Ileocolic Anastomosis Dehiscence in Colorectal Cancer Surgery
Abstract
1. Introduction
2. Results
2.1. Baseline Clinical and Preoperative Data
2.2. Surgical Procedure
2.3. Postoperative Findings
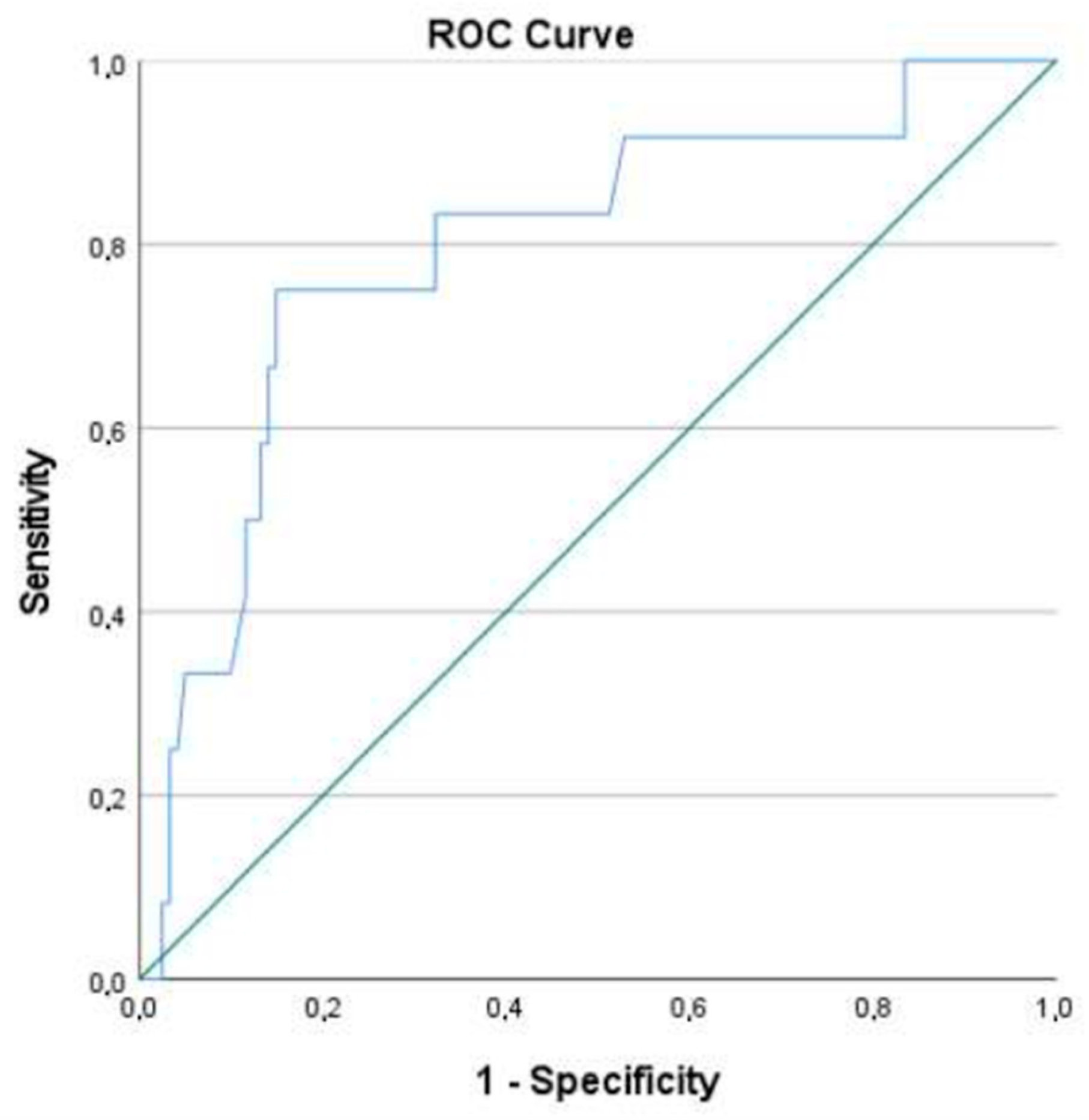
2.4. Association with AL
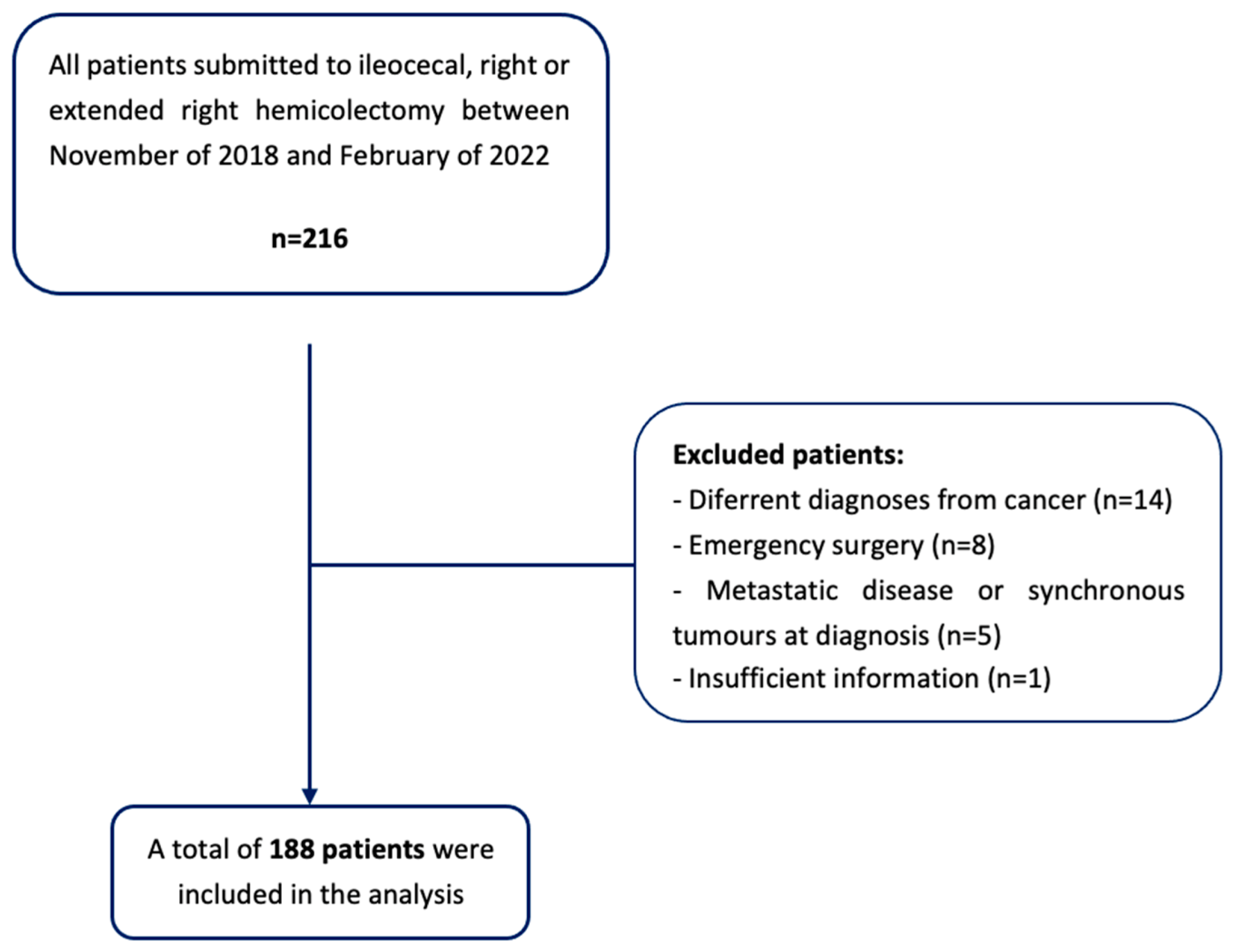
3. Materials and Methods
- Patients undergoing to emergency surgery or surgical re-intervention;
- Patients with a non-cancer diagnosis (i.e., inflammatory bowel disease or diverticular disease);
- The presence of metastatic disease at diagnosis, or synchronous tumors in other locations;
- The presence of a diverting stoma or a tumor resection surgery, with no ileocolic anastomosis;
- Insufficient information in the clinical process to determine the variables used in the study.
3.1. Data Collection
3.2. Definitions
- Grade A: Corresponds to the definition of a ‘‘radiologic leakage’’, used by several authors. This grade of AL is not associated with clinical symptoms or abnormal laboratory tests, and no active therapeutic intervention is required.
- Grade B: Patient’s clinical condition requires an active therapeutic intervention, such as the administration of antibiotics and/or radiologically guided drainage, that can be managed without operative reintervention.
- Grade C: Patients are often quite ill and require operative re-laparotomy.
3.3. Statistical Analysis
3.4. Ethical Considerations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salibasic, M.; Pusina, S.; Bicakcic, E.; Pasic, A.; Gavric, I.; Kulovic, E.; Rovcanin, A.; Beslija, S. Colorectal Cancer Surgical Treatment, our Experience. Med. Arch. 2019, 73, 412–414. [Google Scholar] [CrossRef]
- Rentsch, M.; Schiergens, T.; Khandoga, A.; Werner, J. Surgery for Colorectal Cancer-Trends, Developments, and Future Perspectives. Visc. Med. 2016, 32, 184–1891. [Google Scholar] [CrossRef]
- Golub, R.W.; Cantu, R.; Stein, H.D. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J. Am. Coll. Surg. 1997, 184, 364–372. [Google Scholar]
- Alves, A.; Panis, Y.; Trancart, D.; Regimbeau, J.M.; Pocard, M.; Valleur, P. Factors associated with clinically significant anastomotic leakage after large bowel resection: Multivariate analysis of 707 patients. World J. Surg. 2002, 26, 499–502. [Google Scholar]
- Branagan, G.; Finnis, D. Prognosis After Anastomotic Leakage in Colorectal Surgery. Dis. Colon Rectum 2005, 48, 1021–1026. [Google Scholar] [CrossRef]
- Kube, R.; Mroczkowski, P.; Granowski, D.; Benedix, F.; Sahm, M.; Schmidt, U.; Gastinger, I.; Lippert, H. Anastomotic leakage after colon cancer surgery: A predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur. J. Surg. Oncol. EJSO 2010, 36, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.C.; Gervaz, P.; Secic, M.; Bucher, P.; Mugnier-Konrad, B.; Morel, P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: A prospective monocentric study. Int. J. Color. Dis. 2007, 23, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Golda, T.; Lazzara, C.; Zerpa, C.; Sobrino, L.; Fico, V.; Kreisler, E.; Biondo, S. Risk factors for ileocolic anastomosis dehiscence; a cohort study. Am. J. Surg. 2019, 220, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.T.; Jørgensen, T.; Kirkeby, L.T.; Skovdal, J.; Vennits, B.; Wille-Jørgensen, P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br. J. Surg. 1999, 86, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K.G.M. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef]
- Frasson, M.; ANACO Study Group; Granero-Castro, P.; Rodríguez, J.L.R.; Flor-Lorente, B.; Braithwaite, M.; Martínez, E.M.; Pérez, J.A.Á.; Cazador, A.C.; Espí, A. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: Results from a prospective, multicentric study of 1102 patients. Eur. Soc. Coloproctol. Meet. 2014, 31, 105–114. [Google Scholar] [CrossRef]
- Choy, P.Y.G.; Bissett, I.P.; Docherty, J.G.; Parry, B.R.; Merrie, A.; Fitzgerald, A. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst. Rev. 2011, 9, CD004320. [Google Scholar] [CrossRef]
- European Society of Coloproctology Collaborating Group; Battersby, N.; Bhangu, A.; Chaudhri, S.; El-Hussuna, A.; Frasson, M.; Nepogodiev, D.; Singh, B.; Vennix, S.; Zmora, O.; et al. Relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: An international snapshot audit. Color. Dis. 2017, 19, e296–e311. [Google Scholar] [CrossRef]
- Jessen, M.; Nerstrøm, M.; Wilbek, T.E.; Roepstorff, S.; Rasmussen, M.S.; Krarup, P.-M. Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Color. Dis. 2016, 31, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Jestin, P.; Gunnarsson, U.; Lindforss, U. Higher Frequency of Anastomotic Leakage with Stapled Compared to Hand-Sewn Ileocolic Anastomosis in a Large Population-Based Study. World J. Surg. 2015, 39, 1834–1839. [Google Scholar] [CrossRef]
- Jurowich, C.; Lichthardt, S.; Matthes, N.; Kastner, C.; Haubitz, I.; Prock, A.; Filser, J.; Germer, C.-T.; Wiegering, A. Effects of anastomotic technique on early postoperative outcome in open right-sided hemicolectomy. BJS Open 2018, 3, 203–209. [Google Scholar] [CrossRef]
- Sánchez-Guillén, L.; Frasson, M.; García-Granero, A.; Pellino, G.; Flor-Lorente, B.; Álvarez-Sarrado, E. Risk factors for leak, complications and mortality after ileocolic anastomosis: Comparison of two anastomotic techniques. Ann. R. Coll. Surg. Engl. 2019, 101, 571–578. [Google Scholar] [CrossRef]
- Kwak, H.D.; Kim, S.-H.; Kang, D.W.; Baek, S.-J.; Kwak, J.M.; Kim, J. Risk Factors and Oncologic Outcomes of Anastomosis Leakage After Laparoscopic Right Colectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Ellis, C.T.; Maykel, J.A.; Surgery, C.; Polk, H.C. Defining Anastomotic Leak and the Clinical Relevance of Leaks Definition of Large Bowel Anastomotic Leak Definition. Clin. Colon Rectal Surg. 2021, 34, 359–365. [Google Scholar] [PubMed]
- Kulu, Y.; Ulrich, A.; Bruckner, T.; Contin, P.; Welsch, T.; Rahbari, N.N.; Büchler, M.W.; Weitz, J. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 2013, 153, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; Graf, R.; Cameron, J.; Padbury, R.; Masatoshi, M.D.; et al. The Clavien- Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; Sage: London, UK, 2017. [Google Scholar]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Bracale, U.; Melillo, P.; Lazzara, F.; Andreuccetti, J.; Stabilini, C.; Corcione, F.; Pignata, G. Single-Access Laparoscopic Rectal Resection Versus the Multiport Technique. Surg. Innov. 2014, 22, 46–53. [Google Scholar] [CrossRef]
- Sciuto, A.; Merola, G.; De Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef]
- Dubose, A.C.; Kuy, S. A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer. In 50 Studies Every Surgeon Should Know; Oxford Medicine Online: Oxford, UK, 2017. [Google Scholar]
- Lacy, A.M.; Delgado, S.; García-Valdecasas, J.C.; Castells, A.; Piqué, J.M.; Grande, L.; Fuster, J.; Targarona, E.M.; Pera, M.; Visa, J. Port site metastases and recurrence after laparoscopic colectomy. Surg. Endosc. 1998, 12, 1039–1042. [Google Scholar] [CrossRef]
- Arezzo, A.; Passera, R.; Scozzari, G.; Verra, M.; Morino, M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: Results of a systematic review and meta-analysis. Surg. Endosc. 2012, 27, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Young, J.M.; Solomon, M. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br. J. Surg. 2004, 91, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Mungo, B.; Papageorge, C.M.; Stem, M.; Molena, D.; Lidor, A.O. The Impact of Operative Approach on Postoperative Complications Following Colectomy for Colon Cancer. World J. Surg. 2017, 41, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Elmore, U.; Allaix, M.E.; Bianchi, P.P.; Biondi, A.; Boni, L.; Bracale, U.; Cassinotti, E.; Ceccarelli, G.; Corcione, F.; et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: A multicenter experience. Surg. Endosc. 2019, 34, 557–563. [Google Scholar] [CrossRef]
- Carnuccio, P.; Jimeno, J.; Parés, D. Laparoscopic right colectomy: A systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech. Coloproctol. 2013, 18, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Nors, J.; Sommer, T.; Wara, P. Leakage Rate After Laparoscopic Ileocolic Intracorporeal Anastomosis. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, V.N.N.; Hagendoorn, J.; Van Koeverden, S.; Van Ramshorst, B.; Smits, A.B. Totally Laparoscopic Right Hemicolectomy with Intracorporeal Anastomosis is a Technically and Oncologically Safe Procedure. Acta Chir. Belg. 2013, 113, 439–443. [Google Scholar] [CrossRef]
- Biondi, A.; Santocchi, P.; Pennestrì, F.; Santullo, F.; D’ugo, D.; Persiani, R. Totally laparoscopic right colectomy versus laparoscopically assisted right colectomy: A propensity score analysis. Surg. Endosc. 2017, 31, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Magistro, C.; Di Lernia, S.; Ferrari, G.; Zullino, A.; Mazzola, M.; De Martini, P.; De Carli, S.; Forgione, A.; Bertoglio, C.L.; Pugliese, R. Totally laparoscopic versus laparoscopic-assisted right colectomy for colon cancer: Is there any advantage in short-term outcomes? A prospective comparative assessment in our center. Surg. Endosc. 2013, 27, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, N.; Abrisqueta, J.; Luján, J.; Hernández, Q.; Rufete, M.D.; Parrilla, P. Isoperistaltic versus antiperistaltic ileocolic anastomosis. Does it really matter? Results from a randomised clinical trial (ISOVANTI). Surg. Endosc. 2018, 33, 2850–2857. [Google Scholar] [CrossRef]
- 2015 European Society of Coloproctology Collaborating Group; Glasbey, J.C.; Nepogodiev, D.; Battersby, N.; Bhangu, A.; El-Hussuna, A.; Frasson, M.; Singh, B.; Vennix, S.; Zmora, O.; et al. The impact of stapling technique and surgeon specialism on anastomotic failure after right- sided colorectal resection: An international multicentre, prospective audit. Color. Dis. 2018, 20, 1028–1040. [Google Scholar]
- Fleetwood, V.A.; Gross, K.; Alex, G.C.; Cortina, C.S.; Smolevitz, J.B.; Sarvepalli, S.; Bakhsh, S.R.; Myers, J.A.; Singer, M.A.; Orkin, B.A. Common side closure type but not stapler brand or oversewing influences side-to-side anastomotic leak rates. J. Am. Coll. Surg. 2015, 221, 590–595. [Google Scholar] [CrossRef]
- Vasiliu, E.C.Z.; Zarnescu, N.O.; Costea, R.; Neagu, S. Review of Risk Factors for Anastomotic Leakage in Colorectal Surgery. Chirurgia 2015, 110, 319–326. [Google Scholar]
- Ozben, V.; Stocchi, L.; Ashburn, J.; Liu, X.; Gorgun, E. Impact of a restrictive vs liberal transfusion strategy on anastomotic leakage and infectious complications after restorative surgery for rectal cancer. Color. Dis. 2017, 19, 772–780. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, G.-S.; Kim, S.H.; Kim, H.R.; Kim, N.K.; Lee, K.Y.; Kang, S.B.; Kim, J.Y.; Lee, K.Y.; Kim, B.C.; et al. Multicenter Analysis of Risk Factors for Anastomotic Leakage After Laparoscopic Rectal Cancer Excision. Ann. Surg. 2013, 257, 665–671. [Google Scholar] [CrossRef]
- Tartter, P.I.; Quintero, S.; Barron, D.M. Perioperative blood transfusion associated with infectious complications after colorectal cancer operations. Am. J. Surg. 1986, 152, 479–482. [Google Scholar] [CrossRef]
- Tang, R.; Chen, H.H.; Wang, Y.L.; Changchien, C.R.; Chen, J.-S.; Hsu, K.-C.; Chiang, J.-M.; Wang, J.-Y. Risk Factors for Surgical Site Infection After Elective Resection of the Colon and Rectum: A Single-Center Prospective Study of 2,809 Consecutive Patients. Ann. Surg. 2001, 234, 181–189. [Google Scholar] [CrossRef]
- Ydy, L.R.A.; Slhessarenko, N.; de Aguilar-Nascimento, J.E. Effect of Perioperative Allogeneic Red Blood Cell Transfusion on the Immune-Inflammatory Response After Colorectal Cancer Resection. World J. Surg. 2007, 31, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.D.; Vanmoorleghem, G.; Menlove, R.L. Blood transfusions and postoperative wound infection. Surgery 1993, 113, 603–607. [Google Scholar] [PubMed]
- Gibbs, J.; Cull, W.; Henderson, W.; Daley, J.; Hur, K.; Khuri, S.F. Preoperative Serum Albumin Level as a Predictor of Operative Mortality and Morbidity Results from the National VA Surgical Risk Study. Arch Surg. 1999, 134, 36–42. [Google Scholar] [CrossRef]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef]
- Kudsk, K.; Tolley, E.; DeWitt, R.; Janu, P.; Blackwell, A.; Yeary, S.; King, B. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. J. Parenter. Enter. Nutr. 2003, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.A.; Terzioğlu, H.; Genç, V.; Erkek, A.B.; Özban, M.; Sonyürek, P.; Elhan, A.H.; Torun, N. Preoperative Nutritional Risk Assessment in Predicting Postoperative Outcome in Patients Undergoing Major Surgery. World J. Surg. 2006, 30, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Lohsiriwat, D.; Boonnuch, W.; Chinswangwatanakul, V.; Akaraviputh, T.; Lert-Akayamanee, N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J. Gastroenterol. 2008, 14, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; You, J.-F.; Yeh, C.-Y.; Chen, J.-S.; Tang, R.; Wang, J.-Y.; Chin, C.-C. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int. J. Color. Dis. 2010, 26, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Irvin, T.T.; Hunt, T.K. Effect of Malnutrition on Colonic Healing. Ann. Surg. 1974, 180, 765–772. [Google Scholar] [CrossRef]
- Gonçalves, C.G.; Groth, A.K.; Ferreira, M.; Matias, J.E.F.; Coelho, J.C.U.; Campos, A.C.L. Influence of Preoperative Feeding on the Healing of Colonic Anastomoses in Malnourished Rats. J. Parenter. Enter. Nutr. 2008, 33, 83–89. [Google Scholar] [CrossRef]
- Ward, M.W.N.; Danzi, M.; Lewin, M.R.; Rennie, M.J.; Clark, C.G. The effects of subclinical malnutrition and refeeding on the healing of experimental colonic anastomoses. Br. J. Surg. 1982, 69, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Toiyama, Y.; Hiro, J.; Imaoka, H.; Fujikawa, H.; Kobayashi, M.; Ohi, M.; Inoue, Y.; Mohri, Y.; Kusunoki, M. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J. Surg. 2018, 41, 30–38. [Google Scholar] [CrossRef] [PubMed]
| [Total] N = 188 | ||
|---|---|---|
| Age (years) (Mdn, IQR) | 73 (17) | |
| Gender (n, %) | Female | 68 (36.2) |
| Male | 120 (63.8) | |
| ASA score (n, %) | I | 5 (2.7) |
| II | 109 (58) | |
| III | 64 (34) | |
| IV | 10 (5.3) | |
| COPD (n, %) | No | 178 (94.7) |
| Yes | 10 (5.3) | |
| DM (n, %) | No | 136 (72.3) |
| Yes | 52 (27.7) | |
| HTN (n, %) | No | 61 (32.4) |
| Yes | 127 (67.6) | |
| Smoking Habits (n, %) | Non Smoker | 163 (86.7) |
| Previous Smoker | 9 (4.8) | |
| Active Smoker | 16 (8.5) | |
| BMI (Mdn, IQR) | 32.5 (72.8) | |
| Obesity (body mass index ≥ 30) (n, %) | No Yes | 125 (77.6) 36 (22.4) |
| NO AL | AL | Statistics Test | p-Value | |
|---|---|---|---|---|
| Gender (n, %) | Fisher’s exact test | >0.990 | ||
| Female | 63 (36.0) | 5 (38.5) | ||
| Male | 112 (64.0) | 8 (61.5) | ||
| Age, years (Mdn, IQR) | 73 (17) | 77 (9) | Mann–Whitney test | 0.107 |
| ASA score (n, %) | Fisher’s exact test | 0.046 | ||
| I–II | 107 (61.1) | 7 (53.8) | ||
| III | 61 (34.9) | 3 (23.1) | ||
| ≥IV | 7 (4.0) | 3 (23.1) | ||
| DM (n, %) | Fisher’s exact test | 0.009 | ||
| No | 131 (74.9) | 5 (38.5) | ||
| Yes | 44 (25.1) | 8 (61.5) | ||
| COPD (n, %) | Fisher’s exact test | 0.520 | ||
| No | 166 (94.9) | 12 (92.3) | ||
| Yes | 9 (5.1) | 1 (7.7) | ||
| Smoking habits (n, %) | Fisher’s exact test | 0.685 | ||
| Non Smoker | 152 (86.9) | 11 (84.6) | ||
| Active Smoker/Previous Smoker | 23 (13.1) | 2 (15.4) | ||
| HTN (n, %) | Fisher’s exact test | 0.553 | ||
| No | 58 (33.1) | 3 (23.1) | ||
| Yes | 117 (66.9) | 10 (76.9) | ||
| Obesity (n, %) | Fisher’s exact test | 0.685 | ||
| No | 117 (77.0) | 8 (88.9) | ||
| Yes | 35 (23.0) | 1 (11.1) | ||
| Ca 19.9 (Mdn, IQR) | 6.80 (14.40) | 5.16 (6.74) | Mann–Whitney test | 0.655 |
| CEA (Mdn, IQR) | 1.70 (2.20) | 2.30 (2.80) | Mann–Whitney test | 0.402 |
| Albumin | 4.20 (0.77) | 4.0 (0.92) | Mann–Whitney test | 0.504 |
| (g/dL) (Mdn, IQR) | ||||
| Total proteins (g/dL) (Mdn, IQR) | 7.1 (0.7) | 7.1 (1.45) | Mann–Whitney test | 0.413 |
| Haemoglobin (g/dL) (Mdn, IQR) | 12.6 (3.5) | 10.9 (2.8) | Mann–Whitney test | 0.066 |
| Glucose (g/dL) (Mdn, IQR) | 105.5 (35) | 117 (31) | Mann–Whitney test | 0.992 |
| Blood transfusion (n, %) | Fisher’s exact test | >0.990 | ||
| No | 159 (90.9) | 12 (92.3) | ||
| Yes | 16 (9.1) | 1 (7.7) |
| NO AL | AL | Statistics Test | p-Value | |
|---|---|---|---|---|
| Operating time (min) (Mdn, IQR) | 140 (60) | 120 (75) | Mann–Whitney test | 0.050 |
| Surgical approach (n, %) | Fisher’s exact test | 0.001 | ||
| Laparoscopic | 151 (86.3) | 6 (46.2) | ||
| Laparotomy/Converted to laparotomy | 24 (13.7) | 7 (53.8) | ||
| Suture Reinforcement (n, %) | Fisher’s exact test | 0.553 | ||
| No | 58 (33.1) | 3 (23.1) | ||
| Yes | 117 (66.9) | 10 (76.9) |
| NO AL | AL | Statistics Test | p-Value | |
|---|---|---|---|---|
| Length of stay (days) (Mdn, IQR) | 4.00 (2) | 11.00 (8) | Mann–Whitney test | <0.001 |
| Blood transfusion (n, %) | Fisher’s exact test | 0.005 | ||
| No | 172 (98.3) | 10 (76.9) | ||
| Yes | 3 (1.7) | 3 (23.1) | ||
| Haemoglobin (g/dL) (M, SD) | 10.38 (1.91) | 9.80 (2.17) | t-test | 0.294 |
| Albumin (g/dL) (M, SD) | 3.34 (0.57) | 2.96 (0.54) | t-test | 0.026 |
| Total proteins (g/dL) (M, SD) | 5.67 (0.57) | 5.51 (0.58) | t-test | 0.455 |
| CRP (Mdn, IQR) | 140.7 (107.8) | 171 (111.30) | Mann–Whitney test | 0.07 |
| Mortality (n, %) | Fisher’s exact test | 0.024 | ||
| No | 168 (96.0) | 10 (76.9) | ||
| Yes | 7 (4.0) | 3 (23.1) |
| Multivariate | |||||
|---|---|---|---|---|---|
| B | S.E | p-Value | OR | IC 95% | |
| Operating time (min) (Mdn, AIQ) | −0.015 | 0.007 | 0.038 | 0.985 | 0.972; 0.999 |
| Postop albumin (g/L) (M, SD) | −1.268 | 0.537 | 0.018 | 0.281 | 0.098; 0.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.L.; Santos, P.M.D.d.; Costa Pereira, J.; Martins, S.F. Ileocolic Anastomosis Dehiscence in Colorectal Cancer Surgery. Gastrointest. Disord. 2023, 5, 273-286. https://doi.org/10.3390/gidisord5020022
Gomes SL, Santos PMDd, Costa Pereira J, Martins SF. Ileocolic Anastomosis Dehiscence in Colorectal Cancer Surgery. Gastrointestinal Disorders. 2023; 5(2):273-286. https://doi.org/10.3390/gidisord5020022
Chicago/Turabian StyleGomes, Sara Lima, Pedro Miguel Dias dos Santos, Joaquim Costa Pereira, and Sandra F. Martins. 2023. "Ileocolic Anastomosis Dehiscence in Colorectal Cancer Surgery" Gastrointestinal Disorders 5, no. 2: 273-286. https://doi.org/10.3390/gidisord5020022
APA StyleGomes, S. L., Santos, P. M. D. d., Costa Pereira, J., & Martins, S. F. (2023). Ileocolic Anastomosis Dehiscence in Colorectal Cancer Surgery. Gastrointestinal Disorders, 5(2), 273-286. https://doi.org/10.3390/gidisord5020022






