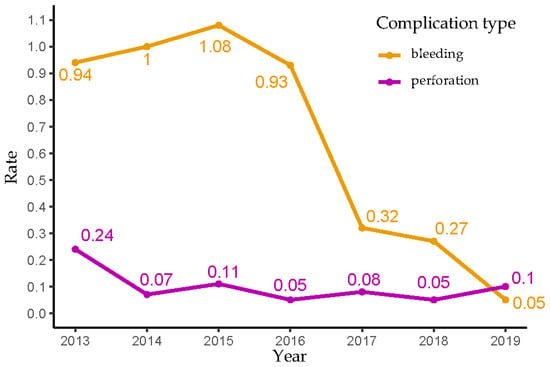
Figure 1.
Bleeding and perforation rates of follow-up colonoscopies after a positive FIT result in Flanders (2013–2019).
2.3. Time Interval between Index Colonoscopy and Bleeding/Perforation Events
Figure 2 and Supplementary Table S2 present the numbers and percentages of bleeding and perforation events grouped by the interval between the index colonoscopy and the complication occurrence. The majority of bleeding and perforation events occurred either on the same day or within two days following the index colonoscopy, accounting for 85% (421/496) of bleeding cases and 83% (43/52) of perforation cases. Notably, the occurrence of perforation exhibited a more delayed pattern compared to bleeding: while almost 70% (345/469) of bleeding events were recorded on the same day as the index colonoscopy, along with an additional 11% (53/496) and 5% (23/496) on the first and second days afterwards, only 46% (24/52) of perforation events occurred on the same day of the index colonoscopy, and an additional 19% (10/52) and 17% (9/52) were noted on the first and second days after the index colonoscopy, respectively.
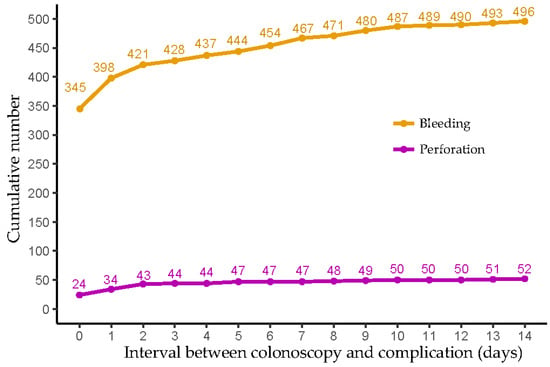
Figure 2.
The cumulative number of bleeding and perforation events based on the time interval between index colonoscopy and complication occurrence.
2.4. Factors Associated with the Occurrence of Bleeding and Perforation following Colonoscopies Performed after a Positive Faecal Immunochemical Test
As mentioned in our Methods (Section 4), due to the small proportions of cases where surgeons and practitioners from other specialties (non-gastroenterologist/internist/surgeon) performed colonoscopies (0.33% and 0.15%, respectively), and considering the absence of any bleeding or perforation events within these subgroups, they were excluded from our logistic regression analyses. Consequently, our logistic regression analyses comprised 69,385 study subjects.
2.4.1. Univariable Analyses
In univariable analyses, eight variables exhibited a significant association with the occurrence of bleeding, including gender, age, endoscopist’s specialty, number of FIT-colonoscopies performed annually, presence of polypectomy, type of anaesthesia, recent use of antiplatelet/antithrombotic drugs, and lesion location. Five variables demonstrated a significant association with the occurrence of perforation, including type of colonoscopy, presence of polypectomy, type of anaesthesia, recent use of antiplatelet/antithrombotic drug, and lesion location (as shown in Table 2). These variables, together with year of colonoscopy performance (categorised as 2013–2016, 2017–2018 and 2019 for bleeding due to observed changes in trends during these periods) and lesion type, were included in the multivariable models to identify factors associated with the occurrence of bleeding and perforation, respectively, after a FIT-colonoscopy.

Table 2.
Results of univariable analyses assessing the association between each determinant of interest and occurrence of bleeding or perforation.
2.4.2. Multivariable Analyses
Bleeding
The results of multivariable analyses for bleeding are presented in Table 3. Men demonstrated 1.5 times higher odds of experiencing bleeding following a FIT-colonoscopy compared to women. The presence of polypectomy increased the odds of experiencing a bleeding event after a FIT-colonoscopy by 2.7 times. Compared to cases without registered anaesthesia, the use of general anaesthesia during colonoscopy increased the odds of having a bleeding event by 1.8 times. A recent use of antiplatelet/antithrombotic drugs increased the odds of experiencing a bleeding event after a FIT-colonoscopy by 1.2 times. Although some categories of the endoscopist’s annual number of FIT-colonoscopies exhibited statistical significance when compared to the reference range of 20–39 FIT-colonoscopies/year, no clear pattern was observed between the number of FIT-colonoscopies performed annually and the odds of having a bleeding event.

Table 3.
Results of multivariable analyses assessing the association between each determinant of interest and bleeding occurrence.
Perforation
The results of the multivariable analyses for perforation are shown in Table 4. Compared to a complete colonoscopy, an incomplete colonoscopy was associated with 43.9 times higher odds of having a perforation event. The presence of polypectomy during colonoscopy increased the odds of experiencing perforation by 3.1 times. Compared to no registration of anaesthesia during colonoscopy, the use of general anaesthesia increased the odds of experiencing a perforation event by 7.6 times. Moreover, recent use of antiplatelet/antithrombotic drugs resulted in 3.6 times higher odds of experiencing perforation after a FIT-colonoscopy.

Table 4.
Results of multivariable analyses assessing the association between each determinant of interest and perforation occurrence.
2.5. Endoscopist’s Volume of Colonoscopies Performed after a Positive Faecal Immunochemical Test and Bleeding/Perforation Rates
Figure 3 and Figure 4 illustrate the relationships between endoscopists’ total number of FIT-colonoscopies performed over the study period of 2013–2019 and their overall rate of bleeding and perforation. To ensure the reliability of the calculated bleeding and perforation rates, the analysis only involved a subset of 322 endoscopists out of the total 528 endoscopists included in the study, each of whom conducted a minimum of 20 colonoscopies. Among these 332 endoscopists, all 492 recorded bleeding events were observed in patients treated by 44.6% of the total endoscopists, who collectively performed 54.1% of the total FIT-colonoscopies (37,328 procedures). Simultaneously, all 51 recorded perforations events occurred in patients treated by 12.0% of the total endoscopists, who performed 16.2% of the total FIT-colonoscopies (11,178 procedures).
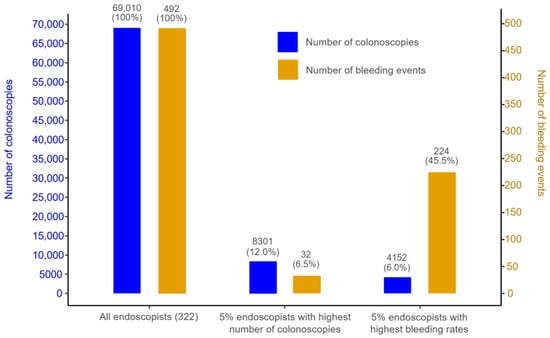
Figure 3.
Number of colonoscopies (proportion) and number of bleeding events (proportion) of three groups: (1) all endoscopists with a minimum of 20 colonoscopies (total 332 endoscopists); (2) the top 5% of endoscopists with the highest number of colonoscopies; and (3) the top 5% of endoscopists with the highest bleeding rates.
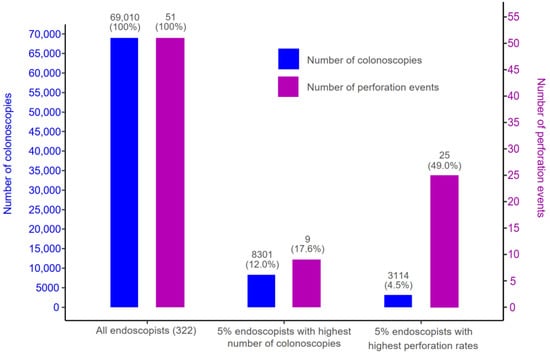
Figure 4.
Number of colonoscopies (proportion) and number of perforation events (proportion) of three groups: (1) all endoscopists with a minimum of 20 colonoscopies (total 332 endoscopists); (2) the top 5% of endoscopists with the highest number of colonoscopies; and (3) the top 5% of endoscopists with the highest perforation rates.
Figure 3 and Figure 4 highlight that the highest rates of bleeding/perforation were associated with a small subset of endoscopists who did not necessarily perform an exceptionally high or low volume of FIT-colonoscopies. Specifically, the 5% of endoscopists (16 individuals) with the highest number of FIT-colonoscopies contributed to 12.0% of total FIT-colonoscopies, while their patients experienced 6.5% of the bleeding and 17.6% of the perforation events. Meanwhile, the endoscopists with the highest bleeding rates (top 5%) contributed to only 6% of total number of FIT-colonoscopies, yet their patients experienced 45.5% of the overall bleeding events. Similarly, the endoscopists with the highest perforation rates (top 5%) performed only 4.5% of the total number of FIT-colonoscopies but had 49.0% of the total perforation events occur in their patients. Notably, there was no overlap between top 5% of endoscopists with the highest bleeding rates and top 5% of endoscopists with the highest perforation rates.
3. Discussion
3.1. Summary of Main Findings
The current study investigated the occurrence and characteristics of bleeding and perforation complications associated with FIT-colonoscopies within the Flemish CRC screening programme, along with factors contributing to the occurrence of these complications. The rates of bleeding and perforation complications were low and in line with the recommended rates outlined in widely recognised guidelines. Notably, bleeding rates decreased, starting in 2017, whereas perforation rates remained stable throughout the study period. Factors such as polypectomy, general anaesthesia, and recent use of antiplatelet/antithrombotic drugs were associated with increased odds of experiencing complications for both bleeding and perforation. Male gender was only linked to a higher occurrence of bleeding, while incomplete colonoscopy was only linked to a higher occurrence of perforation. It is worth noting that the highest rates of bleeding and perforation complications were concentrated in a small group of endoscopists.
3.2. Rates of Bleeding and Perforation Align with the Literature
Our study recorded a substantial decline in bleeding rates, with a range of 0.93% to 1.08% before 2017, decreasing to around 0.3 during 2017–2018, and further declining to 0.05 in 2019. Meanwhile, perforation rates remained consistent at 0.05% to 0.11% between 2014 and 2019. These figures were in line with documented rates of colonoscopy-related bleeding and perforation in the existing literature. Prior studies have reported a broad spectrum of overall bleeding complication rates for all colonoscopy indications, ranging from 0.04% to 6.1% [3,4,5,6,7,8,9,10,11,12,13,14]. Specifically, bleeding complication rates for screening/surveillance colonoscopies without polypectomy have been reported within the range of 0.21%–0.37% [12,21,22]. Conversely, colonoscopies with polypectomy carry a heightened risk, with bleeding complication rates ranging from 0.7% to 1.5% [6,12,17,21,23].
Regarding perforation complications, the literature has reported overall rates ranging from 0.02% to 0.27% for all colonoscopy indications [3,4,5,7,8,9,10,12,13,14,15,16,17]. In the context of screening colonoscopies, the rate of perforation varies between 0.01% and 0.1% [11,20,22], while diagnostic colonoscopies carry rates of 0.04%–0.1% [3,6,12,23]. Notably, colonoscopies with polypectomy could exhibit elevated rates of perforation, reaching up to 0.25% [11,23]. Existing research consistently underscores a substantial four- to six-fold increase in bleeding or perforation complications following colonoscopies with polypectomy, compared to those without [3,12,18].
In recent years, gastrointestinal professional societies worldwide have adopted safety standards for colonoscopy practice. The rates of bleeding and perforation complications following FIT-colonoscopies in our studies align with the ranges recommended by The American Society for Gastrointestinal Endoscopy (ASGE)/American College of Gastroenterology (ACG) Task Force. The ASGE/ACG Task Force recommends that the post-polypectomy “bleeding” rate should remain below 1%, and post-colonoscopy “perforation” should be less than 0.2% for all examinations and even less than 0.1% for screening examinations [24]. Concerning screening colonoscopies, the European Society of Gastrointestinal Endoscopy (ESGE) proposes that fewer than 5% of bleeding complications should require surgical intervention, and the rate of perforation requiring emergency surgery should be below 0.1% [25].
In our study, the rate of bleeding met the norm recommended by ESGE, while the rate of perforation slightly exceeded 0.1% for specific years. This can be attributed to the fact that our study included colonoscopies performed after a positive FIT result, which predominantly comprised diagnostic and therapeutic procedures involving polypectomy. The higher complication rates among FIT-colonoscopies in comparison to screening colonoscopies is anticipated and aligns with the complexity of the procedures performed.
3.3. Declining Bleeding Rates Coupled with Stable Perforation Rates
Our study revealed a significant reduction in bleeding rates starting in 2017, whereas perforation rates remained quite stable throughout the study period. This trend is consistent with previous research that also indicated declining bleeding rates and stable perforation rates over the past 15 years [12,20]. Several factors might account for the declining trend of bleeding rates. Physician-related variables play a role in post-polypectomy bleeding, including the selection of techniques for polyp removal [26]. In recent years, there has been an increasing adoption of cold snare resection compared to hot snare resection. Cold snare, involving the use of a snare without electrocautery, is considered to carry a lower risk of delayed post-polypectomy bleeding. Several case series have reported notably low rates of delayed bleeding following cold snare removal [27,28].
Furthermore, advancements in colonoscopy equipment, such as the integration of high-definition colonoscopies, together with increased experience gained through higher colonoscopy volumes since the start of the organised screening programme, and improved training covering both knowledge and technical skills for endoscopists, have significantly contributed to the reduction in bleeding rates [29,30,31]. Prophylactic clipping has been used more and more frequently nowadays to mitigate delayed bleeding following the resection of proximal colorectal polyps [32]. Systematic training of endoscopic resection techniques through hands-on training and video tutorials has also further spread practical expertise, ultimately enhancing the safety of routine colonoscopy practice [12].
3.4. Risk Factors of Colonoscopy-Related Bleeding and Perforation Occurrence
3.4.1. Patient Sex and Age
In our study, male gender exhibited higher odds (1.5 times) of experiencing a bleeding event compared to females. Prior research has reported inconsistent findings. While Pox et al. [15] and Arana-Arri et al. [33] identified male gender as a predictor of colonoscopy-related complications, Chan et al. [34] observed a significant association between female gender and immediate colonoscopy-related complications.
Age has been consistently reported to be associated with a higher risk of post-colonoscopy perforation [5,10,16] and bleeding [5,10], as well as overall colonoscopy-related complications [15] in the existing literature. However, it is important to note that these studies mainly showed elevated risk of complications in those aged 75 and older. Our study only included individuals between the ages of 50 and 75 (99.8% aged 50–74 years) who underwent a colonoscopy after participating in FIT screening within the Flemish CRC screening programme. Within this age range, we did not observe a significant association between age and the occurrence of colonoscopy-related bleeding and perforation, probably because our study population is younger than those examined in the previously mentioned studies.
3.4.2. Polypectomy
Our study found significant higher odds (2.7–3.1 times) of experiencing a bleeding or perforation event after colonoscopy with polypectomy, in comparison to cases without polypectomy. The literature has extensively reported polypectomy as a risk factor for complications after colonoscopy, particularly bleeding and perforation [5,10,15,17,20,26]. On the other hand, a few studies have shown no significant impact of polypectomy on the risk of colonoscopy-related bleeding and perforation [13,16].
The association between polypectomy and bleeding and perforation complications is expected. Immediate bleeding during or right after polypectomy may result from not adequately sealing the blood vessels where the polyps were removed, occurring in about 1% to 2% of polypectomies [26,35]. During the process of polypectomy, there is also a possibility of causing perforation, due to either inadvertently grabbing deeper layers of the colon wall or excessive heat injury [20]. Delayed bleeding after polypectomy (typically within 1–2 weeks after the procedure) is believed to occur when a scab-like layer forms after the removal of the polyp; and as this scab comes off, it exposes and potentially injures an underlying blood vessel [26].
3.4.3. Anaesthesia
Our study found increased odds of post-colonoscopy bleeding and perforation complications associated with the use of general anaesthesia, compared to cases with no registration of anaesthesia. Prior research has highlighted an elevated risk of perforation following colonoscopy when propofol sedation is utilized, particularly in therapeutic procedures [36,37]. Without feedback from patients, endoscopists might not be aware of increased tension on the colon wall due to pressure from the colonoscopy [38], which could raise the risk of perforation.
The introduction of propofol sedation alters colonoscopy technique, involving fewer pressure manoeuvres and positional adjustments [39]. When patients are under sedation, they are less likely to move or react to the sensations caused by the insertion of the colonoscope. This allows endoscopists to navigate and manipulate the colonoscope more easily and with greater control, enabling a greater application of axial and radial forces during colonoscope insertion [40]. This raises the concerns that propofol sedation might escalate the risk of perforation, particularly due to mechanical injury.
Despite this reasonable consideration, other studies have demonstrated no significant difference in the risk of perforation with anaesthesia assistance and/or propofol sedation [41,42,43]. It is possible that the sample size in these studies was too limited to detect the association [42,43]. Nevertheless, even a comprehensive study by Bielawska et al., involving 3,059,045 outpatient colonoscopies, did not establish a link between anaesthesia and an increased risk of perforation [41]. This suggests a requirement for more intensive research in this area, and possibly a meta-analysis to thoroughly examine this aspect.
3.4.4. Recent Use of Antiplatelet/Antithrombotic Drugs
According to our findings, recent use of antiplatelet/antithrombotic drugs (within seven days before and two days after the colonoscopy) exhibited a 1.2-fold increase in the odds of experiencing colonoscopy-related bleeding, and a 3.6-fold increase in the odds of perforation. This aligns with prior research, which has indicated the associations between the use of these drugs and an elevated risk of bleeding [3,26,44] and both bleeding and perforation [45], as well as complications in general [12,34]. With regard to guidance on antithrombotic management before and after colonoscopy, balancing the risk of post-polypectomy bleeding against the risk of experiencing thromboembolic issues due to the interruption of antithrombotic treatment is the key consideration [46].
Different clinical practice guidelines have provided guidance on the management of antithrombotic medications in patients who are undergoing endoscopic procedures [47,48,49,50]. These guidelines differentiate between procedures with low and high risk of post-procedure bleeding and classify patients based on their risk of developing thromboembolism. All guidelines universally support the safety of performing colonoscopic biopsy without the need to temporarily interrupt antithrombotic drugs [47,48,49,50]. In contrast, polypectomy, conventionally considered a high-risk procedure for bleeding, typically requires temporary interruption of antiplatelet agents (except aspirin) and anticoagulants. Furthermore, according to the Asian guidelines, endoscopic submucosal dissection and endoscopic mucosal resection (EMR) for lesions larger than 2 cm are categorized as ultra-high-risk procedures, implying that careful consideration should be given to discontinuing aspirin in these cases [48]. Individuals scheduled for a colonoscopy while on antiplatelet and/or antithrombotic regiments, or who recently completed such treatment, should consult their GP or gastroenterologist for an evaluation to ensure that appropriate measures are taken regarding their medications and the execution of the colonoscopy.
3.4.5. Incomplete Colonoscopy
Our study found a strong association (43.9 times) between the use of incomplete colonoscopy, compared to complete colonoscopy, and the occurrence of perforation. This observation is consistent with the prior findings of Chan et al.’s study [34]. However, it is important to note that this association does not necessarily imply that incomplete colonoscopy directly led to more perforation complications. It is plausible that the occurrence of perforation could have influenced endoscopists’ decision to conduct an incomplete colonoscopy rather than a complete one. For example, incomplete colonoscopy might be the result of underlying conditions such as a bowel obstruction, which, in itself, is a risk factor for perforation complications [16]. Additionally, factors such as inadequate bowel preparation could contribute to both performance of incomplete colonoscopy and increased risk of perforation. Unfortunately, we were unable to explore the potential impact of bowel preparation or the underlying reasons behind the decision to perform an incomplete colonoscopy on our results due to data unavailability, as discussed in ‘Strengths and limitations’ (Section 3.6).
3.4.6. Endoscopist Experience and Specialty
The endoscopist’s experience has been repeatedly identified as a factor associated with the occurrence of colonoscopy-related complications. Previous studies have consistently indicated that an annual colonoscopy volume exceeding 200 to 300 procedures is linked to notably reduced risks of colonoscopy-related complications [10,18,51,52]. Lorenzo-Zúñiga et al. even demonstrated that colonoscopies performed by low-volume endoscopists (fewer than 591 annually) exhibited a higher likelihood of post-polypectomy bleeding or colonic perforation [17]. Based on the available evidence, the Dutch national CRC screening programme established specific criteria, which require endoscopists to have a minimum lifetime experience of performing 500 colonoscopies, including at least 200 colonoscopies and 50 polypectomies performed in the year preceding the initiation of the accreditation programme [2]. These requirements continue to apply on an annual basis for endoscopists who have attained accreditation within the Dutch screening programme.
Arora et al.’s study reported endoscopist specialty as a determinant of colonoscopy-related perforation, revealing a lower perforation risk when colonoscopies were performed by primary care physicians other than gastroenterologists or surgeons, for which the reasons remained unclear [16]. Potential explanations include allocation bias, where technically demanding procedures are assigned to gastroenterologists or surgeons, leaving relatively simpler procedures to other practitioners. In our studies, where only two categories of endoscopist specialty, namely gastroenterologist and internist, were included, no significant difference in the occurrence of bleeding or perforation after FIT-colonoscopy were found between the two groups.
While our study did not find significant associations between the number of FIT-colonoscopies performed annually by an endoscopist, or the endoscopist’s specialty, and the occurrence of colonoscopy-related bleeding and perforation, it is important to acknowledge that our study specifically focused on FIT-colonoscopies, which did not cover all colonoscopies performed by each endoscopist. Interestingly, we observed a concentration of the highest complication rates within a small group of endoscopists; and these increased complication rates did not consistently correspond to the highest or lowest numbers of FIT-colonoscopies performed. Among the included endoscopists, the top 5% (16 in total) with the highest bleeding rates contributed to only 6.0% of the total FIT-colonoscopies but accounted for 45.5% of all bleeding events. Similarly, the top 5% (16) with the highest perforation rates contributed to just 4.5% of the total FIT-colonoscopies but were associated with 49.0% of all perforation events. This implies that relying solely on the number of colonoscopies performed may not be sufficient for identifying these high-risk practitioners. Future research in Flanders should delve into this subgroup of endoscopists, investigating the underlying factors driving their elevated risks of colonoscopy-related complications. Such insights can guide regulatory authorities in providing appropriate support and interventions to address this issue effectively.
3.5. Issue of the High False Positive Rates of Faecal Immunochemical Tests
Although widely employed in current population-based CRC screening programmes and demonstrating notable effectiveness, FIT presents a drawback, due to its relatively high false positivity rate. Nearly half of the individuals testing positive for FIT do not exhibit colorectal abnormalities, leading to unnecessary colonoscopies, increased costs, and associated complications [19,53,54,55]. Addressing this issue involves considering the implementation of a triage test after a positive FIT and before colonoscopy, which should outperform FIT in specificity, maintain or surpass FIT’s sensitivity, be less invasive than colonoscopy, user-friendly, and cost-effective. While methods such as capsule endoscopy or CT colonoscopy have been proposed as potential triage tests following a positive FIT, conclusive supporting evidence is lacking [54,56]. Despite their high sensitivity in detecting CRC and advanced adenomas, surpassing 85%, the high prevalence of lesions in the positive FIT population could lead to a significant probability of false negatives, thereby impacting the negative predictive value of the tests [54,57]. Additionally, the question of cost-effectiveness concerning the use of these methods as a triage test between a positive FIT result and colonoscopy remains a subject requiring further investigation [54,56,57].
3.6. Strengths and Limitations
The primary strength of this study lies in its utilization of register-based data, which provided high-quality information on a large scale while effectively minimizing selection and recall bias. As Belgium has compulsory health insurance, covering over 99% of the population [58], more than 99% of all performed FIT-colonoscopies and medical procedures associated with complications were included in our study. Another notable strength is the study’s specific focus on FIT-colonoscopy, producing findings that are more relevant for CRC screening programmes.
However, our study has several limitations. We could not take into account the number of endoscopists’ other (non-FIT) colonoscopies, which also contributes to their experience. Consequently, the reported number of endoscopists’ FIT-colonoscopies in this study might not accurately reflect their overall experience. Moreover, the identification of complications relied on nomenclature codes utilized in health insurance claims. It is possible that some bleeding/perforation cases could be registered using other nomenclature codes that were not listed in this study. At the same time, certain procedures were coded using the provided nomenclature codes but might not be directly linked to colonoscopy complications. Nevertheless, the number of such potentially misclassified cases is expected to be minimal and unlikely to have significant impact on our main findings. Additionally, due to data unavailability, we were not able to incorporate several major variables such as polyp characteristics (number, size, morphology), bowel preparation (type, quality), and endoscopists’ number of years of experience. Although not presented in this study, our initial exploration (unpublished results) did not indicate significant associations between the comorbidities included in the Charlson comorbidity index and the occurrence of colonoscopy-related bleeding and perforation in the Flemish CRC screening population. Due to the limited number of bleeding and perforation events in the current study, comorbidities were not included in the list of exploratory variables. Lastly, some degree of selection bias might be present, due to the absence of specific control selection techniques, such as employing matching criteria or random control selection. Nevertheless, we believe this has minimal impact on our study’s findings, as our controls closely resemble the source population from which the cases arose, primarily due to the very small number of cases in comparison to the overall study population.
3.7. Implications of Study Findings
3.7.1. Integrating Information Regarding Colonoscopy Complications into Screening Materials
Our study provides insights into the prevalence and characteristics of bleeding and perforation complications following FIT-colonoscopies in Flanders, along with their associated risk factors. Integrating these findings into CRC screening materials can enhance the target population’s understanding of the risks associated with colonoscopy, thereby facilitating their informed decisions about their participation in CRC screening.
3.7.2. Strategies Based on Identified Risk Factors to Mitigate Bleeding and Perforation Complications
The identified risk factors in this study form a foundation for informed and proactive measures to refine CRC screening practices, mitigate the risk of bleeding and perforation complications associated with colonoscopy, and enhance patient safety. For instance, acknowledging the increased risk associated with polypectomy allows for the refinement of polypectomy techniques, such as adopting cold snare resection, and emphasizing training and guidelines to improve the sealing of blood vessels after polyp removal, thus preventing post-polypectomy bleeding [26,27,28,35]. When considering the association between general anaesthesia and increased odds of bleeding and perforation, it is essential to carefully decide the sedation methods in order to balance sedation depth to minimize patient movements while ensuring adequate assessment of potential complications. Additionally, comprehensive patient evaluations before and after colonoscopy to manage antiplatelet/antithrombotic drugs are crucial. Physicians and gastroenterologists should guide patients on the appropriate management of these medications, considering the risk of colonoscopy-related bleeding and perforation against the threat of thromboembolic events due to treatment interruption [47,48,49,50]. Such considerations should be individualised based on the patient’s risks and procedural complexity.
3.7.3. Further Research to Explore the Subgroup of Endoscopists Who Significantly Contributed to the Recorded Bleeding and Perforation Events
The revelation that a small group of endoscopists contributed substantially to the recorded bleeding and perforation events indicated the need for more in-depth investigation into the factors leading to the increased complication rates in this subgroup. Identifying procedural and skill gaps can inform targeted interventions, ensuring these practitioners receive the necessary support, guidance, and specialised training to address their specific deficiencies. Regulatory bodies should consider adopting standardised best practices across all endoscopists to ensure a uniform level of care and patient safety.
3.7.4. Establishing a Centralised Colonoscopy Register in Belgium
Our study also highlights the importance of introducing a central colonoscopy register in Belgium, as well as in other countries and regions where such a system is lacking. Such a central colonoscopy register would provide a structured platform for systematically gathering and storing data on patient characteristics (age, gender, medical history, medication use, and other individual risk factors), colonoscopy details (bowel preparation, colonoscopy type, anaesthesia type, polypectomy/biopsy procedure, immediate and delayed complications, and corresponding complication interventions), as well as information about the endoscopist (specialty, colonoscopy and polypectomy volume, and years of experience). The collected data can be linked with information on colonoscopy outcomes (the number and characteristics of polyps such as their location, size, and morphology), which is available within the database of the national/regional cancer registry.
By establishing and maintaining a central colonoscopy register, healthcare authorities can monitor the quality of colonoscopy procedures and outcomes. This proactive approach enables the detection of performance variations among different healthcare providers and facilities, ensuring a uniform standard of care, and identifying areas for improvement. Furthermore, a central register facilitates the tracking of colonoscopy-related complications, such as perforations, bleedings, infections, and fatalities. Timely identification and thorough analysis of these complications can contribute to improved patient safety, as healthcare providers can implement measures to prevent future occurrences and ensure that appropriate post-procedure care is provided. Healthcare policymakers can use the comprehensive data from such a register to make well-informed decisions concerning CRC screening guidelines, recommendations, and resource allocation.
3.8. Generalizability of Study Findings
Our findings can be highly generalisable to diverse settings, as most factors examined in this study are not region-specific, except for the FIT cut-off. Specifically, the FIT cut-off used in Flanders is 15 µg Hb/g, which is much lower than the FIT cut-offs applied in other countries, such as 47 µg Hb/g in the Netherlands [59], 30 µg Hb/g in France [60], and 80 µg Hb/g for men and 40 µg Hb/g for women in Sweden [61]. This variance could potentially result in differences in the types of lesions identified between the Flemish CRC screening programme and other screening programmes. A lower FIT cut-off increases the test’s sensitivity, enabling the detection of smaller amounts of blood in the stool, possibly leading to the identification of smaller or potentially less advanced lesions.
However, we believe these differences in FIT cut-offs would have minimal impact on our findings regarding the associations between the determinants of interest (gender, age at colonoscopy performance, endoscopist’s specialty, the annual volume of FIT-colonoscopies by the endoscopist, presence of polypectomy, type of anaesthesia, recent use of antiplatelet/antithrombotic drugs, and lesion location) and the occurrence of colonoscopy-related bleeding and perforation complications. This is because the lesion type was included as a confounding factor for adjustment in our multivariable logistic regression models.
4. Methods
4.1. The Flemish Colorectal Cancer Screening Programme
The population-based CRC screening in Flanders started in October 2013. Screening invitations are sent via mail every two years to all eligible individuals through a centralized invitation procedure, and participation is free of charge. During the study period, the target screening ages were gradually expanded, starting with individuals aged 56–74 as of 2013 and gradually including those aged 55 as of June 2017; 53–54 as of July 2018; 51–52 as of 2019, and finally reaching down to 50 years as of 2020. The cut-off for a positive FIT result (OC-sensor, Eiken Chemical Co., Tokyo, Japan) was set at 75 ng Hb/mL (15 µg Hb/g). Individuals with a positive FIT result are recommended to undergo a follow-up colonoscopy [62].
4.2. Study Population
The study population consisted of individuals who fulfilled the following criteria: (1) received at least one invitation for CRC screening in Flanders between 18 October 2013 (when the first screening invitations were sent out) and 31 December 2018, (2) participated and had a positive FIT result, and (3) underwent at least one complete or incomplete colonoscopy (between 2013 and 2019) following the positive FIT result (referred to as ‘FIT-colonoscopy’ in this study).
Exclusion Criteria
Given our specific focus on investigating bleeding and perforation complications after a FIT-colonoscopy (and thus, in principle, the study population had no complaints/symptoms), we excluded subjects who had been diagnosed with Crohn’s disease, ulcerative colitis, or diverticular disease of the intestine within the 2-year period preceding the index colonoscopy.
Figure 5 presents the process of identifying the index colonoscopy in this study.
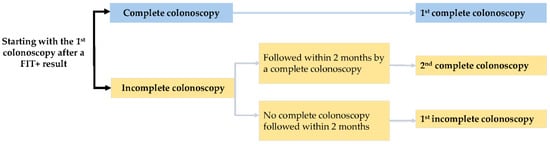
Figure 5.
Identification of the index colonoscopy.
4.3. Study Design
This study adopted a retrospective, case–control study design.
Definitions of Cases and Controls
Cases included individuals who experienced a bleeding or perforation event, while controls consisted of all individuals in the study population without any occurrence of bleeding or perforation, within 14 days after the index FIT-colonoscopy. No specific sampling techniques were employed for control selection; instead, all individuals without a bleeding or perforation event were included as controls for the respective analyses for bleeding or perforation.
4.4. Study Variables, Data Collection, and Data Sources
The data regarding bleeding, perforation, anaesthesia, polypectomy, and the use of antiplatelet/antithrombotic drugs and systemic corticosteroids were obtained from the Insurance Intermutualistic Agency (IMA-AIM), which is responsible for gathering information on healthcare services and medication reimbursements for insured Belgian residents (covering over 99% of the Belgian population) [58]. These data, collected by the seven health insurance funds in Belgium, were processed and made available for research by the Insurance Intermutualistic Agency.
Bleeding and perforation complications were identified through nomenclature codes referring to the procedures to manage bleeding and perforation, respectively, performed within 14 days following the index colonoscopy. The choice of a 14-day follow-up period after the index colonoscopy allows for sufficient confidence that the observed bleeding/perforation events were related to the index colonoscopy, while also offering a sufficiently long window to capture complications that might not occur immediately during or right after the colonoscopy. This timeframe has been recommended and employed in previous research [20,63]. Similarly, the performance of anaesthesia and polypectomy was recognised by nomenclature codes, indicating these procedures were carried out on the same day as the index colonoscopy. In Belgium, nomenclature codes are used to classify healthcare services partially or fully reimbursed by health insurance. The use of antiplatelet/antithrombotic drugs and systemic corticosteroids was identified through CNK codes, which are unique product codes assigned to each pharmaceutical retail package or prescribed drug in Belgium.
FIT results, originally from the Centre for Cancer Detection, and data on the type of colonoscopy, from the Insurance Intermutualistic Agency, were available at the Belgian Cancer Registry, due to routine data sharing and linkage between the organisations. Other details such as subjects’ age at the time of colonoscopy, gender, endoscopist’s specialty, FIT-colonoscopy volume, and the characteristics of lesions found during colonoscopy were retrieved from the Belgian Cancer Registry.
For this study, all the above data were collected and safely stored in a secured environment of the Belgian Cancer Registry. The electronic exchange and linkage of personal data between the Belgian Cancer Registry, the Insurance Intermutualistic Agency, and the Federal Public Service (FPS) Health, Food Chain Safety and Environment was approved by the Information Security Committee (number 21/118, issued on 6 July 2021). Different data sources were linked using subjects’ national registration number and the date of the index colonoscopy. Data analyses for this study were conducted by a designated researcher within the secured environment of the Belgian Cancer Registry.
For more detailed information about each variable, its definition, and the corresponding data collection source, please refer to Table 5. Supplementary Tables S3–S6 contain comprehensive lists of nomenclature codes used for identifying medical procedures and lists of medications included in this study.

Table 5.
Study outcomes, determinants of interest, and additional variables for adjustment: definitions and data sources.
4.5. Statistical Analysis
4.5.1. Population Size
Our study involved a total of 69,723 FIT-colonoscopies, all of which were included in our descriptive analysis. However, for our logistic regression models, we excluded 233 colonoscopies performed by surgeons, and 105 colonoscopies performed by professionals other than gastroenterologists, internists, or surgeons. This exclusion was due to their small proportions, accounting for 0.33% and 0.15% of the total colonoscopies, respectively, coupled with the absence of any recorded bleeding or perforation events within these subgroups. As a result, our logistic regression models were based on a total of 69,385 colonoscopies.
4.5.2. Main Analysis
Descriptive Analysis
Rates of bleeding and perforation after a FIT-colonoscopy, along with their corresponding 95% CI (confidence interval) (exact binomial) were presented. Continuous variables were described with medians (interquartile range) due to their non-normal distributions, while categorical variables were described with numbers (percentages).
Additionally, we examined the relationships between the cumulative number of FIT-colonoscopies performed over the entire study period (2013–2019) and the overall rates of bleeding and perforation among endoscopists who had conducted a minimum of 20 colonoscopies.
Logistic Regression Analysis
Given the dichotomous nature of the study outcomes (bleeding/no bleeding and perforation/no perforation), logistic regression was used to identify factors associated with bleeding or perforation occurrence within 14 days after a FIT-colonoscopy. The logistic regression analyses for bleeding included 496 bleeding events and 68,889 controls (FIT-colonoscopies without bleeding events), while the analyses for perforation involved 52 perforation events and 69,333 controls (FIT-colonoscopies without perforation events). No cases were identified where both bleeding and perforation events occurred within 14 days after the index colonoscopy.
For the logistic regression analyses, initial univariable analyses were conducted to assess the relationship between each determinant of interest and the study outcomes (bleeding/perforation). The likelihood ratio test was applied to compare a model incorporating the determinant of interest as an independent variable with a null model containing only the intercept, in order to determine if the determinant of interest exhibits a significant association with a study outcome. All the variables with a likelihood ratio test p-value of ≤0.2, along with year of colonoscopy (categorised as 2013–2016, 2017–2018 and 2019 for bleeding, due to observed changes in trends during these periods) and lesion type as adjustment variables, were included in the final multivariable analyses. Multicollinearity in the multivariable models for bleeding and perforation was assessed using the variance inflation factor (VIF), with variables displaying a VIF of >5, indicating high multicollinearity, being removed from the models.
When examining the association between a specific independent variable and a study outcome, we treated all the other independent variables in the multivariable model (i.e., variables with a likelihood ratio test p-value of ≤0.2 from the univariable analyses except for the variable under assessment), year of colonoscopy (for bleeding), and lesion type as confounders. For example, the multivariable model for bleeding included gender, age at colonoscopy performance, endoscopist’s specialty, the annual volume of FIT-colonoscopies by the endoscopist, presence of polypectomy, type of anaesthesia, recent use of antiplatelet/antithrombotic drugs, lesion location, year of colonoscopy (categorised as 2013–2016, 2017–2018 and 2019), and lesion type. When assessing the association between gender and the occurrence of bleeding, all the other independent variables except for gender—including age at colonoscopy performance, endoscopist’s specialty, the annual volume of FIT-colonoscopies by the endoscopist, presence of polypectomy, type of anaesthesia, recent use of antiplatelet/antithrombotic drugs, lesion location, year of colonoscopy (categorised as 2013–2016, 2017–2018 and 2019), and lesion type—were considered potential confounders and were adjusted for. The detailed lists of variables included in the final multivariable logistic regression models for bleeding and perforation are specified in the footnotes of the corresponding result Table 3 and Table 4. A p-value of <0.05 (two-sided) in the multivariable analyses was considered statistically significant.
Note that the numbers of colonoscopies in 2013 and 2019 are incomplete compared to the other years (2014–2018). In 2013, only colonoscopies performed in 2013 following a positive FIT result between October and December 2013 could be included, and in 2019 only colonoscopies conducted in 2019 after a positive FIT result in 2018 were included, whereas in the other years, colonoscopies conducted after a positive FIT result in either the same year or the preceding year are included. Therefore, in our logistic regression analyses, the endoscopist’s number of FIT-colonoscopies performed in 2014 was used for 2013, and the endoscopist’s number of FIT-colonoscopies performed in 2018 was used for 2019, for each endoscopist.
All analyses were performed using RStudio (version 1.3.1056; RStudio, PBC, Boston, MA, USA).
5. Conclusions
The rates of bleeding and perforation resulting from colonoscopies performed after a positive FIT result within the Flemish CRC screening programme were generally low and adhered to established guidelines. Polypectomy, general anaesthesia, and recent use of antiplatelet/antithrombotic drugs were associated with an increased likelihood of experiencing complications for both bleeding and perforation. Male gender was only linked to a higher occurrence of bleeding, while incomplete colonoscopy was only associated with a higher occurrence of perforation. Notably, a small group of endoscopists contributed substantially to the recorded bleeding and perforation events.
Integrating information on colonoscopy-associated complications into CRC screening materials can facilitate informed decisions about participation in CRC screening among the target population. Based on the identified risk factors, targeted strategies can be developed to mitigate these complications and improve patient safety. Future research is needed to investigate the subset of endoscopists contributing substantially to the recorded bleeding and perforation events, in order to identify potential procedural and skill gaps and introduce necessary support and guidance in this group. Establishing a centralised colonoscopy register in Belgium is crucial to enable the continuous monitoring and evaluation of the quality of colonoscopy procedures and outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gidisord6010003/s1, Table S1: Number of colonoscopies, rates of colonoscopy-related bleeding and perforation during the study period (2013–2019); Table S2: Numbers and percentages of bleeding and perforation events by interval between index colonoscopy and occurrence of the complication; Table S3: List of nomenclature codes used to identify bleeding and perforation within 14 days after colonoscopy; Table S4: List of nomenclature codes used to identify anaesthesia; Table S5: List of nomenclature codes used to identify polypectomy; Table S6: List of antiplatelet/antithrombotic drugs.
Author Contributions
Conceptualisation, S.H., G.V.H., T.N.T. and M.P.; data curation, J.B. and K.V.H.; formal analysis, T.N.T.; methodology, T.N.T., S.H., G.V.H., M.P., J.B., B.B., E.V.C. and K.V.H.; project administration, T.N.T.; supervision, S.H., G.V.H. and K.V.H.; visualisation, T.N.T.; writing—original draft, T.N.T.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The Flemish colorectal cancer screening programme is funded exclusively by the Agency for Care and Health, part of the Flemish Ministry of Welfare, Public Health and Family (https://www.vlaanderen.be/en, accessed on 30 May 2023). The Flemish Ministry was not involved in any phase of this study (design, data collection, analysis, interpretation, or writing the manuscript).
Institutional Review Board Statement
The secondary use and linkage of the databases involved were approved on 17 September 2013 (updated on 20 March 2018), with reference number 13/091, and on 6 July 2021, with reference number 21/118, by the Information Security Committee (formerly the Committee for the Protection of Privacy). Approval of an ethical committee was not necessary given the fact that this retrospective study does not fall under the Belgian legislation for ethical committee approval (Law of 7 May 2004 regarding experiments on human persons (art. 3, Section 2)). Only pseudonymised data were used for this study, and results are reported in an aggregated way. The study protocol conforms to the principles of the 1964 Helsinki Declaration and its later amendments and to the applicable national guidelines.
Informed Consent Statement
When participating in the Flemish colorectal cancer screening programme, all participants filled out a written informed consent explaining that personal information can be used for scientific research and evaluation to improve the programme.
Data Availability Statement
The data used and analysed during the study are available from the corresponding author upon reasonable request. The pseudonymised data can be provided within the secured environment of the Belgian Cancer Registry after having been guaranteed that the applicable GDPR regulations are applied.
Acknowledgments
We acknowledge the Flemish Colorectal Cancer Screening Task Force for functioning as a sounding board. We would also like to thank Harlinde De Schutter for her valuable guidance in the preparation and submission of the necessary documents to seek permission from the Information Security Committee (Informatieveiligheidscomité—IVC). This permission (number 21/118, issued on 6 July 2021) facilitated a secure electronic exchange and linkage of personal data between the Belgian Cancer Registry (BCR), the Insurance Intermutualistic Agency (IMA-AIM), and the Federal Public Service (FPS) Health, Food Chain Safety and Environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khalid, M.; Khalid, M.; Gayam, V.; Yeddi, A.; Adam, O.; Chakraborty, S.; Abdallah, M.; Abu-Heija, A.; Kaloti, Z.; Mukhtar, O.; et al. The Impact of Hospital Teaching Status on Colonoscopy Perforation Risk: A National Inpatient Sample Study. Gastroenterol. Res. 2020, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bronzwaer, M.E.S.; Depla, A.; van Lelyveld, N.; Spanier, B.W.M.; Oosterhout, Y.H.; van Leerdam, M.E.; Spaander, M.C.W.; Dekker, E. Quality assurance of colonoscopy within the Dutch national colorectal cancer screening program. Gastrointest. Endosc. 2019, 89, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Gralnek, I.M. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Benazzato, L.; Zorzi, M.; Antonelli, G.; Guzzinati, S.; Hassan, C.; Fantin, A. Colonoscopy-related adverse events and mortality in an Italian organized colorectal cancer screening program. Endoscopy 2020, 53, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Laanani, M.; Coste, J.; Blotiere, P.O.; Carbonnel, F.; Weill, A. Patient, Procedure, and Endoscopist Risk Factors for Perforation, Bleeding, and Splenic Injury after Colonoscopies. Clin. Gastroenterol. Hepatol. 2019, 17, 719–727.e13. [Google Scholar] [CrossRef] [PubMed]
- Dafnis, G.; Ekbom, A.; Pahlman, L.; Blomqvist, P. Complications of diagnostic and therapeutic colonoscopy within a defined population in Sweden. Gastrointest. Endosc. 2001, 54, 302–309. [Google Scholar] [CrossRef]
- Ko, C.W.; Riffle, S.; Michaels, L.; Morris, C.; Holub, J.; Shapiro, J.A.; Ciol, M.A.; Kimmey, M.B.; Seeff, L.C.; Lieberman, D. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin. Gastroenterol. Hepatol. 2010, 8, 166–173. [Google Scholar] [CrossRef]
- Levin, T.R.; Zhao, W.; Conell, C.; Seeff, L.C.; Manninen, D.L.; Shapiro, J.A.; Schulman, J. Complications of colonoscopy in an integrated health care delivery system. Ann. Intern. Med. 2006, 145, 880–886. [Google Scholar] [CrossRef]
- Suissa, A.; Bentur, O.S.; Lachter, J.; Yassin, K.; Chermesh, I.; Gralnek, I.; Karban, A.; Khamaysi, I.; Naveh, Y.; Tamir, A.; et al. Outcome and complications of colonoscopy: A prospective multicenter study in northern Israel. Diagn. Ther. Endosc. 2012, 2012, 612542. [Google Scholar] [CrossRef]
- Blotière, P.O.; Weill, A.; Ricordeau, P.; Alla, F.; Allemand, H. Perforations and haemorrhages after colonoscopy in 2010: A study based on comprehensive French health insurance data (SNIIRAM). Clin. Res. Hepatol. Gastroenterol. 2014, 38, 112–117. [Google Scholar] [CrossRef]
- Manta, R.; Tremolaterra, F.; Arezzo, A.; Verra, M.; Galloro, G.; Dioscoridi, L.; Pugliese, F.; Zullo, A.; Mutignani, M.; Bassotti, G. Complications during colonoscopy: Prevention, diagnosis, and management. Tech. Coloproctol. 2015, 19, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Reumkens, A.; Rondagh, E.J.A.; Bakker, M.C.; Winkens, B.; Masclee, A.A.M.; Sanduleanu, S. Post-Colonoscopy Complications: A Systematic Review, Time Trends, and Meta-Analysis of Population-Based Studies. Am. J. Gastroenterol. 2016, 111, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Coser, R.B.; Dalio, M.B.; Martins, L.C.P.; Alvarenga, G.F.; Cruz, C.A.; Imperiale, A.R.; Padovese, C.C.; Paulo, G.A.; Teixeira Junior, J.C. Colonoscopy complications: Experience with 8968 consecutive patients in a single institution. Rev. Col. Bras. Cir. 2018, 45, e1858. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, E.M.; Thomsen, M.K.; Tybjerg, J.; Friis-Hansen, L.; Andersen, B.; Jørgensen, J.C.R.; Baatrup, G.; Njor, S.H.; Mehnert, F.; Rasmussen, M. Colonoscopy-related complications in a nationwide immunochemical fecal occult blood test-based colorectal cancer screening program. Clin. Epidemiol. 2018, 10, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Pox, C.P.; Altenhofen, L.; Brenner, H.; Theilmeier, A.; Von Stillfried, D.; Schmiegel, W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology 2012, 142, 1460–1467.e2. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Mannalithara, A.; Singh, G.; Gerson, L.B.; Triadafilopoulos, G. Risk of perforation from a colonoscopy in adults: A large population-based study. Gastrointest. Endosc. 2009, 69 Pt 2, 654–664. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Moreno de Vega, V.; Doménech, E.; Mañosa, M.; Planas, R.; Boix, J. Endoscopist experience as a risk factor for colonoscopic complications. Color. Dis. 2010, 12, e273–e277. [Google Scholar] [CrossRef]
- Rabeneck, L.; Paszat, L.F.; Hilsden, R.J.; Saskin, R.; Leddin, D.; Grunfeld, E.; Wai, E.; Goldwasser, M.; Sutradhar, R.; Stukel, T.A. Bleeding and Perforation After Outpatient Colonoscopy and Their Risk Factors in Usual Clinical Practice. Gastroenterology 2008, 135, 1899–1906.e1. [Google Scholar] [CrossRef]
- Centre for Cancer Detection. What Are the Possible Outcomes of the Colonoscopy? Available online: https://dikkedarmkanker.bevolkingsonderzoek.be/nl/ddk/wat-zijn-de-mogelijke-resultaten-van-de-coloscopie (accessed on 20 August 2023). (In Dutch).
- Kim, S.Y.; Kim, H.S.; Park, H.J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef]
- Warren, J.L.; Klabunde, C.N.; Mariotto, A.B.; Meekins, A.; Topor, M.; Brown, M.L.; Ransohoff, D.F. Adverse events after outpatient colonoscopy in the Medicare population. Ann. Intern. Med. 2009, 150, 849–857, W152. [Google Scholar] [CrossRef]
- Zwink, N.; Holleczek, B.; Stegmaier, C.; Hoffmeister, M.; Brenner, H. Complication Rates in Colonoscopy Screening for Cancer. Dtsch. Arztebl. Int. 2017, 114, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Saraste, D.; Martling, A.; Nilsson, P.J.; Blom, J.; Tornberg, S.; Hultcrantz, R.; Janson, M. Complications after colonoscopy and surgery in a population-based colorectal cancer screening programme. J. Med. Screen. 2016, 23, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G., 2nd; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Rembacken, B.; Hassan, C.; Riemann, J.F.; Chilton, A.; Rutter, M.; Dumonceau, J.M.; Omar, M.; Ponchon, T. Quality in screening colonoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 2012, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A. Colonoscopy, Polypectomy, and the Risk of Bleeding. Med. Clin. N. Am. 2019, 103, 125–135. [Google Scholar] [CrossRef]
- Muniraj, T.; Sahakian, A.; Ciarleglio, M.M.; Deng, Y.; Aslanian, H.R. Cold snare polypectomy for large sessile colonic polyps: A single-center experience. Gastroenterol. Res. Pract. 2015, 2015, 175959. [Google Scholar] [CrossRef]
- Choksi, N.; Elmunzer, B.J.; Stidham, R.W.; Shuster, D.; Piraka, C. Cold snare piecemeal resection of colonic and duodenal polyps ≥1 cm. Endosc. Int. Open 2015, 3, E508–E513. [Google Scholar] [CrossRef]
- Bielawska, B.; Day, A.G.; Lieberman, D.A.; Hookey, L.C. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: A multivariable analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 85–92. [Google Scholar] [CrossRef]
- Anderloni, A.; Jovani, M.; Hassan, C.; Repici, A. Advances, problems, and complications of polypectomy. Clin. Exp. Gastroenterol. 2014, 7, 285–296. [Google Scholar] [CrossRef]
- Leyden, J.E.; Doherty, G.A.; Hanley, A.; McNamara, D.A.; Shields, C.; Leader, M.; Murray, F.E.; Patchett, S.E.; Harewood, G.C. Quality of colonoscopy performance among gastroenterology and surgical trainees: A need for common training standards for all trainees? Endoscopy 2011, 43, 935–940. [Google Scholar] [CrossRef]
- Turan, A.S.; Pohl, H.; Matsumoto, M.; Lee, B.S.; Aizawa, M.; Desideri, F.; Albeniz, E.; Raju, G.S.; Luba, D.; Barret, M.; et al. The Role of Clips in Preventing Delayed Bleeding after Colorectal Polyp Resection: An Individual Patient Data Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 362–371.e23. [Google Scholar] [CrossRef] [PubMed]
- Arana-Arri, E.; Imaz-Ayo, N.; Fernández, M.J.; Idigoras, I.; Bilbao, I.; Bujanda, L.; Bao, F.; Ojembarrena, E.; Gil, I.; Gutiérrez-Ibarluzea, I.; et al. Screening colonoscopy and risk of adverse events among individuals undergoing fecal immunochemical testing in a population-based program: A nested case-control study. United Eur. Gastroenterol. J. 2018, 6, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Lee, L.N.; Chan, A.C.; Ho, W.N.; Chan, Q.W.; Lau, S.; Chan, J.W. Predictive factors for colonoscopy complications. Hong Kong Med. J. 2015, 21, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Waye, J.D.; Lewis, B.S.; Yessayan, S. Colonoscopy: A prospective report of complications. J. Clin. Gastroenterol. 1992, 15, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Wernli, K.J.; Brenner, A.T.; Rutter, C.M.; Inadomi, J.M. Risks Associated with Anesthesia Services during Colonoscopy. Gastroenterology 2016, 150, 888–894, quiz e818. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.; Bannazadeh, M.; Riggs, T.; Shellnut, J.; Barkel, D.; Wasvary, H. Does sedation type affect colonoscopy perforation rates? Dis. Colon Rectum 2014, 57, 110–114. [Google Scholar] [CrossRef]
- Wernli, K.J.; Inadomi, J.M. Anesthesia for colonoscopy: Too much of a good thing? JAMA Intern. Med. 2013, 173, 556–558. [Google Scholar] [CrossRef]
- Hansen, J.J.; Ulmer, B.J.; Rex, D.K. Technical performance of colonoscopy in patients sedated with nurse-administered propofol. Am. J. Gastroenterol. 2004, 99, 52–56. [Google Scholar] [CrossRef]
- Korman, L.Y.; Haddad, N.G.; Metz, D.C.; Brandt, L.J.; Benjamin, S.B.; Lazerow, S.K.; Miller, H.L.; Mete, M.; Patel, M.; Egorov, V. Effect of propofol anesthesia on force application during colonoscopy. Gastrointest. Endosc. 2014, 79, 657–662. [Google Scholar] [CrossRef]
- Bielawska, B.; Hookey, L.C.; Sutradhar, R.; Whitehead, M.; Xu, J.; Paszat, L.F.; Rabeneck, L.; Tinmouth, J. Anesthesia Assistance in Outpatient Colonoscopy and Risk of Aspiration Pneumonia, Bowel Perforation, and Splenic Injury. Gastroenterology 2018, 154, 77–85.e3. [Google Scholar] [CrossRef]
- Okholm, C.; Hadikhadem, T.; Andersen, L.T.; Donatsky, A.M.; Vilmann, P.; Achiam, M.P. No increased risk of perforation during colonoscopy in patients undergoing Nurse Administered Propofol Sedation. Scand. J. Gastroenterol. 2013, 48, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.K.; Hung, L.; Kang, F.C.; Lan, K.M.; Poon, P.W.; So, E.C. Anesthesia does not increase the rate of bowel perforation during colonoscopy: A retrospective study. Acta Anaesthesiol. Taiwan 2009, 47, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Pigò, F.; Bertani, H.; Grande, G.; Abate, F.; Vavassori, S.; Conigliaro, R.L. Post-polypectomy bleeding after colonoscopy on uninterrupted aspirin/non steroideal antiflammatory drugs: Systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Vanaclocha-Espi, M.; Ibáñez, J.; Molina-Barceló, A.; Valverde-Roig, M.J.; Pérez, E.; Nolasco, A.; de la Vega, M.; de la Lastra-Bosch, I.D.; Oceja, M.E.; Espinàs, J.A.; et al. Risk factors for severe complications of colonoscopy in screening programs. Prev. Med. 2019, 118, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Telford, J.J.; Abraham, N.S. Management of Antiplatelet and Anticoagulant Agents before and after Polypectomy. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Barkun, A.N.; Sauer, B.G.; Douketis, J.; Laine, L.; Noseworthy, P.A.; Telford, J.J.; Leontiadis, G.I. American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline: Management of Anticoagulants and Antiplatelets during Acute Gastrointestinal Bleeding and the Periendoscopic Period. Am. J. Gastroenterol. 2022, 117, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.L.; Goh, K.L.; Reddy, N.; Fujimoto, K.; Ho, K.Y.; Hokimoto, S.; Jeong, Y.H.; Kitazono, T.; Lee, H.S.; Mahachai, V.; et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: Joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut 2018, 67, 405–417. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Acosta, R.D.; Abraham, N.S.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fisher, D.A.; et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest. Endosc. 2016, 83, 3–16. [Google Scholar] [CrossRef]
- Veitch, A.M.; Vanbiervliet, G.; Gershlick, A.H.; Boustiere, C.; Baglin, T.P.; Smith, L.A.; Radaelli, F.; Knight, E.; Gralnek, I.M.; Hassan, C.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut 2016, 65, 374–389. [Google Scholar] [CrossRef]
- Singh, H.; Penfold, R.B.; DeCoster, C.; Kaita, L.; Proulx, C.; Taylor, G.; Bernstein, C.N.; Moffatt, M. Colonoscopy and its complications across a Canadian regional health authority. Gastrointest. Endosc. 2009, 69, 665–671. [Google Scholar] [CrossRef]
- Choung, B.S.; Kim, S.H.; Ahn, D.S.; Kwon, D.H.; Koh, K.H.; Sohn, J.Y.; Park, W.S.; Kim, I.H.; Lee, S.O.; Lee, S.T.; et al. Incidence and risk factors of delayed postpolypectomy bleeding: A retrospective cohort study. J. Clin. Gastroenterol. 2014, 48, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, T.; van der Vlugt, M.; Dekker, E. Future of Colorectal Cancer Screening: From One-Size-FITs-All to Tailor-Made. Front. Gastroenterol. 2022, 1, 906052. [Google Scholar] [CrossRef]
- Sali, L.; Regge, D. CT colonography for population screening of colorectal cancer: Hints from European trials. Br. J. Radiol. 2016, 89, 20160517. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Milà, N.; Binefa, G.; Borràs, J.M.; Espinàs, J.A.; Moreno, V. False-positive results from colorectal cancer screening in Catalonia (Spain), 2000–2010. J. Med. Screen. 2012, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.C.; Pickhardt, P.J.; Stoker, J. CT colonography: Accuracy, acceptance, safety and position in organised population screening. Gut 2015, 64, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; Stoker, J.; Alarcon, O.; Barbaro, F.; Bellini, D.; Bretthauer, M.; De Haan, M.C.; Dumonceau, J.M.; Ferlitsch, M.; Halligan, S.; et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Eur. Radiol. 2015, 25, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Berete, F.; Demarest, S.; Charafeddine, R.; Bruyere, O.; Van der Heyden, J. Comparing health insurance data and health interview survey data for ascertaining chronic disease prevalence in Belgium. Arch. Public Health 2020, 78, 120. [Google Scholar] [CrossRef]
- Breekveldt, E.C.H.; Toes-Zoutendijk, E.; de Jonge, L.; Spaander, M.C.W.; Dekker, E.; van Kemenade, F.J.; van Vuuren, A.J.; Ramakers, C.R.B.; Nagtegaal, I.D.; van Leerdam, M.E.; et al. Personalized colorectal cancer screening: Study protocol of a mixed-methods study on the effectiveness of tailored intervals based on prior f-Hb concentration in a fit-based colorectal cancer screening program (PERFECT-FIT). BMC Gastroenterol. 2023, 23, 45. [Google Scholar] [CrossRef]
- Pellat, A.; Deyra, J.; Husson, M.; Benamouzig, R.; Coriat, R.; Chaussade, S. Colorectal cancer screening programme: Is the French faecal immunological test (FIT) threshold optimal? Ther. Adv. Gastroenterol. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Blom, J.; Lowbeer, C.; Elfstrom, K.M.; Sventelius, M.; Ohman, D.; Saraste, D.; Tornberg, S. Gender-specific cut-offs in colorectal cancer screening with FIT: Increased compliance and equal positivity rate. J. Med. Screen. 2019, 26, 92–97. [Google Scholar] [CrossRef]
- Hoeck, S.; Pringels, S.; Kellen, E.; Van Herck, K.; Martens, P.; Van Limbergen, E.; Francart, J.; Van Hal, G. First results of the Flemish colorectal cancer screening program: Start-up-period late 2013. Acta Gastroenterol. Belg. 2016, 79, 421–428. [Google Scholar] [PubMed]
- Hsu, W.-F.; Chang, C.-Y.; Chang, C.-C.; Chang, L.-C.; Chen, C.-H.; Lin, C.-C.; Lin, Y.-M.; Lee, C.-L.; Wu, H.-Y.; Lee, H.-C.; et al. Risk of colonoscopy-related complications in a fecal immunochemical test-based population colorectal cancer screening program. Endoscopy 2021, 54, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lallana, M.J.; Feja, C.; Aguilar-Palacio, I.; Malo, S.; Rabanaque, M.J. Use of Non-Steroidal Anti-Inflammatory Drugs and Associated Gastroprotection in a Cohort of Workers. Int. J. Environ. Res. Public Health 2018, 15, 1836. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).