Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children
Abstract
1. Introduction
2. Results
2.1. Demographics and Health Information
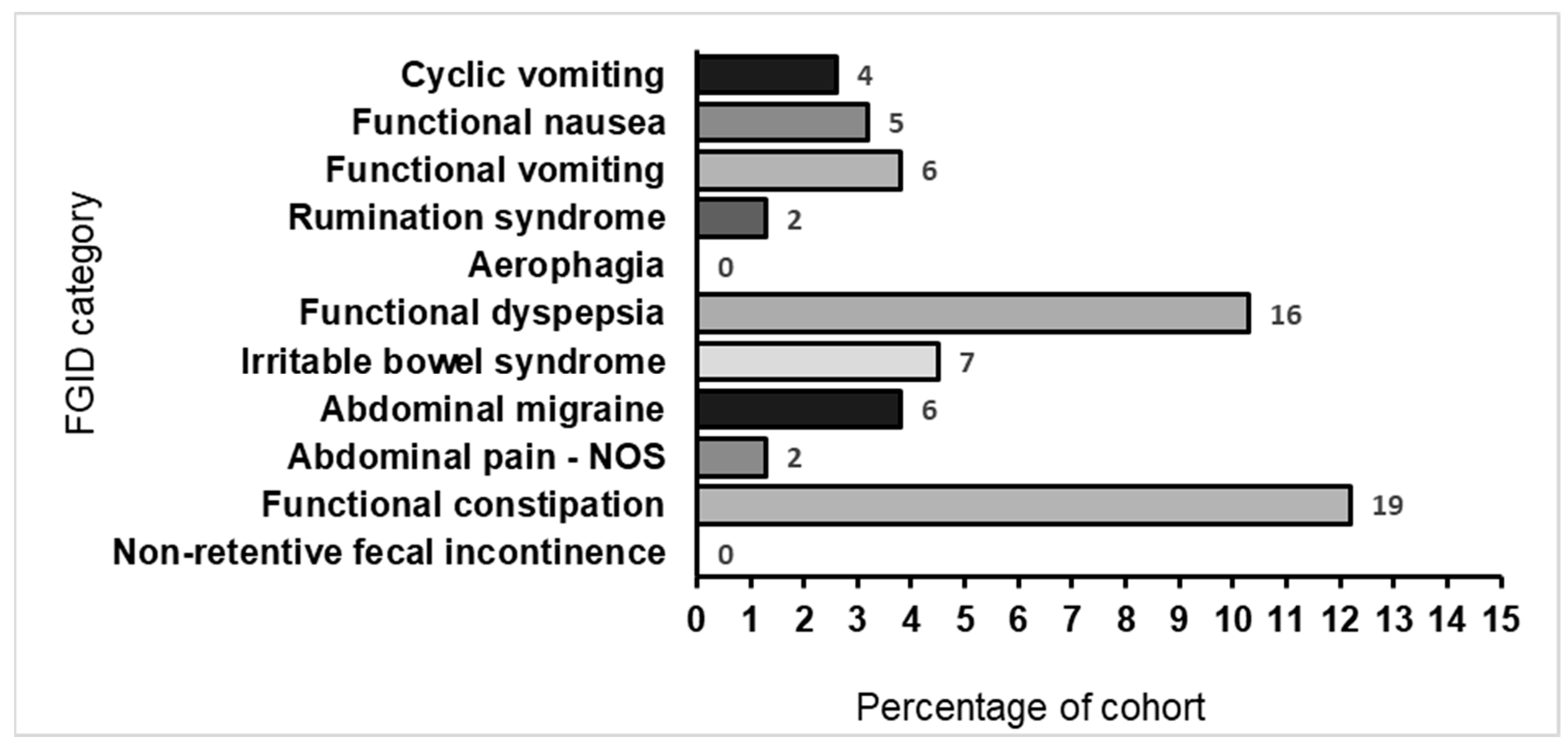
2.2. FGID Prevalence, Categories, and Disorders
2.3. Association of FGID with Independent Variables
2.4. Quality of Life
2.5. Overall Health
3. Discussion
3.1. Strengths
3.2. Limitations
3.3. Conclusions
4. Materials and Methods
4.1. Ethics and Consent
4.2. Population
4.3. Outcome Measures
4.3.1. Demographic Information
4.3.2. FGID Assessment
4.3.3. Quality of Life
4.4. Statistics
4.4.1. Sample Size
4.4.2. Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional Disorders: Children and Adolescents. Gastroenterology 2016, 150, 1456. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.P.; Sandy, N.S.; Alvarenga, L.R.; Lomazi, E.A.; Bellomo-Brandão, M.A. Functional abdominal pain is the main etiology among children referred to tertiary care level for chronic abdominal pain. Arq. Gastroenterol. 2022, 59, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Seshadri, R.; Sztainberg, M.; Schaffer, G.; Marshall, B.M.; Di Lorenzo, C. A Prospective School-based Study of Abdominal Pain and Other Common Somatic Complaints in Children. J. Pediatr. 2009, 154, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Saps, A.M.; Velasco-Benitez, J.J.C.; Blom, A.P.; Benninga, X.M.; Nichols-Vinueza, X.D. Prospective Study of Gastrointestinal Symptoms in School Children of South America. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 391–394. [Google Scholar] [CrossRef]
- Jansen, J.; Shulman, R.; Ward, T.M.; Levy, R.; Self, M.M. Sleep disturbances in children with functional gastrointestinal disorders: Demographic and clinical characteristics. J. Clin. Sleep Med. 2021, 17, 1193–1200. [Google Scholar] [CrossRef]
- Lewis, M.L.; Palsson, O.S.; Whitehead, W.E.; van Tilburg, M.A.L. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. J. Pediatr. 2016, 177, 39–43.e3. [Google Scholar] [CrossRef]
- Robin, S.G.; Keller, C.; Zwiener, R.; Hyman, P.E.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Shulman, R.J.; Hyams, J.S.; Palsson, O.; et al. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria.(Report). J. Pediatr. 2018, 195, 134. [Google Scholar] [CrossRef]
- Varni, J.W.; Bendo, C.B.; Denham, J.; Shulman, R.J.; Self, M.M.; Neigut, D.A.; Nurko, S.; Patel, A.S.; Franciosi, J.P.; Saps, M.; et al. PedsQL™ Gastrointestinal Symptoms Scales and Gastrointestinal Worry Scales in pediatric patients with functional and organic gastrointestinal diseases in comparison to healthy controls. Qual. Life Res. 2015, 24, 363–378. [Google Scholar] [CrossRef]
- Varni, J.W.; Bendo, C.; Nurko, S.; Shulman, R.J.; Self, M.M.; Franciosi, J.P.; Saps, M.; Pohl, J.F. Health-Related Quality of Life in Pediatric Patients with Functional and Organic Gastrointestinal Diseases. J. Pediatr. 2015, 166, 85–90.e2. [Google Scholar] [CrossRef]
- Devanarayana, N.M.; Rajindrajith, S.; Benninga, M.A. Quality of life and health care consultation in 13 to 18 year olds with abdominal pain predominant functional gastrointestinal diseases. BMC Gastroenterol. 2014, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Warschburger, P.; Hänig, J.; Friedt, M.; Posovszky, C.; Schier, M.; Calvano, C. Health-Related Quality of Life in Children With Abdominal Pain Due to Functional or Organic Gastrointestinal Disorders. J. Pediatr. Psychol. 2014, 39, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Mikami, S.; LeClair, J.; Bollom, A.; Lembo, C.; Sethi, S.; Lembo, A.; Jones, M.; Cheng, V.; Friedlander, E.; et al. Inpatient burden of childhood functional GI disorders in the USA: An analysis of national trends in the USA from 1997 to 2009. Neurogastroent. Motil. 2015, 27, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Livitz, M.; Friesen, A.S.; Glynn, E.F.; Schurman, J.V.; Colombo, J.M.; Friesen, C.A. Healthcare System-to-System Cost Variability in the Care of Pediatric Abdominal Pain-Associated Functional Gastrointestinal Disorders. Children 2021, 8, 985. [Google Scholar] [CrossRef]
- Jarrett, M.; Heitkemper, M.; Czyzewski, D.I.; Shulman, R. Recurrent Abdominal Pain in Children: Forerunner to Adult Irritable Bowel Syndrome? J. Spec. Pediatr. Nurs. 2003, 8, 81–89. [Google Scholar] [CrossRef]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef]
- Koppen, I.J.N.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Benninga, M.A. The pediatric Rome IV criteria: What’s new? Expert. Rev. Gastroent. 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Caplan, A.; Walker, L.; Rasquin, A. Development and preliminary validation of the questionnaire on pediatric gastrointestinal symptoms to assess functional gastrointestinal disorders in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 296. [Google Scholar] [CrossRef]
- Vernon-Roberts, A.; Alexander, I.; Day, A.S. Systematic Review of Pediatric Functional Gastrointestinal Disorders (Rome IV Criteria). J. Clin. Med. 2021, 10, 5087. [Google Scholar] [CrossRef]
- Korterink, J.J.; Ockeloen, L.E.; Hilbink, M.; Benninga, M.A.; Deckers-Kocken, J.M. Yoga Therapy for Abdominal Pain-Related Functional Gastrointestinal Disorders in Children: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 481–487. [Google Scholar] [CrossRef]
- Thomassen, R.; Luque, V.; Assa, A.; Borrelli, O.; Broekaert, I.; Dolinsek, J.; Martin-De-Carpi, J.; Mas, E.; Miele, E.; Norsa, L.; et al. An ESPGHAN Position Paper on the Use of Low-FODMAP Diet in Pediatric Gastroenterology. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Barberi, S.; Borrelli, O.; Castellazzi, A.; Di Mauro, D.; Di Mauro, G.; Doria, M.; Francavilla, R.; Landi, M.; Miniello, V.L.; et al. Pharmacological interventions on early functional gastrointestinal disorders. Ital. J. Pediatr. 2016, 42, 68. [Google Scholar] [CrossRef]
- Saps, M.; Youssef, N.; Miranda, A.; Nurko, S.; Hyman, P.; Cocjin, J.; Di Lorenzo, C. Multicenter, Randomized, Placebo-Controlled Trial of Amitriptyline in Children With Functional Gastrointestinal Disorders. Gastroenterology 2009, 137, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Mayer, E.A. The Power of Placebo in Pediatric Functional Gastrointestinal Disease. Gastroenterology 2009, 137, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Boradyn, K.M.; Przybyłowicz, K.E.; Jarocka-Cyrta, E. Low FODMAP Diet Is Not Effective in Children with Functional Abdominal Pain: A Randomized Controlled Trial. Ann. Nutr. Metab. 2020, 76, 334–344. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.; Carter, C.A. Integration of Biomedical and Psychosocial Treatments in Pediatrics Functional Gastrointestinal Disorders. Gastroenterol. Clin. N. Am. 2018, 47, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, D.R.; Zeevenhooven, J.; van Etten-Jamaludin, F.S.; Dekker, I.D.; Benninga, M.A.; Tabbers, M.M.; Vlieger, A.M. The Placebo Response in Pediatric Abdominal Pain-Related Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis. J. Pediatr. 2017, 182, 155–163.e7. [Google Scholar] [CrossRef] [PubMed]
- Beinvogl, B.; Burch, E.; Snyder, J.; Schechter, N.; Hale, A.; Okazaki, Y.; Paul, F.; Warman, K.; Nurko, S. Multidisciplinary Treatment Reduces Pain and Increases Function in Children With Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 994–996. [Google Scholar] [CrossRef]
- Wyeth, J.W. Functional gastrointestinal disorders in New Zealand. J. Gastroenterol. Hepatol. 2011, 26, 15. [Google Scholar] [CrossRef]
- Saps, M.; Velasco-Benitez, C.A.; Langshaw, A.H.; Ramírez-Hernández, C.R. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents: Comparison Between Rome III and Rome IV Criteria. J. Pediatr. 2018, 199, 212–216. [Google Scholar] [CrossRef]
- Velasco-Benitez, C.A.; Axelrod, C.H.; Gutierrez, S.; Saps, M. The Relationship Between Prematurity, Method of Delivery, and Functional Gastrointestinal Disorders in Children. J. Pediatr. Gastroenterol. Nutr. 2020, 70, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Walker, M.M.; Ford, A.C.; Talley, N.J. The overlap of atopy and functional gastrointestinal disorders among 23 471 patients in primary care. Aliment. Pharmacol. Ther. 2014, 40, 382–391. [Google Scholar] [CrossRef]
- Sjölund, J.; Kull, I.; Bergström, A.; Järås, J.; Ludvigsson, J.F.; Törnblom, H.; Simrén, M.; Olén, O. Allergy-related diseases in childhood and risk for abdominal pain-related functional gastrointestinal disorders at 16 years—A birth cohort study. BMC Med. 2021, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, Y.; Carman, K.B.; Yarar, C. Screening for functional gastrointestinal disorders in children with epilepsy. Epilepsy Behav. 2020, 111, 107267. [Google Scholar] [CrossRef] [PubMed]
- Kisla Ekinci, R.M.; Balcı, S.; Mart, O.O.; Tumgor, G.; Yavuz, S.; Celik, H.; Dogruel, D.; Altintas, D.U.; Yilmaz, M. Is Henoch–Schönlein purpura a susceptibility factor for functional gastrointestinal disorders in children? Rheumatol. Int. 2019, 39, 317–322. [Google Scholar] [CrossRef]
- Ekinci, R.M.K.; Balcı, S.; Akay, E.; Tumgor, G.; Dogruel, D.; Altintas, D.U.; Yilmaz, M. Frequency of functional gastrointestinal disorders in children with familial Mediterranean fever. Clin. Rheumatol. 2019, 38, 921–926. [Google Scholar] [CrossRef]
- Kovacic, K.; Chelimsky, T.C.; Sood, M.R.; Simpson, P.; Nugent, M.; Chelimsky, G. Joint Hypermobility: A Common Association with Complex Functional Gastrointestinal Disorders. J. Pediatr. 2014, 165, 973–978. [Google Scholar] [CrossRef]
- Phatak, U.; Pashankar, D. Prevalence of functional gastrointestinal disorders in obese and overweight children. Int. J. Obes. 2014, 38, 1324–1327. [Google Scholar] [CrossRef]
- Bode, G.; Brenner, H.; Adler, G.; Rothenbacher, D. Recurrent abdominal pain in children: Evidence from a population-based study that social and familial factors play a major role but not Helicobacter pylori infection. J. Psychosom. Res. 2003, 54, 417–421. [Google Scholar] [CrossRef]
- Yeh, T.C.; Bai, Y.M.; Tsai, S.J.; Chen, T.J.; Liang, C.S.; Chen, M.H. Risks of Major Mental Disorders and Irritable Bowel Syndrome among the Offspring of Parents with Irritable Bowel Syndrome: A Nationwide Study. Int. J. Environ. Res. Public Health 2021, 18, 4679. [Google Scholar] [CrossRef]
- Levy, R.L.; Whitehead, W.E.; Walker, L.S.; Von Korff, M.; Feld, A.D.; Garner, M.; Christie, D. Increased Somatic Complaints and Health-Care Utilization in Children: Effects of Parent IBS Status and Parent Response to Gastrointestinal Symptoms. Am. J. Gastroenterol. 2004, 99, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.K.; van Diggelen, T.R.; Levy, R.L.; Palermo, T.M. Understanding the Psychosocial and Parenting Needs of Mothers with Irritable Bowel Syndrome with Young Children. Children 2020, 7, 93. [Google Scholar] [CrossRef]
- Newton, E.; Schosheim, A.; Patel, S.; Chitkara, D.K.; van Tilburg, M.A.L. The role of psychological factors in pediatric functional abdominal pain disorders. Neurogastroent. Motil. 2019, 31, e13538. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.L.; Chitkara, D.K.; Palsson, O.S.; Levy, R.L.; Whitehead, W.E. Parental Worries and Beliefs About Abdominal Pain. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Van Oudenhove, L.; Levy, R.L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367.e2. [Google Scholar] [CrossRef]
- Short, P.; Burklow, C.S.; Nylund, C.M.; Susi, A.; Hisle-Gorman, E. Impact of Parental Illness and Injury on Pediatric Disorders of Gut–Brain Interaction. J. Pediatr. 2021, 236, 148–156.e3. [Google Scholar] [CrossRef]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Kalantar, J.; Weltman, M.; Zaguirre, J.; Talley, N.J. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 2012, 61, 1284. [Google Scholar] [CrossRef]
- Ramchandani, P.G.; Stein, A.; Hotopf, M.; Wiles, N.J. Early Parental and Child Predictors of Recurrent Abdominal Pain at School Age: Results of a Large Population-Based Study. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 729–736. [Google Scholar] [CrossRef]
- Heuckendorff, S.; Johansen, M.N.; Overgaard, C.; Johnsen, S.P.; Thomsen, J.L.; Fonager, K. Six-year-old children had greater risks of functional gastrointestinal disorders if their parents had mental health conditions. Acta Paediatr. 2022, 111, 2029–2037. [Google Scholar] [CrossRef]
- Oben, G.; Crengle, S.; Kokaua, J.; Duncanson, M. Deprivation trends in potentially avoidable medical hospitalisations of under-25-year-old Māori and non-Māori non-Pacific in Aotearoa New Zealand: A 20-year perspective. J. R. Soc. N. Z. 2022, 1–15. [Google Scholar] [CrossRef]
- Stoner, L.; Matheson, A.; Hamlin, M.; Skidmore, P. Environmental determinants of childhood obesity: A specific focus on Māori and Pasifika in New Zealand. Perspect. Public Health 2016, 136, 18–20. [Google Scholar] [CrossRef]
- Littlewood, R.; Canfell, O.J.; Walker, J.L. Interventions to prevent or treat childhood obesity in Māori & Pacific Islanders: A systematic review. BMC Public Health 2020, 20, 725. [Google Scholar]
- Tambucci, R.; Quitadamo, P.; Ambrosi, M.; De Angelis, P.; Angelino, G.; Stagi, S.; Verrotti, A.; Staiano, A.; Farello, G. Association Between Obesity/Overweight and Functional Gastrointestinal Disorders in Children. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 517–520. [Google Scholar] [CrossRef]
- Bonilla, S.; Wang, D.; Saps, M. Obesity predicts persistence of pain in children with functional gastrointestinal disorders. Int. J. Obes. 2011, 35, 517–521. [Google Scholar] [CrossRef]
- Wilson, D.; Moloney, E.; Parr, J.M.; Aspinall, C.; Slark, J. Creating an Indigenous Māori-centred model of relational health: A literature review of Māori models of health. J. Clin. Nurs. 2021, 30, 3539–3555. [Google Scholar] [CrossRef]
- Marriott, L.; Alinaghi, N. Closing the gaps: An update on indicators of inequality for Maori and pacific people. J. N. Z. Stud. 2021, 32, 2–39. [Google Scholar] [CrossRef]
- Jansen, P. Non-financial barriers to primary health care services for Maori. J. Prim. Health Care 2009, 1, 240. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Wille, N.; Badia, X.; Bonsel, G.; Burström, K.; Cavrini, G.; Devlin, N.; Egmar, A.-C.; Gusi, N.; Herdman, M.; et al. Feasibility, reliability, and validity of the EQ-5D-Y: Results from a multinational study. Qual. Life Res. 2010, 19, 887–897. [Google Scholar] [CrossRef]
- EuroQol Research Foundation. EQ-5D-Y User Guide 2020. Available online: https://euroqol.org/publications/user-guides (accessed on 20 February 2023).
- IBM Corp. IBM SPSS Statistics for Windows, 27th ed.; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]


| Variable | Category | Value, N (%) |
|---|---|---|
| Child’s age, mean (SD) | 9.5 y (SD 3.3) | |
| Child’s sex | Male | 87 (56) |
| Female | 69 (44) | |
| Ethnicity * (N = 134 (86%)) | NZ European | 123 (79) |
| Māori | 23 (15) | |
| Pacific Islands | 16 (10) | |
| Asian | 9 (6) | |
| MELAA | 5 (3) | |
| Other | 2 (1) | |
| Household income (N = 128 (82%)) | Up to $50,000 | 15 (9) |
| $50–100,000 | 40 (25) | |
| $100–150,000 | 37 (24) | |
| $150–200,000 | 21 (13) | |
| $200,000+ | 15 (9) | |
| Parent education | High school | 43 (28) |
| College | 38 (24) | |
| University | 50 (32) | |
| Post-graduate | 25 (16) | |
| Urban/rural living (N = 153 (98%)) | Rural | 53 (35) |
| Urban | 100 (65) | |
| Chronic health condition | Yes | 47 (30) |
| Taking prescription drugs | Yes | 41 (26) |
| Allergies | Yes | 47 (30) |
| Parent has FGID (all IBS) | Yes | 24 (15) |
| Variable | Category | No FGID | FGID | Mean Difference | p-Value |
|---|---|---|---|---|---|
| Age | 9.4 y (SD 3.2) | 10.0 y (SD 3.5) | 0.6 | 0.313 | |
| Variable | Category | No FGID | FGID | χ2 (Phi) | p-value |
| Sex | Male | 65 (75) | 22 (25) | 1.21 (0.09) | 0.290 |
| Female | 46 (67) | 23 (33) | |||
| Ethnicity | NZ European | 91 (74) | 32 (26) | 2.3 (0.12) | 0.137 |
| Māori | 11 (48) | 12 (52) | 7.2 (0.21) | 0.012 | |
| Pacific Islands | 14 (88) | 2 (12) | 2.3 (0.12) | 0.155 | |
| Asian | 8 (67) | 4 (33) | 0.1 (0.03 | 0.745 | |
| MELAA | 3 (60) | 2 (40) | 0.3 (0.05) | 0.627 | |
| Other | 1 (50) | 1 (50) | 0.4 (0.05) | 0.495 | |
| Household income | Up to NZD 50,000 | 9 (60) | 6 (40) | 3.0 (0.15) | 0.565 |
| NZD 50–100,000 | 25 (62) | 15 (38) | |||
| NZD 100–150,000 | 29 (78) | 8 (22) | |||
| NZD 150–200,000 | 15 (71) | 6 (29) | |||
| NZD 200,000+ | 10 (67) | 5 (33) | |||
| Parent education | High school | 30 (70) | 13 (30) | 1.2 (0.09) | 0.762 |
| College | 26 (68) | 12 (32) | |||
| University | 35 (70) | 15 (30) | |||
| Post-graduate | 20 (80) | 5 (20) | |||
| Urban/rural living | Rural | 69 (69) | 31 (31) | 0.7 (0.07) | 0.456 |
| Urban | 40 (75) | 13 (25) | |||
| Chronic health condition | No | 81 (74) | 28 (26) | 1.8 (0.11) | 0.247 |
| Yes | 30 (64) | 17 (36) | |||
| On medications | No | 85 (75) | 29 (25) | 2.7 (0.13) | 0.112 |
| Yes | 25 (61) | 16 (39) | |||
| Allergies | No | 77 (71) | 32 (29) | 0.05 (0.02) | 0.99 |
| Yes | 34 (72) | 13 (28) | |||
| Parent (overall) has FGID | No | 101 (77) | 31 (23) | 12.0 (0.28) | <0.001 |
| Yes | 10 (42) | 14 (58) | |||
| Mother has FGID | No | 4 (80) | 1 (20) | 2.3 (0.33) | 0.311 |
| Yes | 7 (41) | 10 (59) | |||
| Father has FGID | No | 1 (25) | 3 (75) | 0.69 (0.24) | 0.576 |
| Yes | 4 (50) | 4 (50) |
| EQ-5D-Y Category | Functional Nausea and Vomiting Disorder | Functional Abdominal Pain Disorder | Functional Defecation Disorder | |||
|---|---|---|---|---|---|---|
| χ2 (Phi) | p-Value | χ2 (Phi) | p-Value | χ2 (Phi) | p-Value | |
| Mobility | 4.4 (0.17) | 0.06 | 1.1 (0.09) | 0.37 | 0.4 (0.05) | 0.62 |
| Looking after myself | 2.0 (0.1) | 0.22 | 0.01 (0.01) | 1.0 | 0.6 (0.06) | 0.46 |
| Doing usual activities | 3.8 (0.16) | 0.07 | 2.9 (0.14) | 0.12 | 0.4 (0.05) | 0.63 |
| Having pain or discomfort | 0.7 (0.07) | 0.54 | 5.1 (0.18) | 0.02 | 0.01 (0.01) | 1.0 |
| Feeling Worried, Sad, or Unhappy | 1.7 (0.1) | 0.24 | 14.8 (0.31) | <0.001 | 3.4 (0.15) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernon-Roberts, A.; Alexander, I.; Day, A.S. Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children. Gastrointest. Disord. 2023, 5, 261-272. https://doi.org/10.3390/gidisord5020021
Vernon-Roberts A, Alexander I, Day AS. Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children. Gastrointestinal Disorders. 2023; 5(2):261-272. https://doi.org/10.3390/gidisord5020021
Chicago/Turabian StyleVernon-Roberts, Angharad, India Alexander, and Andrew S. Day. 2023. "Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children" Gastrointestinal Disorders 5, no. 2: 261-272. https://doi.org/10.3390/gidisord5020021
APA StyleVernon-Roberts, A., Alexander, I., & Day, A. S. (2023). Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children. Gastrointestinal Disorders, 5(2), 261-272. https://doi.org/10.3390/gidisord5020021



.png)



