The Treatment Effects of Percutaneous Drainage with or without Sclerotherapy for Symptomatic Liver Cysts
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics upon Initial Treatment
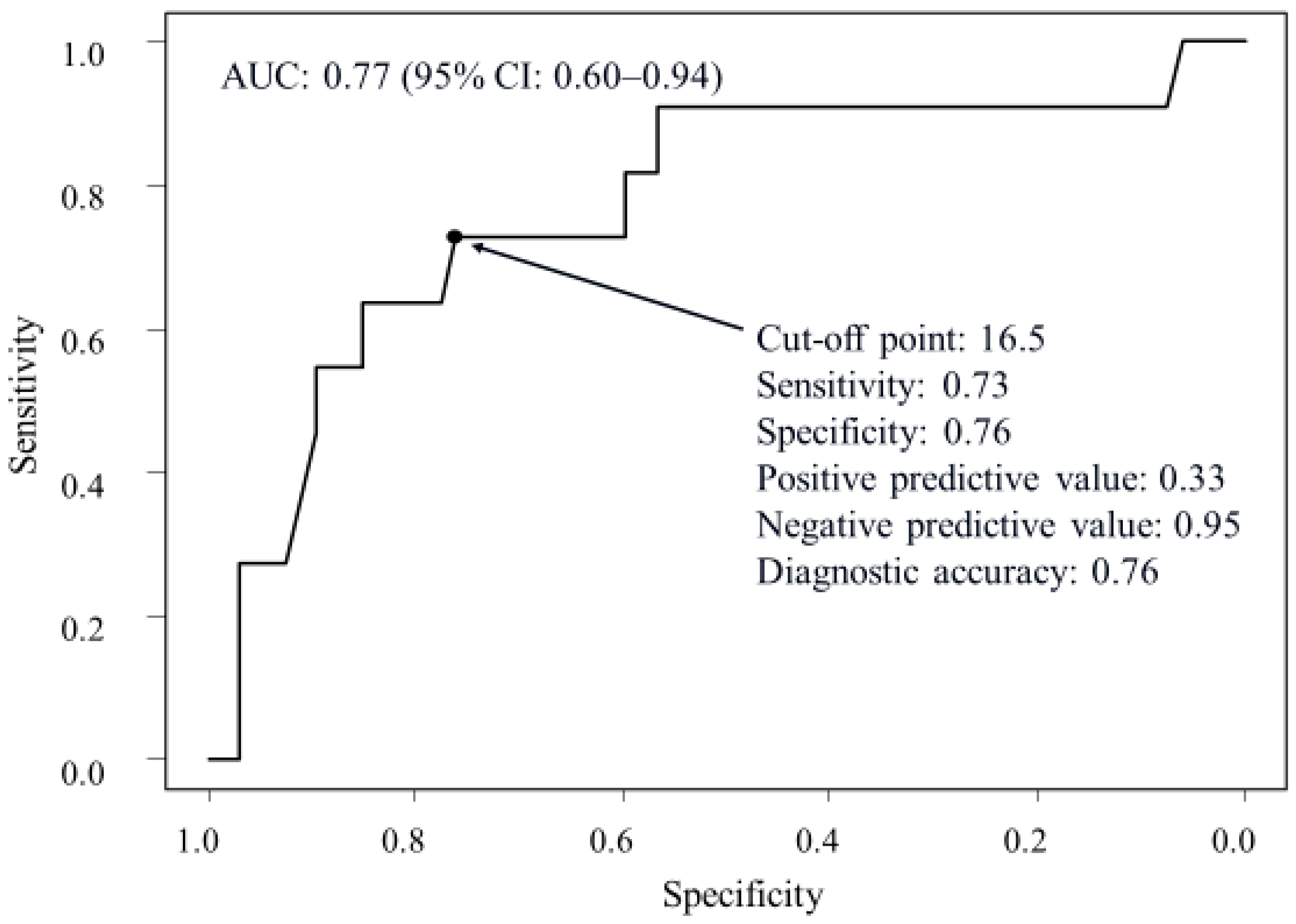
2.2. Factors Associated with Recurrence of Symptoms Due to Liver Cysts in Patients Treated via the Percutaneous Approach
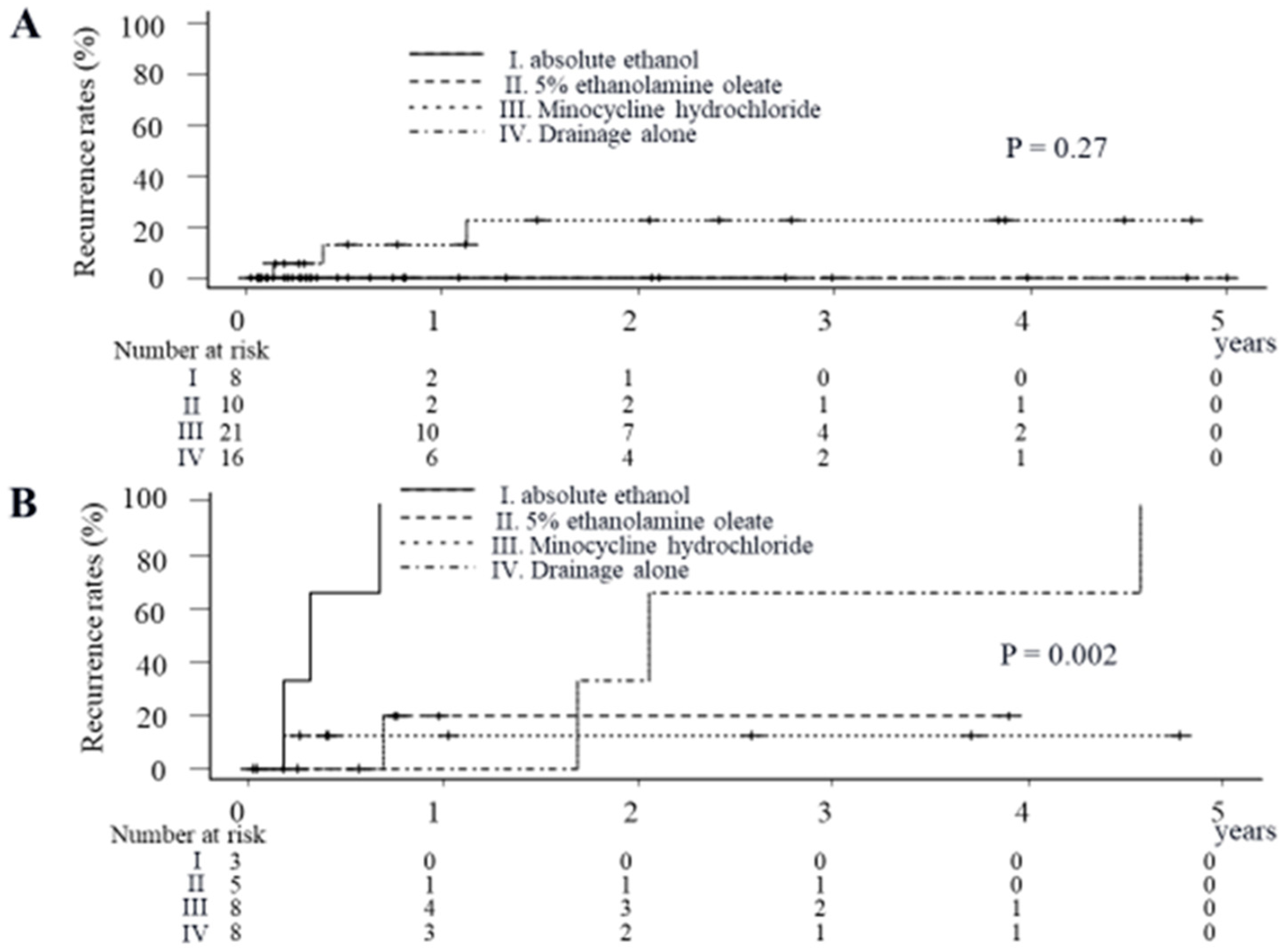
2.3. Impact of Sclerosing Agents on Cumulative Recurrence Rates of Symptoms Due to Liver Cysts in Patients Treated via the Percutaneous Approach
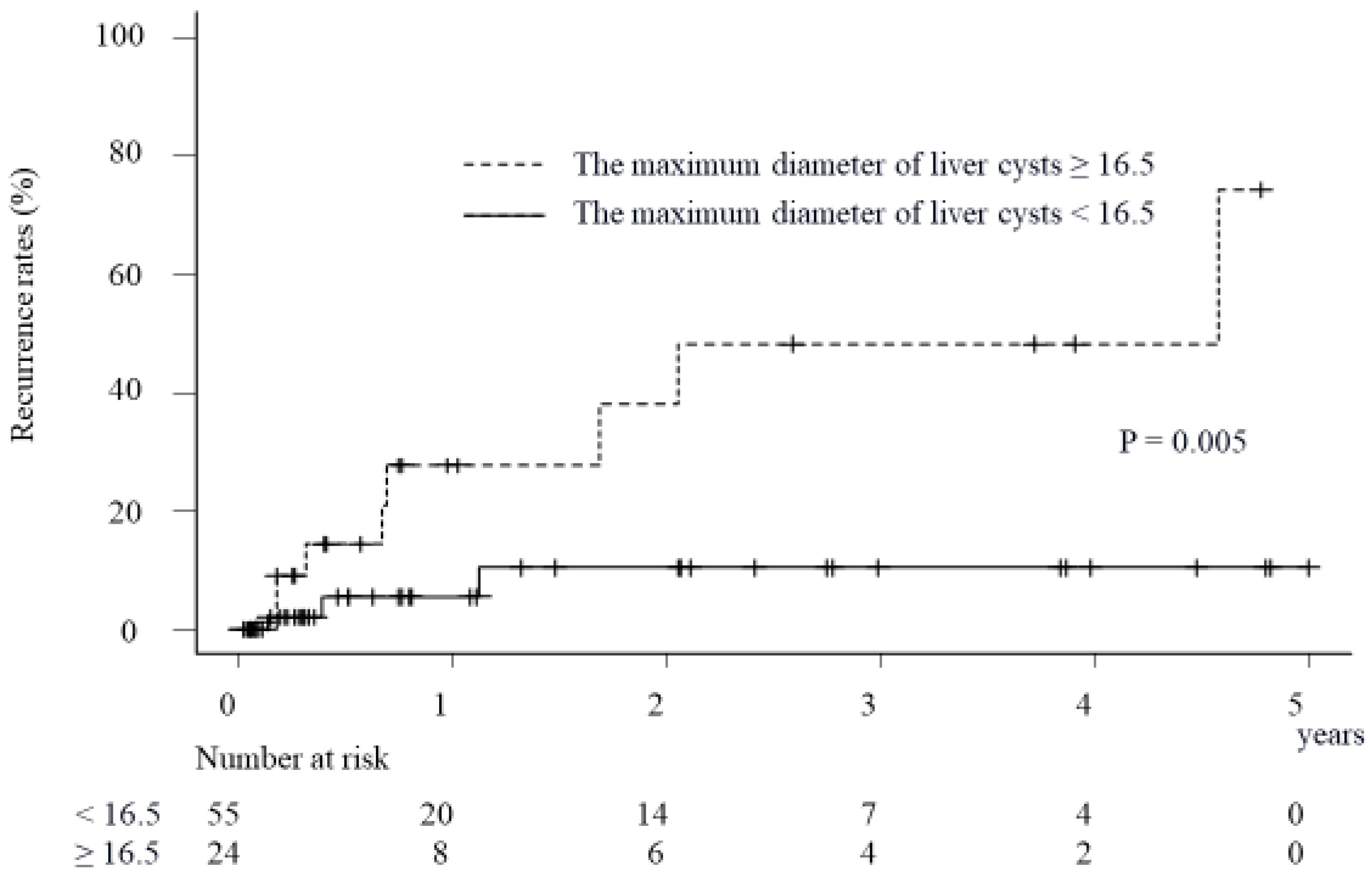
2.4. Impact of Sclerosing Agents on Cumulative Recurrence Rates of Symptoms Due to Liver Cysts in Patients with a Maximum Liver Cyst Diameter of <16.5 cm
2.5. Impact of Sclerosing Agents on Cumulative Recurrence Rates of Symptoms Due to Liver Cysts in the Patients with a Maximum Liver Cyst Diameter of ≥16.5 cm
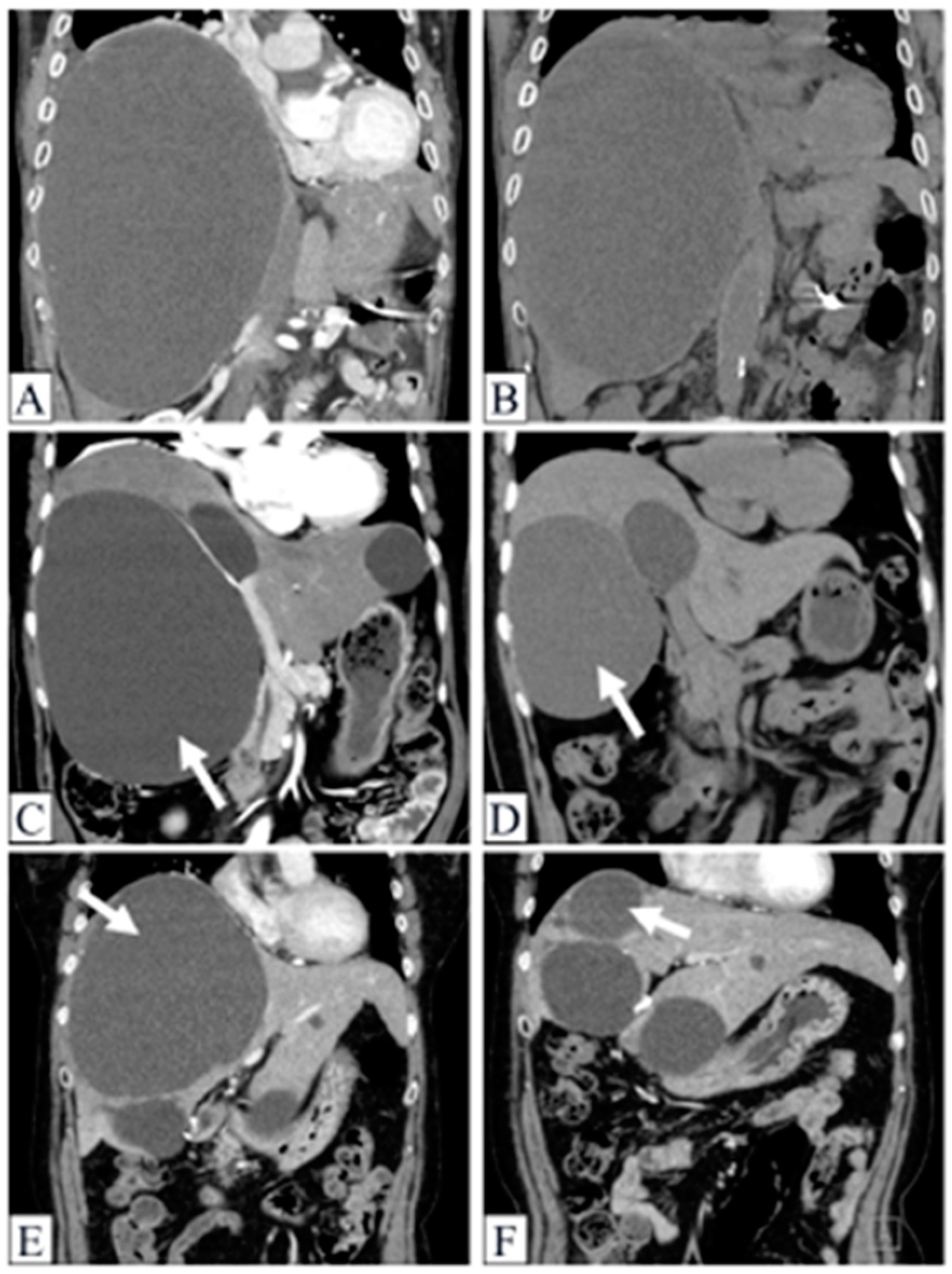
2.6. Demonstrable Cases
2.6.1. Case 1
2.6.2. Case 2
2.6.3. Case 3
2.7. Adverse Events and the Morbid Cases in the Present Study
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Demographics, Laboratory Data and Study Endpoints
4.3. Methods of Administering Each Sclerosing Agent to Liver Cysts
4.4. Ethical Considerations
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrim, Z.I.; Murchison, J.T. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin. Radiol. 2003, 58, 626–629. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J. Hepatol. 2016, 65, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Lantinga, M.A.; Gevers, T.J.; Drenth, J.P. Evaluation of hepatic cystic lesions. World J. Gastroenterol. 2013, 19, 3543–3554. [Google Scholar] [CrossRef] [PubMed]
- Vardakostas, D.; Damaskos, C.; Garmpis, N.; Antoniou, E.A.; Kontzoglou, K.; Kouraklis, G.; Dimitroulis, D. Minimally invasive management of hepatic cysts: Indications and complications. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1387–1396. [Google Scholar] [PubMed]
- Läuffer, J.M.; Baer, H.U.; Maurer, C.A.; Stoupis, C.; Zimmerman, A.; Büchler, M.W. Biliary cystadenocarcinoma of the liver: The need for complete resection. Eur. J. Cancer 1998, 34, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Nguyen, M.H. The diagnosis and management of benign hepatic tumors. J. Clin. Gastroenterol. 2005, 39, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.L.; Mountain, J.C.; Warren, K.W. Symptomatic non-parasitic cysts of the liver. Br. J. Surg. 1974, 61, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Furumaya, A.; van Rosmalen, B.V.; de Graeff, J.J.; Haring, M.P.D.; de Meijer, V.E.; van Gulik, T.M.; Verheij, J.; Besselink, M.G.; van Delden, O.M.; Erdmann, J.I.; et al. Systematic review on percutaneous aspiration and sclerotherapy versus surgery in symptomatic simple hepatic cysts. HPB 2021, 23, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, T.F.; Lantinga, M.A.; Drenth, J.P. Hepatic cyst infection following aspiration sclerotherapy: A case series. J. Gastrointestin. Liver Dis. 2014, 23, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, D.; van Delden, O.M.; Rauws, E.A.; Busch, O.R.; Lameris, J.S.; Gouma, D.J.; van Gulik, T.M. Results of percutaneous sclerotherapy and surgical treatment in patients with symptomatic simple liver cysts and polycystic liver disease. World J. Gastroenterol. 2007, 13, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Mueller, P.R.; Ferrucci, J.T., Jr.; Simeone, J.F.; Wittenberg, J.; Butch, R.J. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. AJR Am. J. Roentgenol. 1983, 141, 559–560. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of cystic liver diseases. J. Hepatol. 2022, 77, 1083–1108. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, R.; Das, K.; Kudo, M.; Chung, H.; Innoue, T. Percutaneous aspiration and ethanolamine oleate sclerotherapy for sustained resolution of symptomatic polycystic liver disease: An initial experience. AJR Am. J. Roentgenol. 2009, 193, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sakaguchi, H.; Anai, H.; Tanaka, T.; Morimoto, K.; Kichikawa, K.; Uchida, H. Sclerotherapy for simple cysts with use of ethanolamine oleate: Preliminary experience. Cardiovasc. Interv. Radiol. 2005, 28, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, M.; Tokunaga, S.; Orito, M.; Shimamura, M.; Hirano, S.; Okasho, A.; Kosaka, S. Percutaneous injection sclerotherapy with minocycline hydrochloride for simple renal cysts. Int. Urol. Nephrol. 1993, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Danza, F.M.; Falcione, M.; Bordonaro, V.; Infante, A.; Paladini, A.; Bonomo, L. Minocycline hydrochloride as a soft sclerotizing agent for symptomatic simple renal and hepatic cysts. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 408–415. [Google Scholar] [PubMed]
- Skolarikos, A.; Laguna, M.P.; de la Rosette, J.J. Conservative and radiological management of simple renal cysts: A comprehensive review. BJU Int. 2012, 110, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, K.; Mihssin, N.; Houghton, P.W. The management of simple hepatic cysts: Sclerotherapy or laparoscopic fenestration. Ann. R. Coll. Surg. Engl. 2001, 83, 409–414. [Google Scholar]

| All Cases (n = 79) | Drainage Alone (n = 24) | Drainage with Sclerotherapy (n = 55) | p | |
|---|---|---|---|---|
| Age (years) | 70 (64–80) | 75 (67–83) | 69 (62–79) | 0.049 |
| Male, n (%) | 20 (25.3) | 11 (45.8) | 9 (16.4) | 0.01 |
| Complication with renal cysts, n (%) | 34 (43.0) | 12 (50) | 22 (40) | 0.46 |
| Number of liver cysts, n (%) | ||||
| <5 | 32 (40.5) | 8 (33.3) | 24 (43.6) | 0.58 |
| 5–10 | 21 (26.6) | 6 (25) | 15 (27.3) | |
| >10 | 26 (32.9) | 10 (41.7) | 16 (29.1) | |
| Maximum diameter of liver cysts (cm) | 13.1 (10.0–17.8) | 12.6 (8.8–18.1) | 13.1 (10.3–16.9) | 0.61 |
| Symptoms, n (%) | ||||
| Poor nutrition status with BMI < 18.5 kg/m2 | 3 (3.8) | 0 (0) | 3 (5.5) | 0.55 |
| Appetite loss | 16 (20.2) | 6 (25) | 10 (18.2) | 0.55 |
| Abdominal distension | 35 (44.3) | 8 (33.3) | 27 (49.1) | 0.23 |
| Ascites | 8 (10.1) | 4 (16.7) | 4 (7.3) | 0.24 |
| Infection of liver cysts | 23 (29.1) | 15 (62.5) | 8 (14.5) | <0.001 |
| ECOG PS, n (%) | ||||
| 0/1/2/3/4 | 61 (77.2)/9 (11.4)/ 5 (6.3)/1 (1.3)/3 (3.8) | 15 (62.5)/4 (16.7)/2 (8.3)/ 1 (4.2)/2 (8.3) | 46 (83.6)/5 (9.1)/3 (5.5)/ 0 (0)/1 (1.8) | 0.13 |
| Platelet count (×109/L) | 207 (166–275) | 243 (171–298) | 204 (166–242) | 0.12 |
| PT (%) | 91.4 (78.3–101.0) | 83.9 (62.6–94.0) | 93.0 (82.0–101.0) | 0.021 |
| Albumin (g/dL) | 3.8 (3.2–4.2) | 3.4 (2.3–3.9) | 3.9 (3.5–4.3) | 0.002 |
| T-Bil (mg/dL) | 0.8 (0.7–1.2) | 0.9 (0.7–1.5) | 0.8 (0.7–1.1) | 0.33 |
| ALT (IU/L) | 20 (15–37) | 35 (20–64) | 18 (12–23) | 0.002 |
| Creatinine (mg/dL) | 0.7 (0.6–0.8) | 0.7 (0.5–1.0) | 0.7 (0.6–0.8) | 0.79 |
| Minocycline Hydrochloride (n = 29) | Ethanolamine Oleate (n = 15) | Absolute Ethanol (n = 11) | p | |
|---|---|---|---|---|
| Age (years) | 70 (65–80) | 64 (60–73) | 69 (63–80) | 0.32 |
| Male, n (%) | 4 (13.8) | 1 (6.7) | 4 (36.4) | 0.14 |
| Complication with renal cysts, n (%) | 10 (34.5) | 8 (53.3) | 4 (36.4) | 0.47 |
| Number of liver cysts, n (%) | ||||
| <5 | 13 (44.8) | 5 (33.3) | 6 (54.5) | 0.83 |
| 5–10 | 7 (24.1) | 5 (33.3) | 3 (27.3) | |
| >10 | 9 (31.0) | 5 (33.3) | 2 (18.2) | |
| Maximum diameter of liver cysts (cm) | 13.1 (11.6–16.5) | 13.8 (10.8–19.2) | 12.0 (10.0–18.0) | 0.9 |
| Symptoms, n (%) | ||||
| Poor nutrition status with BMI < 18.5 kg/m2 | 2 (6.9) | 1 (6.7) | 0 (0) | 1 |
| Appetite loss | 5 (17.2) | 2 (13.3) | 3 (27.3) | 0.72 |
| Abdominal distension | 14 (48.3) | 8 (53.3) | 5 (45.5) | 1 |
| Ascites | 3 (10.3) | 1 (6.7) | 0 (0) | 0.8 |
| Infection of liver cysts | 6 (20.7) | 0 (0) | 2 (18.2) | 0.15 |
| ECOG PS, n (%) | ||||
| 0/1/2/3/4 | 24 (82.8)/2 (6.9)/ 2 (6.9)/0 (0)/1 (3.4) | 13 (86.7)/2 (13.3)/ 0 (0)/0 (0)/0 (0) | 9 (81.8)/1 (9.1)/ 1 (9.1)/0 (0)/0 (0) | 0.92 |
| Platelet count (×109/L) | 204 (166–229) | 196 (181–263) | 208 (158–238) | 0.91 |
| PT (%) | 95.5 (78.7–107.5) | 93 (87.7–100.8) | 85.6 (80.5–99.4) | 0.72 |
| Albumin (g/dL) | 4.0 (3.7–4.4) | 3.9 (3.5–4.2) | 3.7 (3.2–4.1) | 0.14 |
| T-Bil (mg/dL) | 0.8 (0.7–1.0) | 0.7 (0.6–0.9) | 1.1 (0.8–1.4) | 0.1 |
| ALT (IU/L) | 17 (12–21) | 18 (15–22) | 22 (14–43) | 0.29 |
| Creatinine (mg/dL) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.9) | 0.9 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Recurrence (−) (n = 68) | Recurrence (+) (n = 11) | p | Odds Ratio (95% Confidence Interval) | p | |
| Age (years) | 70 (64–79) | 80 (66–86) | 0.3 | ||
| Male, n (%) | 16 (23.5) | 4 (36.4) | 0.46 | ||
| Complication with renal cysts, n (%) | 30 (44.1) | 4 (36.4) | 0.75 | ||
| Number of liver cysts, n (%) | |||||
| <5 | 26 (38.2) | 6 (54.5) | 0.42 | ||
| 5–10 | 20 (29.4) | 1 (9.1) | |||
| >10 | 22 (32.4) | 4 (36.4) | |||
| Maximum diameter of liver cysts (cm) | 12.0 (9.8–16.0) | 19.7 (15.0–21.5) | 0.004 | 1.21 (1.06–1.38) | 0.005 |
| Symptoms, n (%) | |||||
| Poor nutrition status with BMI < 18.5 kg/m2 | 3 (4.4) | 0 (0) | 1 | ||
| Appetite loss | 13 (19.1) | 3 (27.3) | 0.69 | ||
| Abdominal distension | 29 (42.6) | 6 (54.5) | 0.52 | ||
| Ascites | 8 (11.8) | 0 (0) | 0.59 | ||
| Infection of liver cysts | 20 (29.4) | 3 (27.3) | 1 | ||
| ECOG PS | 0 (0–0) | 0 (0–1) | 0.29 | ||
| Treatment methods | |||||
| Drainage alone | 21 (30.9) | 3 (27.3) | 0.57 | ||
| Drainage and injection of absolute ethanol | 8 (11.8) | 3 (27.3) | |||
| Drainage and injection of 5% ethanolamine oleate | 14 (20.6) | 1 (9.1) | |||
| Drainage and injection of minocycline hydrochloride | 25 (36.8) | 4 (36.4) | |||
| Platelet count (×109/L) | 208 (173–280) | 146 (134–234) | 0.099 | ||
| PT (%) | 92.0 (81.0–101.0) | 85.6 (64.8–92.3) | 0.16 | ||
| Albumin (g/dL) | 3.9 (3.4–4.2) | 3.0 (2.6–3.9) | 0.12 | ||
| T-Bil (mg/dL) | 0.8 (0.6–1.2) | 1.0 (0.8–1.6) | 0.11 | ||
| ALT (IU/L) | 19 (15–35) | 23 (14–54) | 0.44 | ||
| Creatinine (mg/dL) | 0.7 (0.6–0.8) | 0.7 (0.5–0.9) | 0.42 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakusagi, S.; Kakizaki, S.; Saito, N.; Kohga, T.; Ueno, T.; Hatanaka, T.; Namikawa, M.; Tojima, H.; Naganuma, A.; Kosone, T.; et al. The Treatment Effects of Percutaneous Drainage with or without Sclerotherapy for Symptomatic Liver Cysts. Gastrointest. Disord. 2024, 6, 13-25. https://doi.org/10.3390/gidisord6010002
Takakusagi S, Kakizaki S, Saito N, Kohga T, Ueno T, Hatanaka T, Namikawa M, Tojima H, Naganuma A, Kosone T, et al. The Treatment Effects of Percutaneous Drainage with or without Sclerotherapy for Symptomatic Liver Cysts. Gastrointestinal Disorders. 2024; 6(1):13-25. https://doi.org/10.3390/gidisord6010002
Chicago/Turabian StyleTakakusagi, Satoshi, Satoru Kakizaki, Naoto Saito, Tatsuya Kohga, Takashi Ueno, Takeshi Hatanaka, Masashi Namikawa, Hiroki Tojima, Atsushi Naganuma, Takashi Kosone, and et al. 2024. "The Treatment Effects of Percutaneous Drainage with or without Sclerotherapy for Symptomatic Liver Cysts" Gastrointestinal Disorders 6, no. 1: 13-25. https://doi.org/10.3390/gidisord6010002
APA StyleTakakusagi, S., Kakizaki, S., Saito, N., Kohga, T., Ueno, T., Hatanaka, T., Namikawa, M., Tojima, H., Naganuma, A., Kosone, T., Uraoka, T., & Takagi, H. (2024). The Treatment Effects of Percutaneous Drainage with or without Sclerotherapy for Symptomatic Liver Cysts. Gastrointestinal Disorders, 6(1), 13-25. https://doi.org/10.3390/gidisord6010002







