Allopurinol Suppresses Azoxymethane-Induced Colorectal Tumorigenesis in C57BL/KsJ-db/db Mice
Abstract
1. Introduction
2. Results
2.1. General Observations
2.2. Serum Parameters
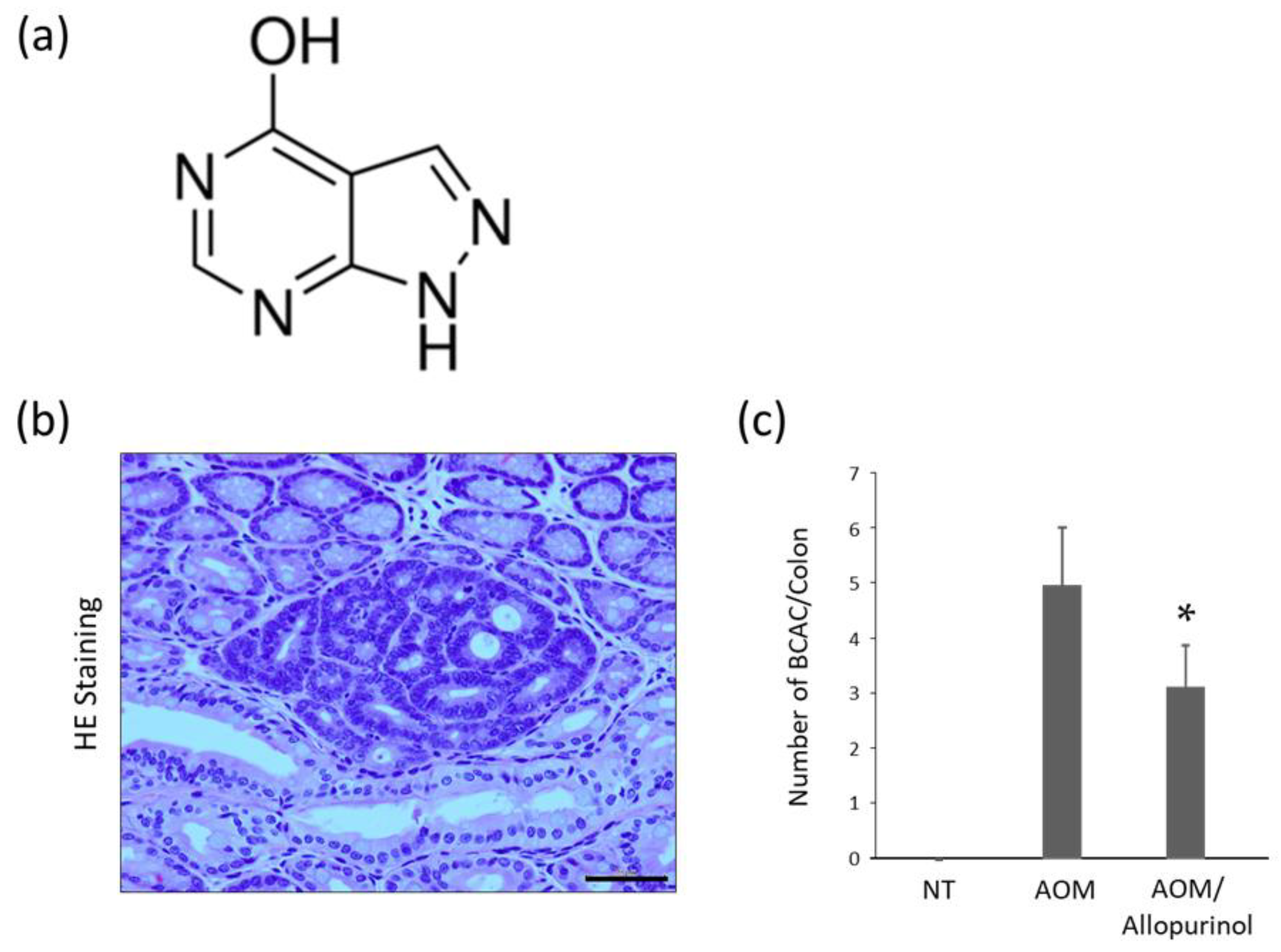
2.3. AOM-Induced Colorectal Pre-Neoplastic Lesions in the Experimental Mice
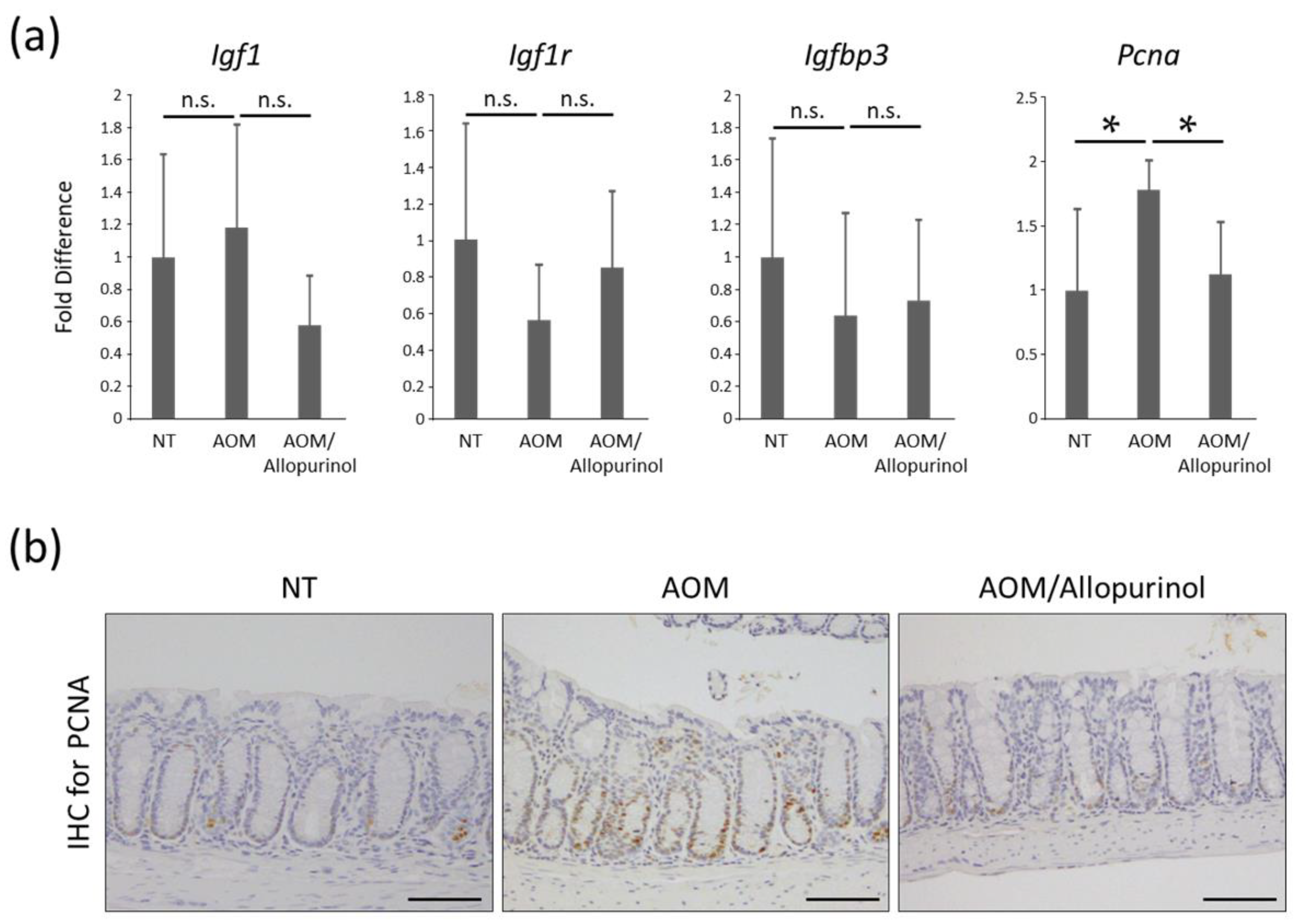
2.4. The Expression Levels of mRNAs in Colonic Mucosa of Experimental Mice
2.5. Cellular Proliferation in Colonic Mucosa of Experimental Mice
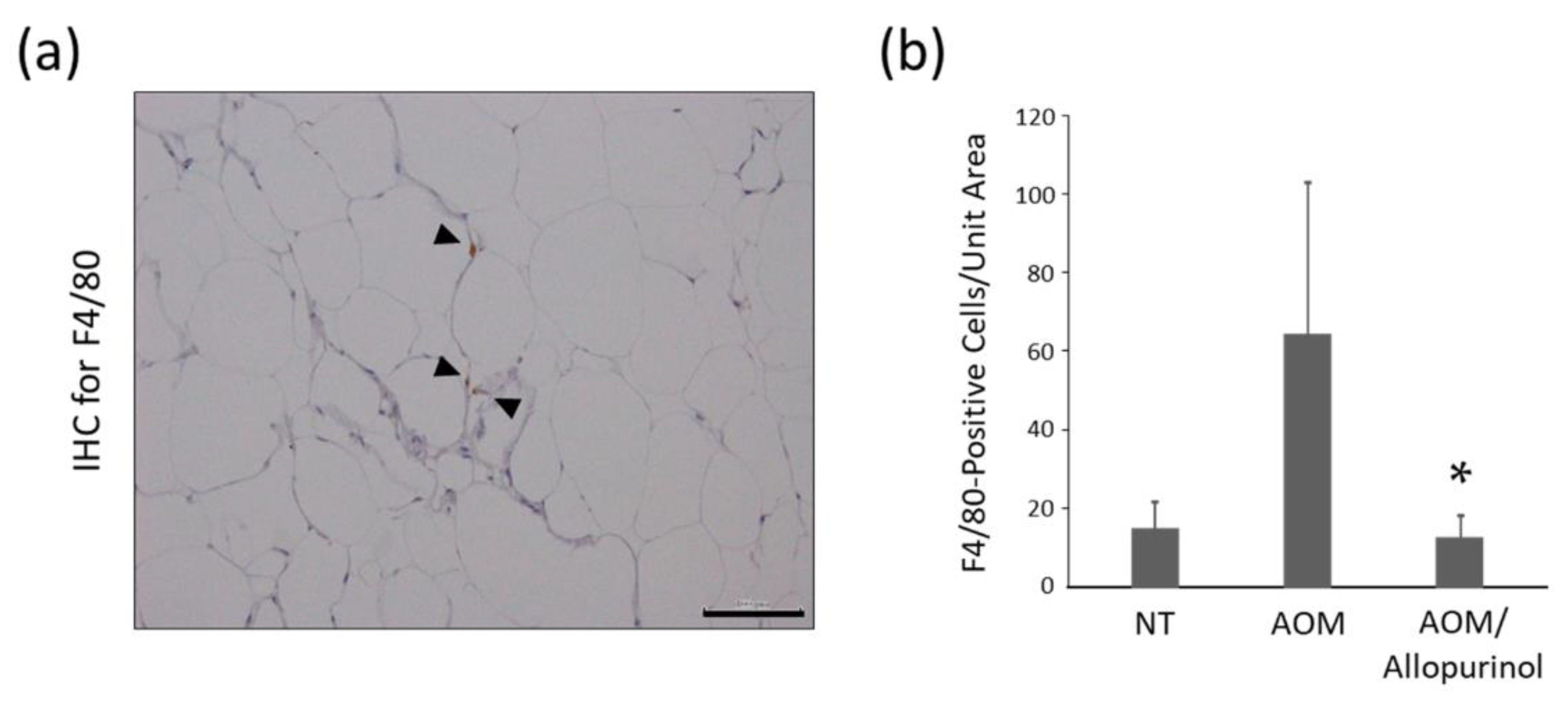
2.6. Immunohistochemistry for F4/80 in Adipose Tissue of the Experimental Mice
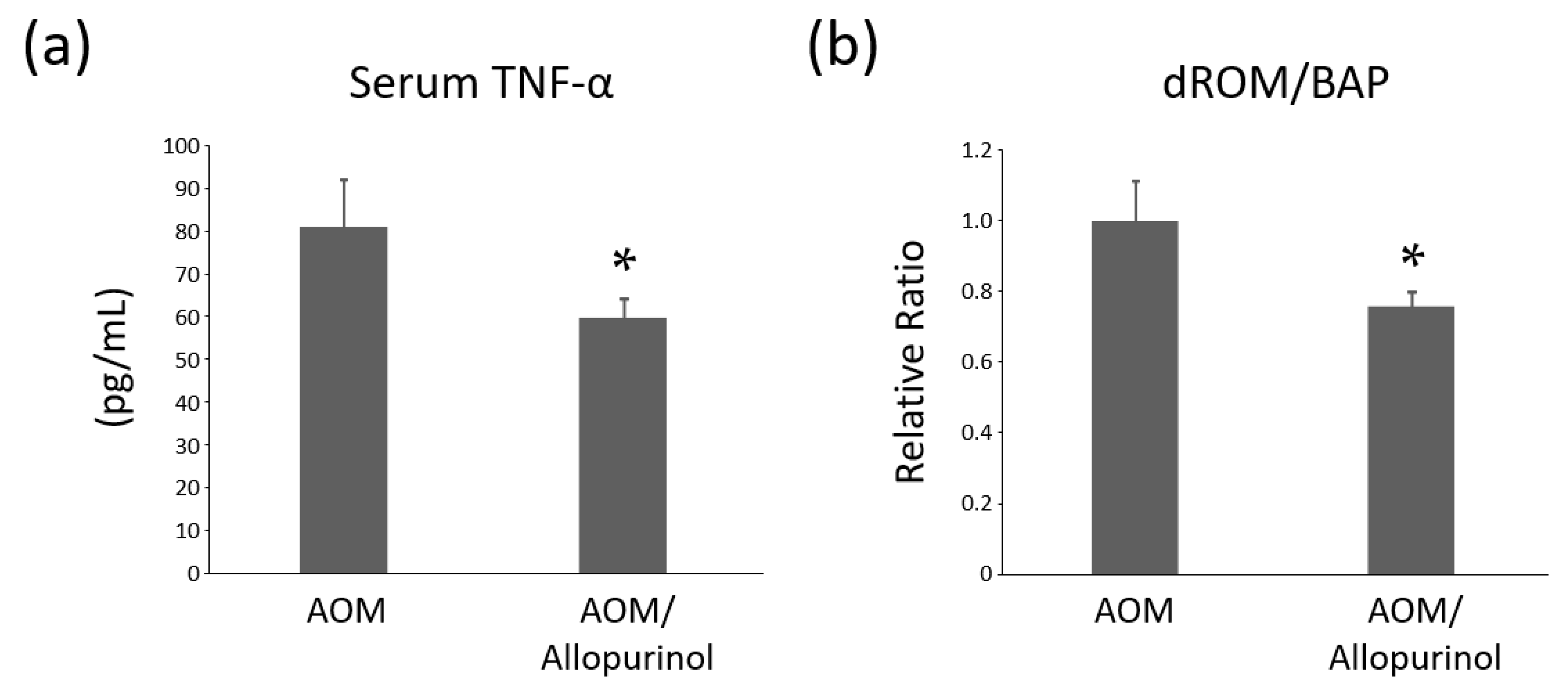
2.7. Serum Levels of TNF-α in the Experimental Mice
2.8. Systemic Oxidative Stress Levels of the Experimental Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Care Conditions
4.2. Chemicals and Administration Methods
4.3. Experimental Procedure
4.4. Histopathological Examination
4.5. Clinical Chemistry
4.6. Extraction of mRNA and Quantitative Real-Time RT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- González, N.; Prieto, I.; Del Puerto-Nevado, L.; Portal-Nuñez, S.; Ardura, J.A.; Corton, M.; Fernández-Fernández, B.; Aguilera, O.; Gomez-Guerrero, C.; Mas, S.; et al. 2017 update on the relationship between diabetes and colorectal cancer: Epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget 2017, 8, 18456–18485. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am. J. Clin. Nutr. 2007, 86, s836–s842. [Google Scholar] [CrossRef]
- Giovannucci, E.; Michaud, D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007, 132, 2208–2225. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: A multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Mikami, T.; Sorimachi, M. Uric acid contributes greatly to hepatic antioxidant capacity besides protein. Physiol. Res. 2017, 66, 1001–1007. [Google Scholar] [CrossRef]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Trisha, A.D.; Hasan, A.; Islam, F. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020, 10, 7841. [Google Scholar] [CrossRef]
- Hammarsten, J.; Damber, J.-E.; Peeker, R.; Mellström, D.; Högstedt, B. A higher prediagnostic insulin level is a prospective risk factor for incident prostate cancer. Cancer Epidemiol. 2010, 34, 574–579. [Google Scholar] [CrossRef]
- Siddiqui, A.A. Metabolic syndrome and its association with colorectal cancer: A review. Am. J. Med. Sci. 2011, 341, 227–231. [Google Scholar] [CrossRef]
- Bjørge, T.; Lukanova, A.; Jonsson, H.; Tretli, S.; Ulmer, H.; Manjer, J.; Stocks, T.; Selmer, R.; Nagel, G.; Almquist, M.; et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2010, 19, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.S. Oxygen-derived free-radical scavengers prolong survival in colonic cancer. Chemotherapy 1992, 38, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-J.; Kao, M.-C.; Tsai, P.-S.; Fan, Y.-C.; Huang, C.-J. Long-term allopurinol use decreases the risk of prostate cancer in patients with gout: A population-based study. Prostate Cancer Prostatic Dis. 2017, 20, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kukko, V.; Kaipia, A.; Talala, K.; Taari, K.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Allopurinol and the risk of prostate cancer in a Finnish population-based cohort. Prostate Cancer Prostatic Dis. 2019, 22, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Yoshida, T.; Goda, A.E.; Horinaka, M.; Yano, K.; Shiraishi, T.; Wakada, M.; Mizutani, Y.; Miki, T.; Sakai, T. Anti-gout agent allopurinol exerts cytotoxicity to human hormone-refractory prostate cancer cells in combination with tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Res. 2008, 6, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, K.; Shirakami, Y.; Maruta, A.; Obara, K.; Iritani, S.; Nakamura, N.; Kochi, T.; Kubota, M.; Sakai, H.; Tanaka, T.; et al. Preventive Effects of Pentoxifylline on the Development of Colonic Premalignant Lesions in Obese and Diabetic Mice. Int. J. Mol. Sci. 2017, 18, 413. [Google Scholar] [CrossRef]
- Kato, J.; Shirakami, Y.; Ohnishi, M.; Mizutani, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Tanaka, T.; Shimizu, M. Suppressive effects of the sodium-glucose cotransporter 2 inhibitor tofogliflozin on colorectal tumorigenesis in diabetic and obese mice. Oncol. Rep. 2019, 42, 2797–2805. [Google Scholar] [CrossRef]
- Kato, J.; Shirakami, Y.; Mizutani, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Shimizu, M. Alpha-Glucosidase Inhibitor Voglibose Suppresses Azoxymethane-Induced Colonic Preneoplastic Lesions in Diabetic and Obese Mice. Int. J. Mol. Sci. 2020, 21, 2226. [Google Scholar] [CrossRef]
- Kosugi, T.; Nakayama, T.; Heinig, M.; Zhang, L.; Yuzawa, Y.; Sanchez-Lozada, L.G.; Roncal, C.; Johnson, R.J.; Nakagawa, T. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am. J. Physiol. Renal Physiol. 2009, 297, F481–F488. [Google Scholar] [CrossRef]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112–113, 395–402. [Google Scholar] [CrossRef]
- Yamada, Y.; Mori, H. Pre-cancerous lesions for colorectal cancers in rodents: A new concept. Carcinogenesis 2003, 24, 1015–1019. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db Mice. Cancer Prev. Res. 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kochi, T.; Shimizu, M.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tanaka, T.; Moriwaki, H. Inhibitory effects of astaxanthin on azoxymethaneinduced colonic preneoplastic lesions in C57/BL/KsJ-db/db mice. BMC Gastroenterol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-α as a tumour promoter. Eur. J. Cancer 2006. [Google Scholar] [CrossRef]
- Kochi, T.; Shimizu, M.; Ohno, T.; Baba, A.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Preventive effects of the angiotensin-converting enzyme inhibitor, captopril, on the development of azoxymethane-induced colonic preneoplastic lesions in diabetic and hypertensive rats. Oncol. Lett. 2014, 8, 223–229. [Google Scholar] [CrossRef]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Leitzmann, M.F. Obesity and colorectal cancer: Epidemiology, mechanisms and candidate genes. J. Nutr. Biochem. 2006, 17, 145–156. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W.J. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H.J. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Schetter, A.J.; Heegaard, N.H.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.-Q.; Gu, T.-T.; Song, L.; Li, J.-M.; Xue, Q.-C.; Kong, L.-D. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic. Biol. Med. 2015, 83, 214–226. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.-P.; Qu, Y.; Jie, L.-G.; Deng, J.-X.; Yu, Q.-H. Efficacy of uric acid-lowering therapy on hypercholesterolemia and hypertriglyceridemia in gouty patients. Int. J. Rheum. Dis. 2019, 22, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- de Castro, V.M.F.; de Melo, A.C.; Belo, V.S.; Chaves, V.E. Effect of allopurinol and uric acid normalization on serum lipids hyperuricemic subjects: A systematic review with meta-analysis. Clin. Biochem. 2017, 50, 1289–1297. [Google Scholar] [CrossRef]
- Hirose, Y.; Hata, K.; Kuno, T.; Yoshida, K.; Sakata, K.; Yamada, Y.; Tanaka, T.; Reddy, B.S.; Mori, H. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis 2004, 25, 821–825. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Iwasa, J.; Shiraki, M.; Yasuda, Y.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin. Cancer Res. 2009, 15. [Google Scholar] [CrossRef]
- Yasuda, Y.; Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010, 101, 1701–1707. [Google Scholar] [CrossRef]
- Kubota, M.; Shimizu, M.; Sakai, H.; Yasuda, Y.; Ohno, T.; Kochi, T.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 2011, 410, 108–113. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Kubota, M.; Ohno, T.; Kochi, T.; Nakamura, N.; Sumi, T.; Tanaka, T.; Moriwaki, H.; Seishima, M. Pentoxifylline prevents nonalcoholic steatohepatitis-related liver pre-neoplasms by inhibiting hepatic inflammation and lipogenesis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ 2016, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Gottesman, M.E.; Blaner, W.S. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin: Retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis 2012, 33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Group | Treatment | Number of Mice | Body Weight (g) | Relative Weight (g/100 g Body Weight) of: | ||
|---|---|---|---|---|---|---|
| Liver | Kidneys | Adipose Tissue a | ||||
| 1 | No treatment | 6 | 41.4 ± 12.5 b | 6.9 ± 1.2 | 1.4 ± 0.5 | 5.1 ± 1.0 |
| 2 | AOM | 12 | 46.8 ± 2.3 | 4.9 ± 0.3 d | 1.0 ± 0.1 | 5.9 ± 0.6 |
| 3 | AOM/allopurinol | 12 | 39.6 ± 3.8 c | 4.9 ± 0.3 d | 1.2 ± 0.1 | 5.6 ± 0.5 |
| 4 | Allopurinol | 6 | 34.0 ± 3.0 | 5.2 ± 0.7 | 1.3 ± 0.2 | 6.3 ± 0.7 |
| Group | Treatment | ALT (IU/L) | FFA (µEQ/L) | Triglyceride (mg/dL) | UA (mg/mL) |
|---|---|---|---|---|---|
| 1 | No treatment | 11.3 ± 7.5 a | 246.2 ± 129.0 | 6.5 ± 2.3 | 8.5 ± 2.0 |
| 2 | AOM | 15.7 ± 4.5 | 340.1 ± 59.0 | 5.0 ± 2.4 | 7.4 ± 0.5 |
| 3 | AOM/allopurinol | 9.9 ± 2.9 b | 249.6 ± 65.7 b | 4.5 ± 2.0 | 2.2 ± 2.0 b,c |
| 4 | Allopurinol | 5.7 ± 3.4 | 299.5 ± 75.7 | 5.0 ± 1.8 | 1.5 ± 0.8 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, J.; Shirakami, Y.; Yamaguchi, K.; Mizutani, T.; Ideta, T.; Nakamura, H.; Ninomiya, S.; Kubota, M.; Sakai, H.; Ibuka, T.; et al. Allopurinol Suppresses Azoxymethane-Induced Colorectal Tumorigenesis in C57BL/KsJ-db/db Mice. Gastrointest. Disord. 2020, 2, 385-396. https://doi.org/10.3390/gidisord2040035
Kato J, Shirakami Y, Yamaguchi K, Mizutani T, Ideta T, Nakamura H, Ninomiya S, Kubota M, Sakai H, Ibuka T, et al. Allopurinol Suppresses Azoxymethane-Induced Colorectal Tumorigenesis in C57BL/KsJ-db/db Mice. Gastrointestinal Disorders. 2020; 2(4):385-396. https://doi.org/10.3390/gidisord2040035
Chicago/Turabian StyleKato, Junichi, Yohei Shirakami, Kimihiro Yamaguchi, Taku Mizutani, Takayasu Ideta, Hiroshi Nakamura, Soranobu Ninomiya, Masaya Kubota, Hiroyasu Sakai, Takashi Ibuka, and et al. 2020. "Allopurinol Suppresses Azoxymethane-Induced Colorectal Tumorigenesis in C57BL/KsJ-db/db Mice" Gastrointestinal Disorders 2, no. 4: 385-396. https://doi.org/10.3390/gidisord2040035
APA StyleKato, J., Shirakami, Y., Yamaguchi, K., Mizutani, T., Ideta, T., Nakamura, H., Ninomiya, S., Kubota, M., Sakai, H., Ibuka, T., Tanaka, T., & Shimizu, M. (2020). Allopurinol Suppresses Azoxymethane-Induced Colorectal Tumorigenesis in C57BL/KsJ-db/db Mice. Gastrointestinal Disorders, 2(4), 385-396. https://doi.org/10.3390/gidisord2040035







