Tumor Regression in Lymph Node Metastases of Esophageal Adenocarcinomas after Neoadjuvant Therapy
Abstract
1. Introduction
2. Results
2.1. Pathologic Data
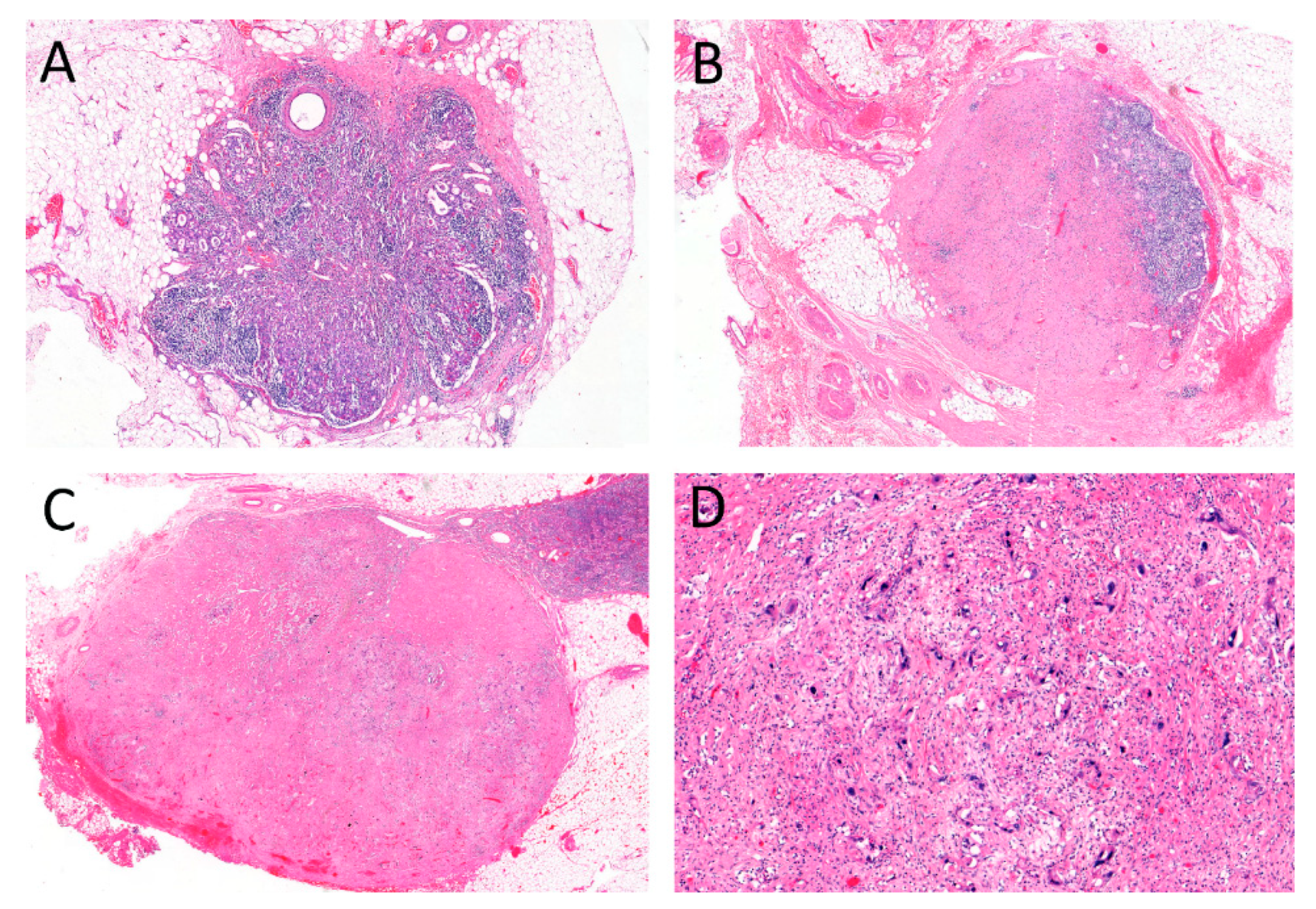
2.2. Regressive Changes in Lymph Nodes
2.3. Correlation with Pathological Data
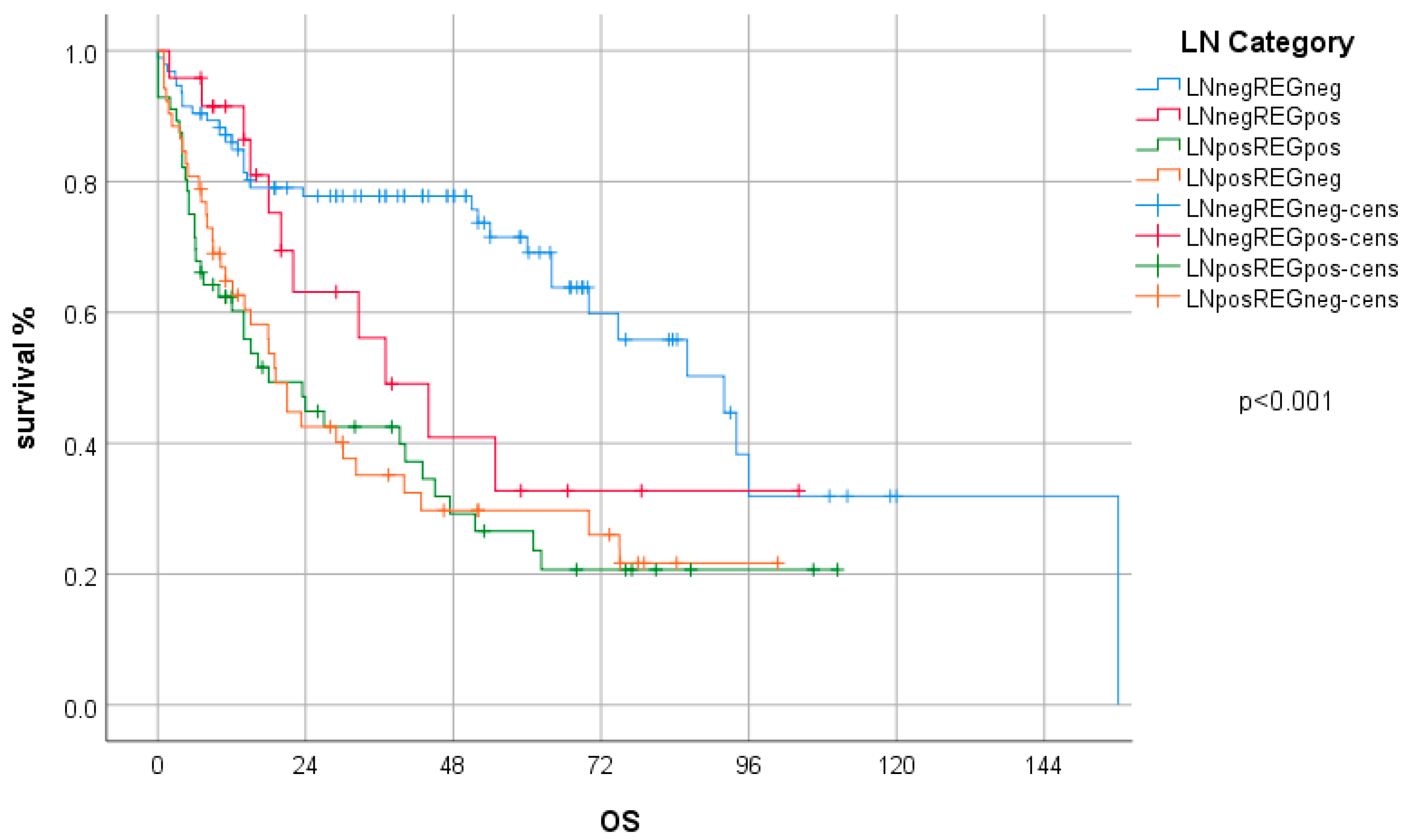
2.4. Survival Analysis
2.5. Subgroup Analysis—Case Collections and Type of Neoadjuvant Treatment
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Post Neoadjuvant TNM Staging and Tumor Regression Grading
4.3. Evaluation of Tumor Regression in Lymph Node Metastases
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Pasquali, S.; Yim, G.; Vohra, R.S.; Mocellin, S.; Nyanhongo, G.S.; Marriott, P.; Geh, J.I.; Griffiths, E.A. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma. Ann. Surg. 2017, 265, 481–491. [Google Scholar] [CrossRef]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Kieser, M.; Slanger, T.E.; Jensen, K. GE Adenocarcinoma meta-Analysis Group. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Gebski, V.; Burmeister, B.; Smithers, B.M.; Foo, K.; Zalcberg, J.; Simes, J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007, 8, 226–234. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Klevebro, F.; Johnsen, G.; Johnson, E.; Viste, A.; Myrnäs, T.; Szabo, E.; Jacobsen, A.-B.; Friesland, S.; Tsai, J.; Persson, S.; et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 920–926. [Google Scholar] [CrossRef]
- Klevebro, F.; Von Döbeln, G.A.; Wang, N.; Johnsen, G.; Jacobsen, A.-B.; Friesland, S.; Hatlevoll, I.; Glenjen, N.I.; Lind, P.; Tsai, J.A.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.-C.; Marnay, J.; Henry-Amar, M.; Petiot, J.-F.; Roussel, A.; Jacob, J.-H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Becker, K.; Mueller, J.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Böttcher, K.; Siewert, J.R.; Höfler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef]
- Davies, A.; Myoteri, D.; Zylstra, J.; Baker, C.R.; Wulaningsih, W.; Van Hemelrijck, M.; Maisey, N.; Allum, W.H.; Smyth, E.; A Gossage, J.; et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br. J. Surg. 2018, 105, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Langer, R.; Reim, D.; Novotny, A.; Buschenfelde, C.M.Z.; Engel, J.; Friess, H.; Hofler, H. Significance of Histopathological Tumor Regression After Neoadjuvant Chemotherapy in Gastric Adenocarcinomas. Ann. Surg. 2011, 253, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Fassan, M.; Cunningham, D.; Allum, W.H.; Okines, A.F.; Lampis, A.; Hahne, J.C.; Rugge, M.; Peckitt, C.; Nankivell, M.; et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J. Clin. Oncol. 2016, 34, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Talsma, A.; Ong, C.-A.J.; Liu, X.; Van Hagen, P.; Van Lanschot, J.J.B.; Tilanus, H.W.; Hardwick, R.H.; Carroll, N.R.; Spaander, M.C.W.; Fitzgerald, R.C.; et al. Location of Lymph Node Involvement in Patients with Esophageal Adenocarcinoma Predicts Survival. World J. Surg. 2013, 38, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Leers, J.M.; Demeester, S.R.; Chan, N.; Ayazi, S.; Oezcelik, A.; Abate, E.; Banki, F.; Lipham, J.C.; Hagen, J.A.; Demeester, T.R. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. J. Thorac. Cardiovasc. Surg. 2009, 138, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Jones, M.; Gonen, M.; Gotley, D.C.; Thomas, J.; Thomson, D.B.; Burmeister, B.; Smithers, B.M. Refining Esophageal Cancer Staging After Neoadjuvant Therapy: Importance of Treatment Response. Ann. Surg. Oncol. 2008, 15, 2894–2902. [Google Scholar] [CrossRef]
- Hoelscher, A.; Bollschweiler, E.; Bogoevski, D.; Schmidt, H.; Semrau, R.; Izbicki, J. Prognostic impact of neoadjuvant chemoradiation in cT3 oesophageal cancer—A propensity score matched analysis. Eur. J. Cancer 2014, 50, 2950–2957. [Google Scholar] [CrossRef]
- Bollschweiler, E.; Metzger, R.; Drebber, U.; Baldus, S.; Vallböhmer, D.; Kocher, M.; Hölscher, A.H. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann. Oncol. 2009, 20, 231–238. [Google Scholar] [CrossRef]
- Meredith, K.L.; Weber, J.M.; Turaga, K.K.; Siegel, E.M.; McLoughlin, J.; Hoffe, S.; Marcovalerio, M.; Shah, N.; Kelley, S.; Karl, R. Pathologic Response after Neoadjuvant Therapy is the Major Determinant of Survival in Patients with Esophageal Cancer. Ann. Surg. Oncol. 2010, 17, 1159–1167. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Detlefsen, S.; Riddell, R.; Conner, J.; Mastracci, L.; Sheahan, K.; Shetye, J.; Lundell, L.; Vieth, M. Histopathologic tumor regression grading in patients with gastric carcinoma submitted to neoadjuvant treatment: Results of a Delphi survey. Hum. Pathol. 2019, 84, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.R.; Peyre, C.G.; Watson, T.J.; Cao, W.; Lunt, M.D.; Lada, M.J.; Han, M.S.; Jones, C.E.; Peters, J.H. Neoadjuvant Treatment Response in Negative Nodes Is an Important Prognosticator After Esophagectomy. Ann. Thorac. Surg. 2015, 99, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Becker, K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018, 472, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ott, K.; Weber, W.A.; Lordick, F.; Becker, K.; Busch, R.; Herrmann, K.; Wieder, H.; Fink, U.; Schwaiger, M.; Siewert, J.-R. Metabolic Imaging Predicts Response, Survival, and Recurrence in Adenocarcinomas of the Esophagogastric Junction. J. Clin. Oncol. 2006, 24, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Van Heijl, M.; Omloo, J.M.; Henegouwen, M.I.V.B.; Hoekstra, O.S.; Boellaard, R.; Bossuyt, P.M.; Busch, O.; Tilanus, H.W.; Hulshof, M.C.; Van Der Gaast, A.; et al. Fluorodeoxyglucose Positron Emission Tomography for Evaluating Early Response During Neoadjuvant Chemoradiotherapy in Patients With Potentially Curable Esophageal Cancer. Ann. Surg. 2011, 253, 56–63. [Google Scholar] [CrossRef]
- Omloo, J.M.T.; Van Heijl, M.; Hoekstra, O.S.; Henegouwen, M.I.V.B.; Van Lanschot, J.J.B.; Sloof, G.W. FDG-PET parameters as prognostic factor in esophageal cancer patients: A review. Ann. Surg. Oncol. 2011, 18, 3338–3352. [Google Scholar] [CrossRef]
- Piessen, G.; Petyt, G.; Duhamel, A.; Mirabel, X.; Huglo, D.; Mariette, C. Ineffectiveness of 18F-Fluorodeoxyglucose Positron Emission Tomography in the Evaluation of Tumor Response After Completion of Neoadjuvant Chemoradiation in Esophageal Cancer. Ann. Surg. 2013, 258, 66–76. [Google Scholar] [CrossRef]
- Reim, D.; Novotny, A.; Friess, H.; Slotta-Huspenina, J.; Weichert, W.; Ott, K.; Dislich, B.; Lorenzen, S.; Becker, K.; Langer, R. Significance of tumour regression in lymph node metastases of gastric and gastro-oesophageal junction adenocarcinomas. J. Pathol. Clin. Res. 2020. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Granja, M.; Sastre, J.; García-Paredes, B.; Estrada, L. Prognostic significance of tumor regression in lymph nodes after neoadjuvant therapy for rectal carcinoma. Virchows Arch. 2016, 468, 425–430. [Google Scholar] [CrossRef]
- Philippron, A.; Bollschweiler, E.; Kunikata, A.; Plum, P.; Schmidt, C.; Favi, F.; Drebber, U.; Hölscher, A.H. Prognostic Relevance of Lymph Node Regression After Neoadjuvant Chemoradiation for Esophageal Cancer. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chang, H.J.; Kim, D.Y.; Park, J.W.; Baek, J.Y.; Kim, S.Y.; Park, S.C.; Oh, J.H.; Yu, A.; Nam, B.-H. What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy? Cancer Res. Treat. 2016, 48, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Sannier, A.; Lefevre, J.H.; Panis, Y.; Cazals-Hatem, D.; Bedossa, P.; Guedj, N. Pathological prognostic factors in locally advanced rectal carcinoma after neoadjuvant radiochemotherapy: Analysis of 113 cases. Histopathology 2014, 65, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Noble, F.; Lloyd, M.A.; Turkington, R.; Griffiths, E.; O’Donovan, M.; O’Neill, J.R.; Mercer, S.; Parsons, S.L.; Fitzgerald, R.C.; Underwood, T.J.; et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br. J. Surg. 2017, 104, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Bollschweiler, E.; Hölscher, A.H.; Metzger, R.; Besch, S.; Moenig, S.P.; E Baldus, S.; Drebber, U. Prognostic Significance of a New Grading System of Lymph Node Morphology After Neoadjuvant Radiochemotherapy for Esophageal Cancer. Ann. Thorac. Surg. 2011, 92, 2020–2027. [Google Scholar] [CrossRef]
- Donohoe, C.L.; O’farrell, N.J.; Grant, T.; King, S.; Clarke, L.; Muldoon, C.; Reynolds, J.V. Classification of Pathologic Response to Neoadjuvant Therapy in Esophageal and Junctional Cancer. Ann. Surg. 2013, 258, 784–792. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Sun, Y.-K.; Xue, X.-M.; Yue, J.-Y.; Yang, L.; Xue, L. Unnecessity of lymph node regression evaluation for predicting gastric adenocarcinoma outcome after neoadjuvant chemotherapy. World J. Gastrointest. Oncol. 2019, 11, 48–58. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Sola, J.J.; A Diaz-Gonzalez, J.; Chopitea, A.; Iragorri, Y.; Martínez-Regueira, F.; Ponz-Sarvise, M.; Arbea, L.; Subtil, J.C.; Cano, D.; et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br. J. Cancer 2016, 115, 655–663. [Google Scholar] [CrossRef]
- Westerhoff, M.; Osecky, M.; Langer, R. Varying practices in tumor regression grading of gastrointestinal carcinomas after neoadjuvant therapy: Results of an international survey. Mod. Pathol. 2019, 33, 676–689. [Google Scholar] [CrossRef]
- Digestive System Tumours. WHO Classification of Tumours Editorial Board, 5th ed.; WHO: Geneva, Switzerland, 2019; Volume 1. [Google Scholar]
- Kröll, D.; Noser, L.; Erdem, S.; Storni, F.; Arnold, D.; Dislich, B.; Zlobec, I.; Candinas, D.; Seiler, C.A.; Langer, R.; et al. Application of the 8th edition of the AJCC yTNM staging system shows improved prognostication in a single center cohort of esophageal carcinomas. Surg. Oncol. 2018, 27, 100–105. [Google Scholar] [CrossRef]
- Langer, R.; Ott, K.; Feith, M.; Lordick, F.; Siewert, J.-R.; Becker, K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod. Pathol. 2009, 22, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley: Hoboken, NI, USA, 2017. [Google Scholar]
| Total | Cohorts | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Bern | Munich | |||||
| N | % | N | % | N | % | ||
| ypT category | ypT0 | 43 | 18 | 36 | 24.5 | 7 | 7.6 |
| ypT1 | 29 | 12.1 | 20 | 13.6 | 9 | 9.8 | |
| ypT2 | 47 | 19.7 | 28 | 19 | 19 | 20.7 | |
| ypT3 | 116 | 48.5 | 59 | 40.1 | 57 | 61.9 | |
| ypT4 | 4 | 1.7 | 4 | 20.7 | 0 | 0 | |
| ypN category | ypN0 | 122 | 51 | 87 | 59.2 | 35 | 38 |
| ypN1 | 50 | 20.9 | 31 | 21.1 | 19 | 20.7 | |
| ypN2 | 38 | 15.9 | 20 | 13.6 | 18 | 19.6 | |
| ypN3 | 29 | 12.1 | 9 | 6.1 | 20 | 21.7 | |
| distant Metastases | M0 | 215 | 90 | 136 | 92.5 | 79 | 85.9 |
| M1 | 24 | 10 | 11 | 7.5 | 13 | 14.1 | |
| resection status | R0 | 201 | 84.1 | 138 | 93.9 | 63 | 68.5 |
| R1/2 | 38 | 15.9 | 9 | 6.1 | 29 | 31.5 | |
| neoadjuvant therapy | CTX | 114 | 47.7 | 22 | 15 | 92 | 100 |
| RCTX | 125 | 52.3 | 125 | 85 | 0 | 0 | |
| TRG (Becker) primary tumor | 1a | 43 | 18 | 36 | 24.5 | 7 | 7.6 |
| 1b | 78 | 32 | 56 | 38.1 | 22 | 23.9 | |
| 2 | 51 | 21.3 | 25 | 17 | 26 | 28.3 | |
| 3 | 67 | 28 | 30 | 20.4 | 37 | 40.2 | |
| LN category | LN−/REG− | 97 | 40.6 | 69 | 46.9 | 28 | 30.4 |
| LN−/REG+ | 25 | 10.5 | 18 | 12.2 | 7 | 7.6 | |
| LN+/REG+ | 60 | 25.1 | 36 | 24.5 | 24 | 26.1 | |
| LN+/REG− | 57 | 23.8 | 24 | 16.3 | 33 | 35.9 | |
| TRG (Becker) | Total | |||||
|---|---|---|---|---|---|---|
| 1a | 1b | 2 | 3 | |||
| LN category | LN−/REG− | 32 | 38 | 17 | 10 | 97 |
| LN−/REG+ | 7 | 12 | 2 | 4 | 25 | |
| LN+/REG+ | 4 | 17 | 16 | 23 | 60 | |
| LN+/REG− | 0 | 11 | 16 | 30 | 57 | |
| total | 43 | 78 | 51 | 67 | 239 | |
| HR | 95% CI for HR | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| ypTcategory | 1.118 | 0.874 | 1.430 | 0.376 |
| TRG (Becker) | 1.094 | 0.845 | 1.417 | 0.496 |
| LN category | 1.324 | 1.108 | 1.583 | 0.002 |
| distant metastases | 2.492 | 1.412 | 4.400 | 0.002 |
| resection status | 0.754 | 0.444 | 1.280 | 0.295 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osecky, M.; Kröll, D.; Feith, M.; Reim, D.; Dislich, B.; Becker, K.; Langer, R. Tumor Regression in Lymph Node Metastases of Esophageal Adenocarcinomas after Neoadjuvant Therapy. Gastrointest. Disord. 2020, 2, 397-407. https://doi.org/10.3390/gidisord2040036
Osecky M, Kröll D, Feith M, Reim D, Dislich B, Becker K, Langer R. Tumor Regression in Lymph Node Metastases of Esophageal Adenocarcinomas after Neoadjuvant Therapy. Gastrointestinal Disorders. 2020; 2(4):397-407. https://doi.org/10.3390/gidisord2040036
Chicago/Turabian StyleOsecky, Marek, Dino Kröll, Marcus Feith, Daniel Reim, Bastian Dislich, Karen Becker, and Rupert Langer. 2020. "Tumor Regression in Lymph Node Metastases of Esophageal Adenocarcinomas after Neoadjuvant Therapy" Gastrointestinal Disorders 2, no. 4: 397-407. https://doi.org/10.3390/gidisord2040036
APA StyleOsecky, M., Kröll, D., Feith, M., Reim, D., Dislich, B., Becker, K., & Langer, R. (2020). Tumor Regression in Lymph Node Metastases of Esophageal Adenocarcinomas after Neoadjuvant Therapy. Gastrointestinal Disorders, 2(4), 397-407. https://doi.org/10.3390/gidisord2040036







