Participation in Colorectal Cancer Screening among Migrants and Non-Migrants in Germany: Results of a Population Survey
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Study Variables
2.3. Analysis
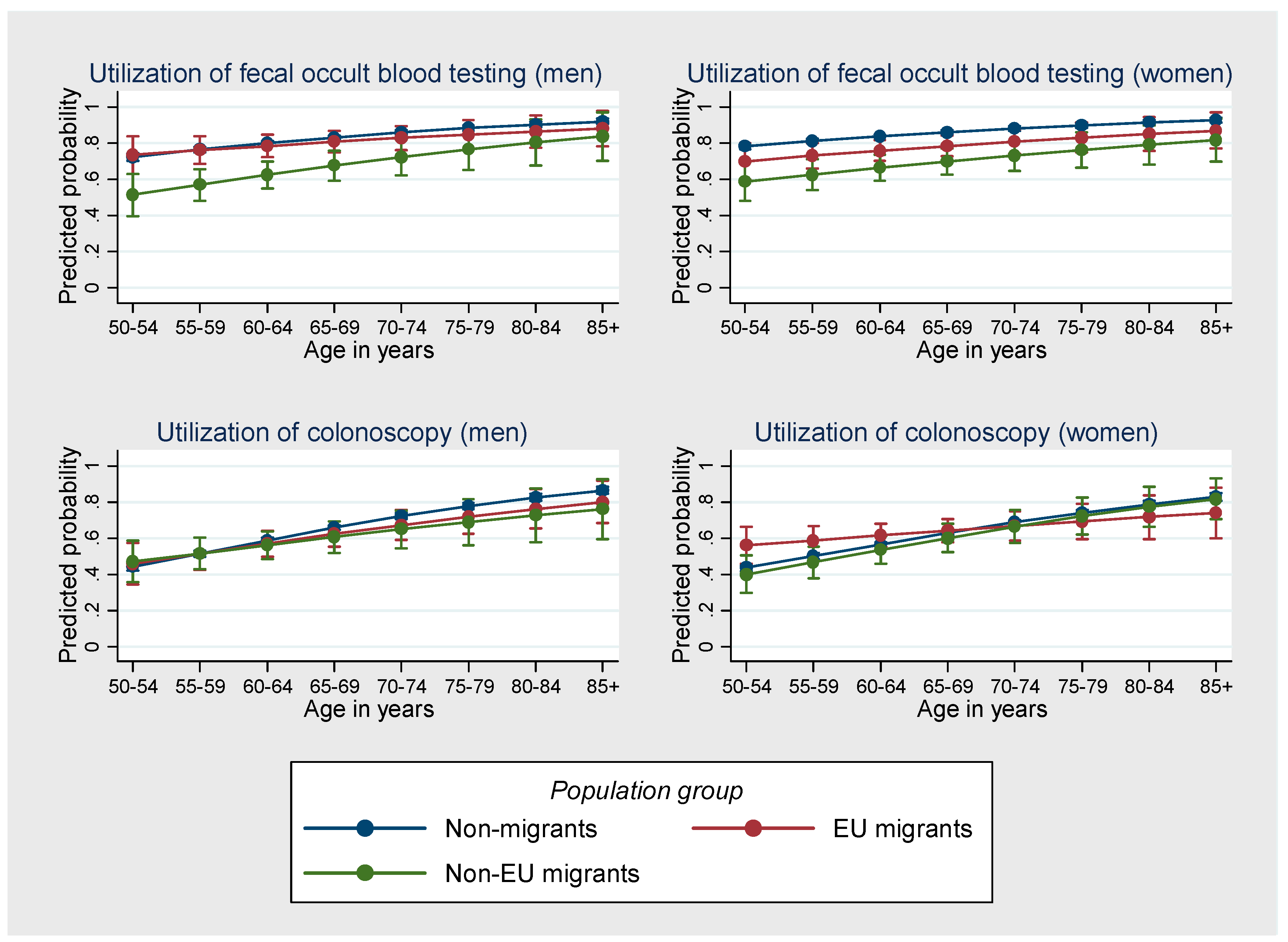
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cinar, D.; Tas, D. Cancer in the elderly. North Clin. Istanb. 2015, 2, 73–80. [Google Scholar] [CrossRef]
- Gutlic, I.; Schyman, T.; Lydrup, M.-L.; Buchwald, P. Increasing colorectal cancer incidence in individuals aged <50 years-a population-based study. Int. J. Colorectal Dis. 2019, 34, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- EuropaColon. Colorectal Cancer in Europe. A Framework for Improving Outcomes for Patients. Available online: https://webgate.ec.europa.eu/chafea_pdb/assets/files/pdb/20124301/20124301_d04-00_en_ps_ecwhitepaper.pdf (accessed on 31 May 2022).
- European Cancer Information System. Colorectal Cancer Burden in EU-27. Available online: https://ecis.jrc.ec.europa.eu/factsheets.php (accessed on 31 May 2022).
- Roche. Colorectal cancer Colorectal Cancer. A Guide for Journalists on Colorectal Cancer and Its Treatment. Available online: https://assets.cwp.roche.com/f/126832/x/6a772fdf01/med-colorectal-cancer.pdf (accessed on 31 May 2022).
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H. Survival from colorectal cancer in Germany in the early 21st century. Br. J. Cancer 2012, 106, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. Inanspruchnahme der Darmspiegelung in Deutschland; Robert Koch Institute: Berlin, Germany, 2017. [Google Scholar]
- Weigl, K.; Hoffmeister, M. Darmkrebs-Screening. TumorDiagn. Ther. 2019, 40, 360–363. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Nowalk, M.P.; Tabbarah, M.; Grufferman, S. Predictors of colorectal cancer screening in diverse primary care practices. BMC Health Serv. Res. 2006, 6, 116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shapiro, J.A.; Seeff, L.; Nadel, M. Colorectal cancer-screening tests and associated health behaviors. Am. J. Prev. Med. 2001, 21, 132–137. [Google Scholar] [CrossRef]
- Wools, A.; Dapper, E.A.; de Leeuw, J.R.J. Colorectal cancer screening participation: A systematic review. Eur. J. Public Health 2016, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, I.; Mendizabal, N.; Martín, U.; Bacigalupe, A.; Aldasoro, E.; Portillo, I. Inequalities in participation in colorectal cancer screening programmes: A systematic review. Eur. J. Public Health 2020, 30, 416–425. [Google Scholar] [CrossRef]
- van Jaarsveld, C.H.M.; Miles, A.; Edwards, R.; Wardle, J. Marriage and cancer prevention: Does marital status and inviting both spouses together influence colorectal cancer screening participation? J. Med. Screen. 2006, 13, 172–176. [Google Scholar] [CrossRef]
- Gimeno Garcia, A.Z.; Buylla, N.H.A.; Nicolas-Perez, D.; Quintero, E. Public awareness of colorectal cancer screening: Knowledge, attitudes, and interventions for increasing screening uptake. ISRN Oncol. 2014, 2014, 425787. [Google Scholar] [CrossRef]
- Gede, N.; Reményi Kiss, D.; Kiss, I. Colorectal cancer and screening awareness and sources of information in the Hungarian population. BMC Fam. Pract. 2018, 19, 106. [Google Scholar] [CrossRef]
- Baron, R.C.; Rimer, B.K.; Coates, R.J.; Kerner, J.; Kalra, G.P.; Melillo, S.; Habarta, N.; Wilson, K.M.; Chattopadhyay, S.; Leeks, K. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening a systematic review. Am. J. Prev. Med. 2008, 35, S56–S66. [Google Scholar] [CrossRef]
- Azerkan, F.; Sparén, P.; Sandin, S.; Tillgren, P.; Faxelid, E.; Zendehdel, K. Cervical screening participation and risk among Swedish-born and immigrant women in Sweden. Int. J. Cancer 2012, 130, 937–947. [Google Scholar] [CrossRef]
- Brunner-Ziegler, S.; Rieder, A.; Stein, K.V.; Koppensteiner, R.; Hoffmann, K.; Dorner, T.E. Predictors of participation in preventive health examinations in Austria. BMC Public Health 2013, 13, 1138. [Google Scholar] [CrossRef]
- Brzoska, P.; Aksakal, T.; Yilmaz-Aslan, Y. Disparities in the use of regular pap smears among migrant and non-migrant women in Austria: A population-based survey of 7633 women. J. Med. Screen. 2021, 28, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, P.; Aksakal, T.; Yilmaz-Aslan, Y. Utilization of cervical cancer screening among migrants and non-migrants in Germany: Results from a large-scale population survey. BMC Public Health 2020, 20, 5. [Google Scholar] [CrossRef]
- Carrasco-Garrido, P.; Hernandez-Barrera, V.; Lopez de Andres, A.; Jimenez-Trujillo, I.; Gallardo Pino, C.; Jimenez-Garcıa, R. Awareness and uptake of colorectal, breast, cervical and prostate cancer screening tests in Spain. Eur. J. Public Health 2014, 24, 264–270. [Google Scholar] [CrossRef]
- Frederiksen, B.L.; Jørgensen, T.; Brasso, K.; Holten, I.; Osler, M. Socioeconomic position and participation in colorectal cancer screening. Br. J. Cancer 2010, 103, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.F.; Pedersen, A.F.; Andersen, B.; Vedsted, P. Identifying specific non-attending groups in breast cancer screening--population-based registry study of participation and socio-demography. BMC Cancer 2012, 12, 518. [Google Scholar] [CrossRef]
- Kristiansen, M.; Thorsted, B.L.; Krasnik, A.; von Euler-Chelpin, M. Participation in mammography screening among migrants and non-migrants in Denmark. Acta Oncol. 2012, 51, 28–36. [Google Scholar] [CrossRef]
- Rodríguez-Salés, V.; Roura, E.; Ibañez, R.; Peris, M.; Bosch, F.X.; de Sanjosé, S. Coverage of Cervical Cancer Screening in Catalonia for the Period 2008-2011 among Immigrants and Spanish-Born Women. Front. Oncol. 2013, 3, 297. [Google Scholar] [CrossRef]
- Fischer, R.; Collet, T.-H.; Zeller, A.; Zimmerli, L.; Gaspoz, J.-M.; Giraudon, K.; Rodondi, N.; Cornuz, J. Obesity and overweight associated with lower rates of colorectal cancer screening in Switzerland. Eur. J. Cancer Prev. 2013, 22, 425–430. [Google Scholar] [CrossRef]
- Deutekom, M.; van Rijn, A.F.; Dekker, E.; Blaauwgeers, H.; Stronks, K.; Fockens, P.; Essink-Bot, M.-L. Uptake of faecal occult blood test colorectal cancer screening by different ethnic groups in the Netherlands. Eur. J. Public Health 2009, 19, 400–402. [Google Scholar] [CrossRef]
- Sewitch, M.J.; Fournier, C.; Ciampi, A.; Dyachenko, A. Adherence to colorectal cancer screening guidelines in Canada. BMC Gastroenterol. 2007, 7, 39. [Google Scholar] [CrossRef]
- Wilf-Miron, R.; Peled, R.; Yaari, E.; Vainer, A.; Porath, A.; Kokia, E. The association between socio-demographic characteristics and adherence to breast and colorectal cancer screening: Analysis of large sub populations. BMC Cancer 2011, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.A.; Blackman, D.K.; Wilson, K.M.; Seeff, L.C.; Nadel, M.R. Colorectal cancer test use among Hispanic and non-Hispanic U.S. populations. Prev. Chronic Dis. 2006, 3, A50. [Google Scholar] [PubMed]
- James, T.M.; Greiner, K.A.; Ellerbeck, E.F.; Feng, C.; Ahluwalia, J.S. Disparities in colorectal cancer screening: A guideline-based analysis of adherence. Ethn. Dis. 2006, 16, 228–233. [Google Scholar] [PubMed]
- Ananthakrishnan, A.N.; Schellhase, K.G.; Sparapani, R.A.; Laud, P.W.; Neuner, J.M. Disparities in colon cancer screening in the Medicare population. Arch. Intern. Med. 2007, 167, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt. Bevölkerung und Erwerbstätigkeit. Bevölkerung mit Migrationshintergrund. Ergebnisse des Mikrozensus 2020 (Fachserie 1 Reihe 2.2); Statistisches Bundesamt: Wiesbaden, Germany, 2021. [Google Scholar]
- Berens, E.-M.; Klinger, J.; Mensing, M.; Carol, S.; Schaeffer, D. Gesundheitskompetenz von Menschen mit Migrationshintergrund in Deutschland; Universität Bielefeld: Bielefeld, Germany, 2022. [Google Scholar]
- Woudstra, A.J.; Dekker, E.; Essink-Bot, M.-L.; Suurmond, J. Knowledge, attitudes and beliefs regarding colorectal cancer screening among ethnic minority groups in the Netherlands—A qualitative study. Health Expect. 2016, 19, 1312–1323. [Google Scholar] [CrossRef]
- Brzoska, P.; Wahidie, D.; Yilmaz-Aslan, Y. An Intersectional Perspective on the Utilization of Cervical Cancer Screening among Migrants. A Cross-Sectional Analysis of Survey Data from Austria. Cancers 2021, 13, 6082. [Google Scholar] [CrossRef]
- Cofie, L.E.; Hirth, J.M.; Cuevas, A.G.; Farr, D. A national study of gender and racial differences in colorectal cancer screening among foreign-born older adults living in the US. J. Behav. Med. 2020, 43, 460–467. [Google Scholar] [CrossRef]
- Negi, J.; Nambiar, D. Intersectional social-economic inequalities in breast cancer screening in India: Analysis of the National Family Health Survey. BMC Women’s Health 2021, 21, 324. [Google Scholar] [CrossRef]
- Berens, E.-M.; Mohwinkel, L.-M.; van Eckert, S.; Reder, M.; Kolip, P.; Spallek, J. Uptake of Gynecological Cancer Screening and Performance of Breast Self-Examination Among 50-Year-Old Migrant and Non-migrant Women in Germany: Results of a Cross-Sectional Study (InEMa). J. Immigr. Minority Health 2019, 21, 674–677. [Google Scholar] [CrossRef]
- Berens, E.-M.; Stahl, L.; Yilmaz-Aslan, Y.; Sauzet, O.; Spallek, J.; Razum, O. Participation in breast cancer screening among women of Turkish origin in Germany—A register-based study. BMC Women’s Health 2014, 14, 24. [Google Scholar] [CrossRef][Green Version]
- Kassenärztliche Bundesvereinigung. Organisiertes Darmkrebsscreening: Was Sich in der Praxis Ändert—Ein Überblick. Available online: https://www.kbv.de/media/sp/PraxisInfo_Darmkrebsscreening.pdf (accessed on 31 May 2022).
- AOK Gesundheitsmagazin. Darmkrebsfrüherkennung: Wie zuverlässig sind Stuhltests? Available online: https://www.aok.de/pk/magazin/koerper-psyche/krebs/stuhltest-wie-er-funktioniert-und-was-er-ueber-darmkrebs-aussagt/ (accessed on 31 May 2022).
- Riemann, J.F.; Hüppe, D. Darmkrebsvorsorge—Bewährtes und Neues. Gynäk Praxis 2021, 47, 391–396. [Google Scholar]
- Wiessner, C.; Keil, T.; Krist, L.; Zeeb, H.; Dragano, N.; Schmidt, B.; Ahrens, W.; Berger, K.; Castell, S.; Fricke, J.; et al. Personen mit Migrationshintergrund in der NAKO Gesundheitsstudie–soziodemografische Merkmale und Vergleiche mit der autochthonen deutschen Bevölkerung. Bundesgesundheitsblatt-Gesundh. -Gesundh. 2020, 63, 279–289. [Google Scholar] [CrossRef]
- Yildirim, T. Inanspruchnahme von Präventionsangeboten in der GKV durch türkischstämmige Migranten am Beispiel von Früherkennungs-und U- Untersuchungen; Bielefeld University: Bielefeld, Germany, 2017. [Google Scholar]
- Lange, C.; Jentsch, F.; Allen, J.; Hoebel, J.; Kratz, A.L.; von der Lippe, E.; Müters, S.; Schmich, P.; Thelen, J.; Wetzstein, M.; et al. Data Resource Profile: German Health Update (GEDA)—The health interview survey for adults in Germany. Int. J. Epidemiol. 2015, 44, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. German Health Update: New Data for Germany and Europe the Background to and Methodology Applied in GEDA 2014/2015-EHIS; Robert Koch Institute: Berlin, Germany, 2017. [Google Scholar]
- Lampert, T.; Kroll, L.E.; Müters, S.; Stolzenberg, H. Messung des sozioökonomischen Status in der Studie “Gesundheit in Deutschland aktuell” (GEDA). Bundesgesundheitsblatt-Gesundh. Gesundh. 2013, 56, 131–143. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LP.: College Station, TX, USA, 2017. [Google Scholar]
- Gesink, D.; Mihic, A.; Antal, J.; Filsinger, B.; Racey, C.S.; Perez, D.F.; Norwood, T.; Ahmad, F.; Kreiger, N.; Ritvo, P. Who are the under- and never-screened for cancer in Ontario: A qualitative investigation. BMC Public Health 2014, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.A.; Solarin, I.; Power, E.; Atkin, W.; Wardle, J. Attitudes to colorectal cancer screening among ethnic minority groups in the UK. BMC Public Health 2008, 8, 34. [Google Scholar] [CrossRef]
- Mikkelsen, E.M.; Thomsen, M.K.; Tybjerg, J.; Friis-Hansen, L.; Andersen, B.; Jørgensen, J.C.R.; Baatrup, G.; Njor, S.H.; Mehnert, F.; Rasmussen, M. Colonoscopy-related complications in a nationwide immunochemical fecal occult blood test-based colorectal cancer screening program. Clin. Epidemiol. 2018, 10, 1649–1655. [Google Scholar] [CrossRef]
- Michie, S.; Dormandy, E.; Marteau, T.M. The multi-dimensional measure of informed choice: A validation study. Patient Educ. Couns. 2002, 48, 87–91. [Google Scholar] [CrossRef]
- Marteau, T.M.; Dormandy, E.; Michie, S. A measure of informed choice. Health Expect. 2001, 4, 99–108. [Google Scholar] [CrossRef]
- Berens, E.-M.; Reder, M.; Razum, O.; Kolip, P.; Spallek, J. Informed Choice in the German Mammography Screening Program by Education and Migrant Status: Survey among First-Time Invitees. PLoS ONE 2015, 10, e0142316. [Google Scholar] [CrossRef]
- Gabel, P.; Larsen, M.B.; Edwards, A.; Kirkegaard, P.; Andersen, B. Effectiveness of a decision aid for colorectal cancer screening on components of informed choice according to educational attainment: A randomised controlled trial. PLoS ONE 2020, 15, e0241703. [Google Scholar] [CrossRef]
- Christou, A.; Thompson, S.C. Colorectal cancer screening knowledge, attitudes and behavioural intention among Indigenous Western Australians. BMC Public Health 2012, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Turrin, A.; Zorzi, M.; Giorgi Rossi, P.; Senore, C.; Campari, C.; Fedato, C.; Naldoni, C.; Anghinoni, E.; Carrozzi, G.; De’ Bianchi, P.S.; et al. Colorectal cancer screening of immigrants to Italy. Figures from the 2013 National Survey. Prev. Med. 2015, 81, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.C.; Midgette, L.A.; Mullan, I.D. Colorectal cancer screening preferences among African Americans: Which screening test is preferred? J. Cancer Educ. 2010, 25, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, P.; Razum, O. Migration and occupational health: High work-related burden. Public Health Forum 2015, 23, 113–115. [Google Scholar] [CrossRef]
- Baier, M.; Calonge, N.; Cutter, G.; McClatchey, M.; Schoentgen, S.; Hines, S.; Marcus, A.; Ahnen, D. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol. Biomark. Prev. 2000, 9, 229–232. [Google Scholar]
- Rauscher, G.H.; Johnson, T.P.; Cho, Y.I.; Walk, J.A. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Hoebel, J.; Richter, M.; Lampert, T. Social status and participation in health checks in men and women in Germany: Results from the German Health Update (GEDA), 2009 and 2010. Dtsch. Ärzteblatt Int. 2013, 110, 679–685. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Bevölkerung und Erwerbstätigkeit. Bevölkerung mit Migrationshintergrund. Ergebnisse des Mikrozensus 2015 (Fachserie 1 Reihe 2.2); Statistisches Bundesamt: Wiesbaden, Germany, 2016. [Google Scholar]
- Wang, A.M.Q.; Yung, E.M.; Nitti, N.; Shakya, Y.; Alamgir, A.K.M.; Lofters, A.K. Breast and Colorectal Cancer Screening Barriers Among Immigrants and Refugees: A Mixed-Methods Study at Three Community Health Centres in Toronto, Canada. J. Immigr. Minority Health 2019, 21, 473–482. [Google Scholar] [CrossRef]
- Shapiro, E. Barriers to Colorectal Cancer Screening Among Russian-speaking Immigrants: The Importance of Culture and Home Country Experiences. J. Immigr. Minority Health 2021. [Google Scholar] [CrossRef]
| Population Group | p-Value * | |||
|---|---|---|---|---|
| Non-Migrants | Migrants from EU Countries | Migrants from Non-EU Countries | ||
| N | 10,997 | 426 | 334 | |
| Sex | 0.71 | |||
| Male | 5297 (48.2%) | 197 (46.2%) | 163 (48.8%) | |
| Female | 5700 (51.8%) | 229 (53.8%) | 171 (51.2%) | |
| Age (years) | 0.02 | |||
| 50–54 | 2470 (22.5%) | 89 (20.9%) | 96 (28.7%) | |
| 55–59 | 1766 (16.1%) | 71 (16.7%) | 59 (17.7%) | |
| 60–64 | 1602 (14.6%) | 58 (13.6%) | 48 (14.4%) | |
| 65–69 | 1498 (13.6%) | 74 (17.4%) | 39 (11.7%) | |
| 70–74 | 1496 (13.6%) | 67 (15.7%) | 30 (9.0%) | |
| 75–79 | 1203 (10.9%) | 29 (6.8%) | 34 (10.2%) | |
| 80–84 | 506 (4.6%) | 19 (4.5%) | 12 (3.6%) | |
| 85+ | 456 (4.1%) | 19 (4.5%) | 16 (4.8%) | |
| Partnership status | 0.18 | |||
| Partner | 7807 (71.0%) | 318 (74.6%) | 245 (73.4%) | |
| No partner | 3190 (29.0%) | 108 (25.4%) | 89 (26.6%) | |
| Socioeconomic status | <0.01 | |||
| Low | 2070 (18.8%) | 88 (20.7%) | 110 (32.9%) | |
| Moderate | 6127 (55.7%) | 212 (49.8%) | 141 (42.2%) | |
| High | 2800 (25.5%) | 126 (29.6%) | 83 (24.9%) | |
| Region | <0.01 | |||
| Western Germany | 8079 (73.5%) | 376 (88.3%) | 275 (82.3%) | |
| Eastern Germany | 2918 (26.5%) | 50 (11.7%) | 59 (17.7%) | |
| Type of residential area | <0.01 | |||
| Cities | 3325 (30.2%) | 169 (39.7%) | 165 (49.4%) | |
| Medium-sized towns | 3881 (35.3%) | 185 (43.4%) | 123 (36.8%) | |
| Small towns | 1733 (15.8%) | 40 (9.4%) | 25 (7.5%) | |
| Rural | 2058 (18.7%) | 32 (7.5%) | 21 (6.3%) | |
| Self-rated health status [1 ‘very good’ to 5 ‘very poor’], mean (SD) | 2.4 (0.8) | 2.4 (0.9) | 2.6 (0.9) | <0.01 |
| Utilization of fecal occult blood testing | <0.01 | |||
| No | 1920 (17.5%) | 90 (21.1%) | 116 (34.7%) | |
| Yes (at least once in life time) | 9077 (82.5%) | 336 (78.9%) | 218 (65.3%) | |
| Utilization of colonoscopy | 0.27 | |||
| No | 4388 (39.9%) | 162 (38.0%) | 146 (43.7%) | |
| Yes (at least once in life time) | 6609 (60.1%) | 264 (62.0%) | 188 (56.3%) | |
| Fecal Occult Blood Testing | Colonoscopy | |||
|---|---|---|---|---|
| aOR | [95%-CI] | aOR | [95%-CI] | |
| Population group (Ref.: Non-migrants) | ||||
| Migrants from EU countries | 0.73 | 0.57,0.94 | 1.06 | 0.86,1.31 |
| Migrants from non-EU countries | 0.39 | 0.31,0.50 | 0.88 | 0.70,1.11 |
| Sex (Ref.: Male) | ||||
| Female | 1.27 | 1.15,1.40 | 0.91 | 0.85,0.99 |
| Age | 1.22 | 1.19,1.25 | 1.32 | 1.30,1.35 |
| Partnership status (Ref.: No partner) | ||||
| Partner | 1.41 | 1.27,1.57 | 1.32 | 1.21,1.44 |
| Socioeconomic status (Ref.: Low) | ||||
| Moderate | 1.36 | 1.20,1.54 | 1.32 | 1.19,1.46 |
| High | 1.51 | 1.31,1.76 | 1.45 | 1.28,1.63 |
| Region (Ref.: Western Germany) | ||||
| Easters Germany | 0.70 | 0.62,0.79 | 0.89 | 0.80,0.98 |
| Type of residential area (Ref.: Cities) | ||||
| Medium-sized towns | 0.96 | 0.85,1.09 | 0.99 | 0.89,1.09 |
| Small towns | 0.99 | 0.85,1.15 | 0.97 | 0.86,1.10 |
| Rural | 0.99 | 0.85,1.15 | 0.91 | 0.81,1.03 |
| Self-rated health status [1 ‘very good’ to 5 ‘very poor’] | 1.20 | 1.12,1.28 | 1.25 | 1.18,1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahidie, D.; Yilmaz-Aslan, Y.; Brzoska, P. Participation in Colorectal Cancer Screening among Migrants and Non-Migrants in Germany: Results of a Population Survey. Gastrointest. Disord. 2022, 4, 97-107. https://doi.org/10.3390/gidisord4030011
Wahidie D, Yilmaz-Aslan Y, Brzoska P. Participation in Colorectal Cancer Screening among Migrants and Non-Migrants in Germany: Results of a Population Survey. Gastrointestinal Disorders. 2022; 4(3):97-107. https://doi.org/10.3390/gidisord4030011
Chicago/Turabian StyleWahidie, Diana, Yüce Yilmaz-Aslan, and Patrick Brzoska. 2022. "Participation in Colorectal Cancer Screening among Migrants and Non-Migrants in Germany: Results of a Population Survey" Gastrointestinal Disorders 4, no. 3: 97-107. https://doi.org/10.3390/gidisord4030011
APA StyleWahidie, D., Yilmaz-Aslan, Y., & Brzoska, P. (2022). Participation in Colorectal Cancer Screening among Migrants and Non-Migrants in Germany: Results of a Population Survey. Gastrointestinal Disorders, 4(3), 97-107. https://doi.org/10.3390/gidisord4030011





