Identification of a Five-MiRNA Expression Assay to Aid Colorectal Cancer Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumour Staging
2.2. Immunohistochemical Tumour Evaluation
2.3. Radiological Staging
2.4. Calculating Follow-Up
2.5. Identification of MiRNA Targets
2.6. RNA Isolation and Biobanking
2.7. Efficiency Calculations
2.8. Reverse Transcription Polymerase Chain Reactions
2.9. Endogenous and Inter-Assay Controls
2.10. Statistical Evaluation
3. Results
3.1. Included Patients
3.2. Target miRNA as Oncogenic Biomarkers in Colorectal Cancer
3.3. Development and Validation of a Five-miRNA Oncogenic Signature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Sheahan, K.; Creavin, B.; Mohan, H.; Winter, D. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit. Rev. Oncol. 2017, 116, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann. Transl. Med. 2019, 7, 609. [Google Scholar] [CrossRef]
- Brouwer, N.P.; Bos, A.C.; Lemmens, V.E.; Tanis, P.; Hugen, N.; Nagtegaal, I.; De Wilt, J.H.; Verhoeven, R. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Shinkins, B.; Nicholson, B.D.; Primrose, J.; Perera, R.; James, T.; Pugh, S.; Mant, D. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: Results from the FACS trial. PLoS ONE 2017, 12, e0171810. [Google Scholar] [CrossRef]
- Eleftheriadis, N.; Papaloukas, C.; Pistevou-Gompaki, K. Diagnostic value of serum tumor markers in asymptomatic individuals. J. BU ON Off. J. Balk. Union Oncol. 2009, 14, 707–710. [Google Scholar]
- Tan, E.; Gouvas, N.; Nicholls, R.J.; Ziprin, P.; Xynos, E.; Tekkis, P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009, 18, 15–24. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yu, M.-H.; Kim, H.-G.; Byun, J.-H.; Lee, C. Noninvasive molecular biomarkers for the detection of colorectal cancer. BMB Rep. 2008, 41, 685–692. [Google Scholar] [CrossRef]
- Iwanicki-Caron, I.; Di Fiore, F.; Roque, I.; Astruc, E.; Stetiu, M.; Duclos, A.; Tougeron, D.; Saillard, S.; Thureau, S.; Benichou, J.; et al. Usefulness of the Serum Carcinoembryonic Antigen Kinetic for Chemotherapy Monitoring in Patients With Unresectable Metastasis of Colorectal Cancer. J. Clin. Oncol. 2008, 26, 3681–3686. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, N.K.; Sohn, S.K.; Kim, Y.W.; Kim, K.J.S.; Hur, H.; Min, B.S.; Cho, C.H. Prognostic Value of Postoperative CEA Clearance in Rectal Cancer Patients with High Preoperative CEA Levels. Ann. Surg. Oncol. 2009, 16, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Kelly, R.; Newell, J.; Kerin, M.J. Systemic miRNA-195 Differentiates Breast Cancer from Other Malignancies and Is a Potential Biomarker for Detecting Noninvasive and Early Stage Disease. Oncologist 2010, 15, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Erson, A.; Petty, E. MicroRNAs in development and disease. Clin. Genet. 2008, 74, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Danac, J.M.C.; Uy, A.G.G.; Garcia, R.L. Exosomal microRNAs in colorectal cancer: Overcoming barriers of the metastatic cascade (Review). Int. J. Mol. Med. 2021, 47, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, Y.; Liu, S.; Qiao, L.; Sun, J.; Zhao, Q. MicroRNAs Associated with Colon Cancer: New Potential Prognostic Markers and Targets for Therapy. Front. Bioeng. Biotechnol. 2020, 8, 176. [Google Scholar] [CrossRef]
- Zhou, F.; Tang, D.; Xu, Y.; He, H.; Wu, Y.; Lin, L.; Dong, J.; Tan, W.; Dai, Y. Identification of microRNAs and their Endonucleolytic Cleavaged target mRNAs in colorectal cancer. BMC Cancer 2020, 20, 242. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, F.; Wang, X.; Ouyang, G. The roles of microRNAs in the regulation of tumor metastasis. Cell Biosci. 2015, 5, 32. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- McGuire, A.; Casey, M.-C.; Waldron, R.M.; Heneghan, H.; Kalinina, O.; Holian, E.; McDermott, A.; Lowery, A.J.; Newell, J.; Dwyer, R.M.; et al. Prospective Assessment of Systemic MicroRNAs as Markers of Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2020, 12, 1820. [Google Scholar] [CrossRef]
- Waldron, R.M.; Moloney, B.M.; Gilligan, K.; Lowery, A.J.; Joyce, M.R.; Holian, E.; Kerin, M.J.; Miller, N. MicroRNAs as biomarkers of multimodal treatment for rectal cancer. Br. J. Surg. 2021, 108, e260–e261. [Google Scholar] [CrossRef]
- Davey, M.G.; Feeney, G.; Annuk, H.; Paganga, M.; Holian, E.; Lowery, A.J.; Kerin, M.J.; Miller, N. MicroRNA Expression Profiling Predicts Nodal Status and Disease Recurrence in Patients Treated with Curative Intent for Colorectal Cancer. Cancers 2022, 14, 2109. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, A highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef]
- Kahn, H.J.; Marks, A. A New Monoclonal Antibody, D2-40, for Detection of Lymphatic Invasion in Primary Tumors. Lab. Investig. 2002, 82, 1255–1257. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, S.; Xu, W.; Huang, J.; Zhang, G.; Lei, L.; Shao, X.; Wang, X. Expression of cluster of differentiation 34 and vascular endothelial growth factor in breast cancer, and their prognostic significance. Oncol. Lett. 2015, 10, 723–729. [Google Scholar] [CrossRef]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol. Surg. Part B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef]
- Roxburgh, C.S.D.; McMillan, D.C.; Anderson, J.H.; McKee, R.F.; Horgan, P.G.; Foulis, A.K. Elastica Staining for Venous Invasion Results in Superior Prediction of Cancer-Specific Survival in Colorectal Cancer. Ann. Surg. 2010, 252, 989–997. [Google Scholar] [CrossRef]
- Xue, X.; Agalliu, I.; Kim, M.Y.; Wang, T.; Lin, J.; Ghavamian, R.; Strickler, H.D. New methods for estimating follow-up rates in cohort studies. BMC Med. Res. Methodol. 2017, 17, 155. [Google Scholar] [CrossRef]
- Knudsen, K.N.; Nielsen, B.S.; Lindebjerg, J.; Hansen, T.F.; Holst, R.; Sørensen, F.B. microRNA-17 Is the Most Up-Regulated Member of the miR-17-92 Cluster during Early Colon Cancer Evolution. PLoS ONE 2015, 10, e0140503. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Dvoretskiy, S.; An, R.; Mantha, S.; Pan, Y.-X. The Efficacy of miR-20a as a Diagnostic and Prognostic Biomarker for Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.B.; Luo, H.P.; Shi, Q.; Hao, Z.N.; Ding, Y.; Wang, Q.S.; Li, S.B.; Xiao, G.C.; Tong, S.L. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J. Gastroenterol. 2014, 20, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- Nezu, Y.; Hagiwara, K.; Yamamoto, Y.; Fujiwara, T.; Matsuo, K.; Yoshida, A.; Kawai, A.; Saito, T.; Ochiya, T. miR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene 2016, 35, 6177–6188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Y.; Zhu, N.; Tsoi, H.; Zhao, Z.; Wu, C.W.; Wang, K.; Zheng, S.; Ng, S.S.; Chan, F.K.; et al. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol. Cancer 2014, 13, 124. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, J.; Ou, C.; Deng, Z.; Chen, M.; Huang, W.; Li, L. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br. J. Cancer 2014, 110, 2300–2309. [Google Scholar] [CrossRef]
- Takahashi, M.; Cuatrecasas, M.; Balaguer, F.; Hur, K.; Toiyama, Y.; Castells, A.; Boland, C.R.; Goel, A. The Clinical Significance of MiR-148a as a Predictive Biomarker in Patients with Advanced Colorectal Cancer. PLoS ONE 2012, 7, e46684. [Google Scholar] [CrossRef]
- Aherne, S.T.; Madden, S.F.; Hughes, D.J.; Pardini, B.; Naccarati, A.; Levy, M.; Vodicka, P.; Neary, P.; Dowling, P.; Clynes, M. Circulating miRNAs miR-34a and miR-150 associated with colorectal cancer progression. BMC Cancer 2015, 15, 329. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Xiang, J.; Gu, X. MicroRNA-155 acts as a tumor suppressor in colorectal cancer by targeting CTHRC1 in vitro. Oncol. Lett. 2018, 15, 5561–5568. [Google Scholar] [CrossRef]
- Sui, H.; Cai, G.X.; Pan, S.F.; Deng, W.L.; Wang, Y.W.; Chen, Z.S.; Cai, S.J.; Zhu, H.R.; Li, Q. miR200c attenuates P-gp-mediated MDR and metastasis by targeting JNK2/c-Jun signaling pathway in colorectal cancer. Mol. Cancer Ther. 2014, 13, 3137–3151. [Google Scholar] [CrossRef]
- Ye, H.; Hao, H.; Wang, J.; Chen, R.; Huang, Z. miR-203 as a novel biomarker for the diagnosis and prognosis of colorectal cancer: A systematic review and meta-analysis. Oncol. Targets Ther. 2017, 10, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Pal, T.; Song, B.; Zhang, C.; Georgakopoulos, P.; Mehmood, S.; Burke, S.; Shroyer, K.; Ju, J. Prognostic Significance of miR-215 in Colon Cancer. Clin. Color Cancer 2011, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Svoboda, M.; Fabian, P.; Smerdova, T.; Knoflickova, D.; Bednarikova, M.; Nenutil, R.; Vyzula, R. Altered Expression of miR-21, miR-31, miR-143 and miR-145 Is Related to Clinicopathologic Features of Colorectal Cancer. Oncology 2007, 72, 397–402. [Google Scholar] [CrossRef]
- McDermott, A.M.; Kerin, M.; Miller, N. Identification and Validation of miRNAs as Endogenous Controls for RQ-PCR in Blood Specimens for Breast Cancer Studies. PLoS ONE 2014, 8, e83718. [Google Scholar] [CrossRef]
- Chang, K.H.; Mestdagh, P.; Vandesompele, J.; Kerin, M.J.; Miller, N. MicroRNA expression profiling to identify and validate reference genes for relative quantification in colorectal cancer. BMC Cancer 2010, 10, 173. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Shinkins, B.; Nicholson, B.D.; James, T.; Pathiraja, I.; Pugh, S.; Perera, R.; Primrose, J.; Mant, D. What carcinoembryonic antigen level should trigger further investigation during colorectal cancer follow-up? A systematic review and secondary analysis of a randomised controlled trial. Health Technol. Assess. 2017, 21, 1–60. [Google Scholar] [CrossRef]
- Davey, M.S.; Davey, M.G.; Ryan, É.J.; Hogan, A.M.; Kerin, M.J.; Joyce, M. The use of radiomic analysis of magnetic resonance imaging in predicting distant metastases of rectal carcinoma following surgical resection: A systematic review and meta-analysis. Colorectal Dis. 2021, 23, 3065–3072. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef]
- Schee, K.; Boye, K.; Abrahamsen, T.W.; Fodstad, Ø.; Flatmark, K. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC Cancer 2012, 12, 505. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, X.; Min, M.; Zou, L.; Shen, P.; Zhu, Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 44893–44909. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhu, W.; Gao, C.; Jiang, R.; Li, W.; Hu, Q.; Zhang, B. MicroRNA-21 and the clinical outcomes of various carcinomas: A systematic review and meta-analysis. BMC Cancer 2014, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-X.; Huang, X.-F.; Shao, Q.; Huang, M.-Y.; Deng, L.; Wu, Q.-L.; Zeng, Y.-X.; Shao, J.-Y. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008, 14, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Winther, M.; Alsner, J.; Tramm, T.; Baeksgaard, L.; Holtved, E.; Nordsmark, M. Evaluation of miR-21 and miR-375 as prognostic biomarkers in esophageal cancer. Acta Oncol. 2015, 54, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Yu, J.; Chen, N.; Wang, X.Y.; Liu, X.Y.; Wang, S.; Ding, Y.Q. Elevated MicroRNA-31 Expression Regulates Colorectal Cancer Progression by Repressing Its Target Gene SATB2. PLoS ONE 2014, 8, e85353. [Google Scholar] [CrossRef]
- Wang, C.J.; Zhou, Z.G.; Wang, L.; Yang, L.; Zhou, B.; Gu, J.; Chen, H.Y.; Sun, X.F. Clinicopathological Significance of microRNA-31, -143 and -145 Expression in Colorectal Cancer. Dis. Markers 2009, 26, 921907. [Google Scholar] [CrossRef]
- Laurila, E.; Kallioniemi, A. The diverse role of miR-31 in regulating cancer associated phenotypes. Genes Chromosom. Cancer 2013, 52, 1103–1113. [Google Scholar] [CrossRef]
- Yu, T.; Ma, P.; Wu, D.; Shu, Y.; Gao, W. Functions and mechanisms of microRNA-31 in human cancers. Biomed. Pharmacother. 2018, 108, 1162–1169. [Google Scholar] [CrossRef]
- Shen-Ong, G.L. The myb oncogene. Biochim. Biophys. Acta 1990, 1032, 39–52. [Google Scholar] [CrossRef]
- Weston, K.M. The myb genes. Semin. Cancer Biol. 1990, 1, 371–382. [Google Scholar]
- Gryfe, R.; Bapat, B.; Gallinger, S.; Swallow, C.; Redston, M.; Couture, J. Molecular biology of colorectal cancer. Curr. Probl. Cancer 1997, 21, 233–2990. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Pan, B.; Zeng, K.; Xu, M.; Liu, X.; He, B.; Pan, Y.; Sun, H.; Wang, S. miR-150-5p suppresses tumor progression by targeting. VEGFA Colorectal Cancer Aging 2018, 10, 3421–3437. [Google Scholar]
- Feng, J.; Yang, Y.; Zhang, P.; Wang, F.; Ma, Y.; Qin, H.; Wang, Y. miR-150 functions as a tumour suppressor in human colorectal cancer by targeting c-Myb. J. Cell. Mol. Med. 2014, 18, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Zhang, H.; Yang, J.; Peng, J.; Liu, W.; Qin, H. miR-150 as a potential biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Gut 2012, 61, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Lowery, A.J.; Miller, N.; McNeill, R.E.; Kerin, M.J. MicroRNAs as Prognostic Indicators and Therapeutic Targets: Potential Effect on Breast Cancer Management. Clin. Cancer Res. 2008, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Lowery, A.; Sweeney, K.J.; Kerin, M.J. MicroRNAs as Novel Biomarkers for Breast Cancer. J. Oncol. 2009, 2010, 950201. [Google Scholar] [CrossRef] [PubMed]
- Schetter, A.J.; Okayama, H.; Harris, C.C. The Role of MicroRNAs in Colorectal Cancer. Cancer J. 2012, 18, 244–252. [Google Scholar] [CrossRef]
- Davey, M.G.; Lowery, A.J.; Miller, N.; Kerin, M.J. MicroRNA Expression Profiles and Breast Cancer Chemotherapy. Int. J. Mol. Sci. 2021, 22, 10812. [Google Scholar] [CrossRef]
- Davey, M.; Davies, M.; Lowery, A.; Miller, N.; Kerin, M. The Role of MicroRNA as Clinical Biomarkers for Breast Cancer Surgery and Treatment. Int. J. Mol. Sci. 2021, 22, 8290. [Google Scholar] [CrossRef]
- You, Y.N.; Rustin, R.B.; Sullivan, J.D. Oncotype DX® colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surg. Oncol. 2015, 24, 61–66. [Google Scholar] [CrossRef]
- O’Dushlaine, C.T.; Dolan, C.; Weale, M.E.; Stanton, A.; Croke, D.T.; Kälviäinen, R.; Eriksson, K.; Kantanen, A.-M.; Gibson, R.A.; Hosford, D.; et al. An assessment of the Irish population for large-scale genetic mapping studies involving epilepsy and other complex diseases. Eur. J. Hum. Genet. 2008, 16, 176–183. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, T.P.; Irwin, R.; Cody, N.; Miller, N.; McDevitt, T.; Sweeney, K.J.; Green, A.; Kerin, M. Familial breast cancer genetic testing in the West of Ireland. Ir. J. Med. Sci. 2014, 183, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
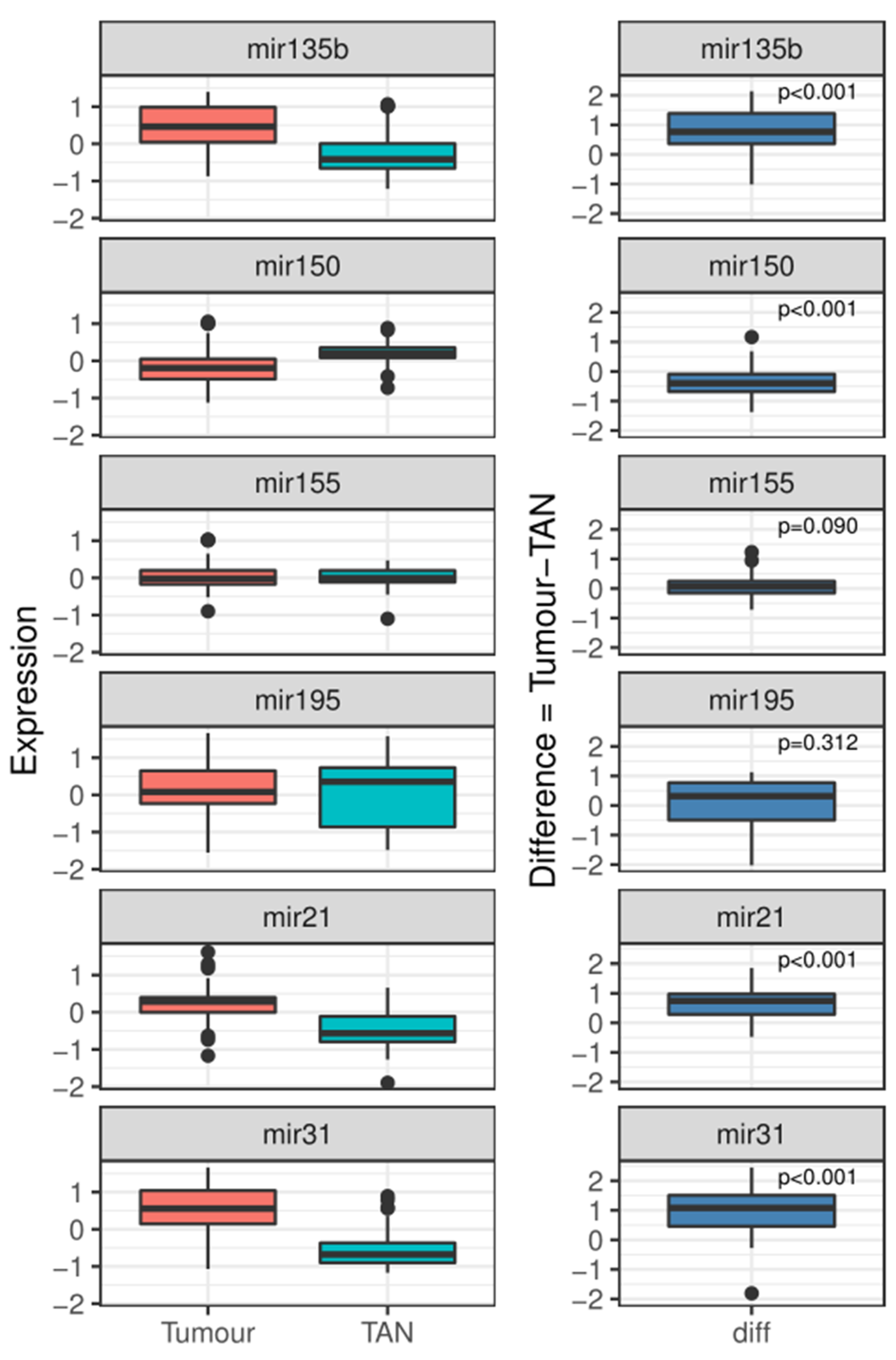

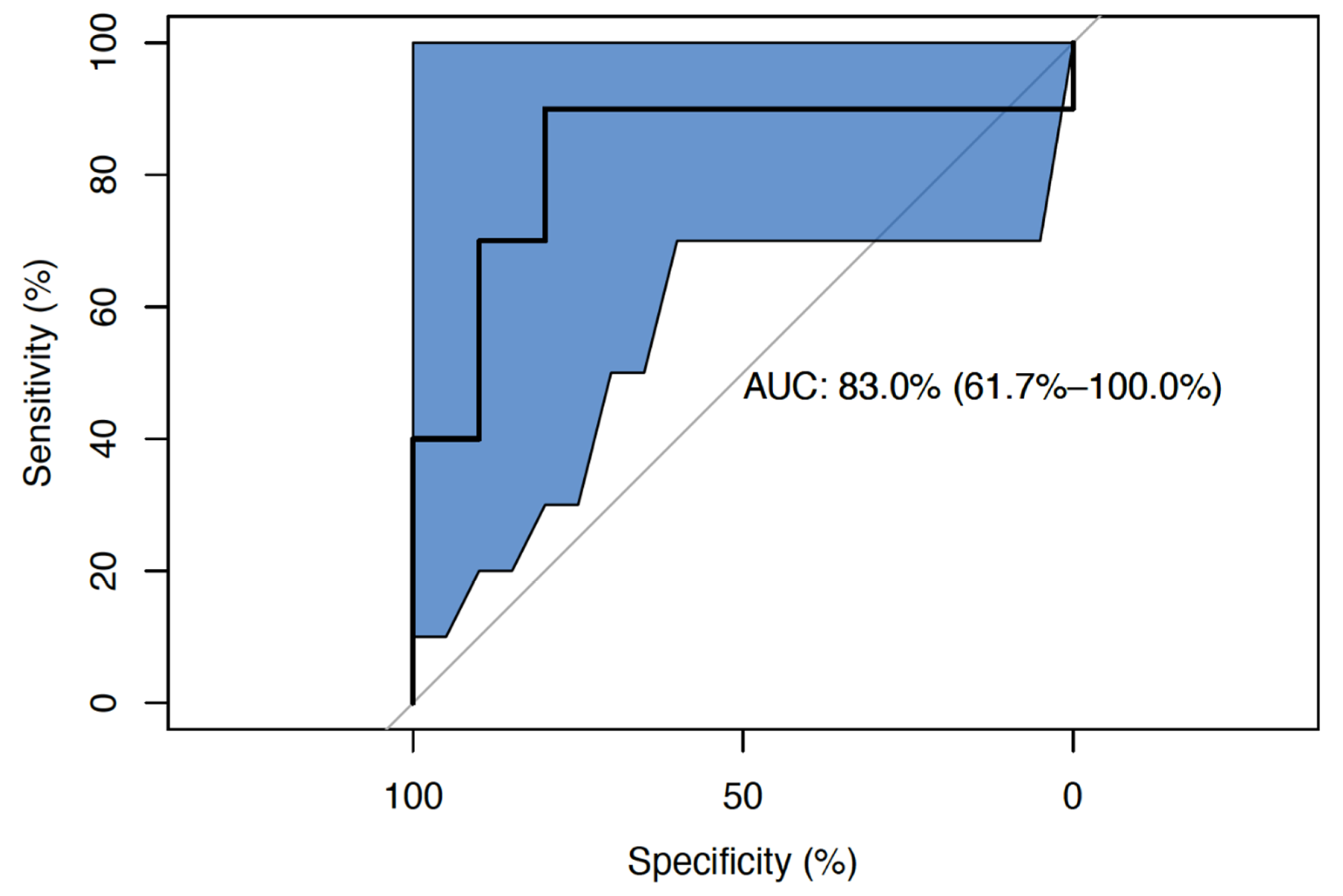
| Clinicopathological Parameter | Patients with Colorectal Cancer (N = 74) |
|---|---|
| Mean age (±standard deviation, range) | 67.8 years (±12.5, 38–90 years) |
| Gender | |
| 51 (68.9%) 23 (31.1%) |
| Tumour Location | |
| 52 (70.3%) 22 (29.7%) |
| Presentation | |
| 12 (16.2%) 62 (83.8%) |
| Histological subtype | |
| 53 (68.0%) 3 (3.8%) 22 (28.2) |
| Tumour Stage | |
| 2 (2.7%) 5 (6.8%) 25 (33.8%) 18 (24.3%) 24 (32.4%) |
| Nodal Stage | |
| 15 (20.3%) 20 (27.0%) 10 (13.5%) 29 (39.2%) |
| Target | MiRNA Function | Expression Levels | CT Difference | Efficiencies | p-Value |
|---|---|---|---|---|---|
| miR-17 | Upregulated in early CRC (31) | Increased | 11.15 | 93% | 0.089☐ |
| miR-20a | Reported as prognostic biomarker (32) | Increased | 15.46 | 91% | 0.325☐ |
| miR-21 | Known oncogene (20) | Increased | 12.19 | 97% | 0.158☐ |
| miR-31 | Oncogenic miRNA in CRC (43) | Increased | 14.42 | 101% | <0.001 *☐ |
| miR-132 | Inhibitory role in CRC invasion and metastasis (33) | Increased | 11.36 | 105% | 0.058☐ |
| miR-135b | Modulatory role in malignancy (34) | Increased | 14.13 | 99% | 0.036 *☐ |
| miR-139-5p | Tumour suppressor miRNA (35) | Decreased | 9.25 | 91% | 0.752☐ |
| miR-145 | Known inhibitive role in growth and metastasis in CRC (36) | Decreased | 11.72 | 92% | 0.358☐ |
| miR-148a | Predictive biomarker in stage IV CRC (37) | Increased | 9.86 | 109% | 0.242☐ |
| miR-150 | Associated with CRC progression (38) | Decreased | 10.88 | 106% | 0.003 *☐ |
| miR-155 | Tumour suppressor miRNA in CRC (39) | Decreased | 13.83 | 108% | 0.016 *☐ |
| miR-195 | Known oncogenic biomarker in malignancy (12) | Decreased | 11.88 | 93% | 0.245☐ |
| miR-200c | Regulator of metastasis within CRC (40) | Decreased | 11.88 | 101% | 0.323☐ |
| miR-203 | Diagnostic and prognostic biomarker in CRC (41) | Increased | 12.84 | 104% | 0.146☐ |
| miR-215 | Prognostic biomarker in CRC (42) | Decreased | 10.46 | 93% | 0.139☐ |
| miR-16 | Endogenous control (44) | Stable | 0.00 | - | - |
| miR-345 | Endogenous control (44) | Stable | 0.00 | - | - |
| Target | Tumour Mean Log2 Fold Change | TAN Mean Log2 Fold Change | p-Value |
|---|---|---|---|
| miR-21 | 0.23 (SE: 0.47) | −0.43 (SE: 0.53) | <0.001 *† |
| miR-31 | 0.48 (SE: 0.73) | −0.54 (SE: 0.49) | <0.001 *† |
| miR-135b | 0.46 (SE: 0.55) | −0.26 (SE: 0.63) | <0.001 *† |
| miR-150 | −0.17 (SE: 0.46) | 0.22 (SE: 0.27) | <0.001 *† |
| miR-155 | 0.04 (SE: 0.37) | 0.01 (SE: 0.27) | 0.312 † |
| miR-195 | 0.17 (SE: 0.60) | 0.02 (SE: 0.83) | 0.090 † |
| Parameter | β-Coefficient (SE) | p-Value | β-Coefficient (SE) | p-Value |
|---|---|---|---|---|
| Univariable | Multivariable | |||
| MiR-21 | 3.661 (1.720) | 0.033 * | ||
| MiR-31 | 2.783 (0.918) | 0.002 * | 2.431 (0.715) | <0.001 * |
| MiR-135b | 0.155 (0.882) | 0.861 | ||
| MiR-150 | −4.404 (0.526) | 0.004 * | −4.620 (1.319) | <0.001 * |
| MiR-155 | 2.850 (2.960) | 0.336 | ||
| MiR-195 | −0.694 (1.017) | 0.495 |
| Target | Cancer Patient Mean Log2 Fold Change | Control Mean Log2 Fold Change | p-Value | Cancer Patient Median Log2 Fold Change | Control Median Log2 Fold Change | p-Value |
|---|---|---|---|---|---|---|
| miR-21 | −0.64 | 0.42 | 0.001 *☐ | 0.25 | 1.02 | 0.476 † |
| miR-31 | 0.01 | −0.05 | 0.001 *☐ | 0.23 | 0.25 | 0.933 † |
| miR-150 | −0.10 | 0.69 | <0.001 *☐ | 0.18 | 1.03 | 0.148 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davey, M.G.; Feeney, G.; Annuk, H.; Paganga, M.; Holian, E.; Lowery, A.J.; Kerin, M.J.; Miller, N. Identification of a Five-MiRNA Expression Assay to Aid Colorectal Cancer Diagnosis. Gastrointest. Disord. 2022, 4, 190-204. https://doi.org/10.3390/gidisord4030018
Davey MG, Feeney G, Annuk H, Paganga M, Holian E, Lowery AJ, Kerin MJ, Miller N. Identification of a Five-MiRNA Expression Assay to Aid Colorectal Cancer Diagnosis. Gastrointestinal Disorders. 2022; 4(3):190-204. https://doi.org/10.3390/gidisord4030018
Chicago/Turabian StyleDavey, Matthew G., Gerard Feeney, Heidi Annuk, Maxwell Paganga, Emma Holian, Aoife J. Lowery, Michael J. Kerin, and Nicola Miller. 2022. "Identification of a Five-MiRNA Expression Assay to Aid Colorectal Cancer Diagnosis" Gastrointestinal Disorders 4, no. 3: 190-204. https://doi.org/10.3390/gidisord4030018
APA StyleDavey, M. G., Feeney, G., Annuk, H., Paganga, M., Holian, E., Lowery, A. J., Kerin, M. J., & Miller, N. (2022). Identification of a Five-MiRNA Expression Assay to Aid Colorectal Cancer Diagnosis. Gastrointestinal Disorders, 4(3), 190-204. https://doi.org/10.3390/gidisord4030018






