Preparation and Characterization of a Dual-Layer Coating with Synergistic Ionic Selectivity and Photocathodic Protection Property
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Surface and Chemical Characterization
2.3. Photoelectrochemical Properties Characterization
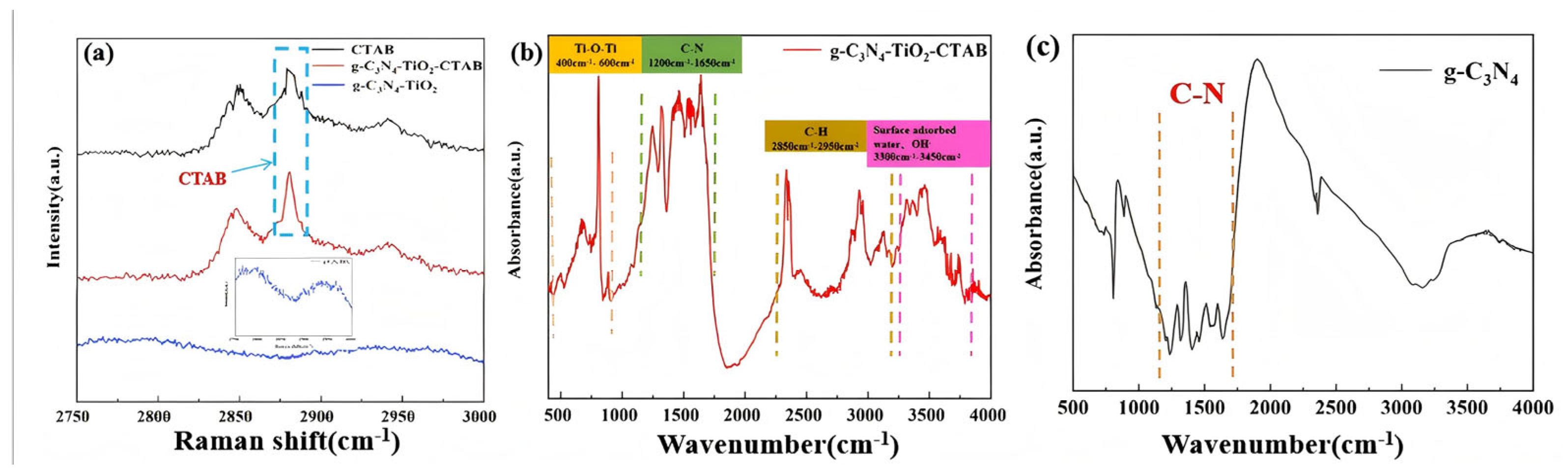
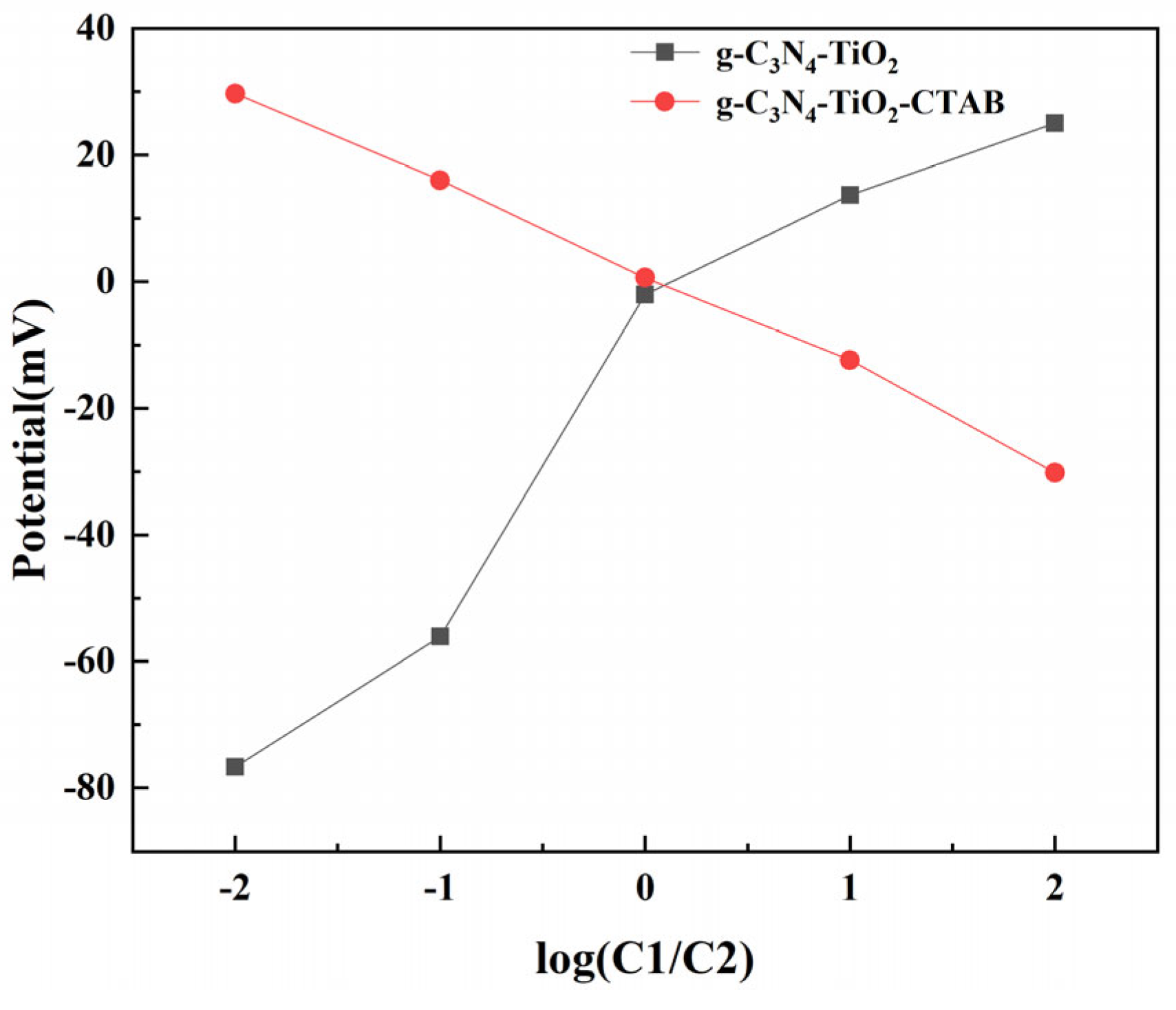
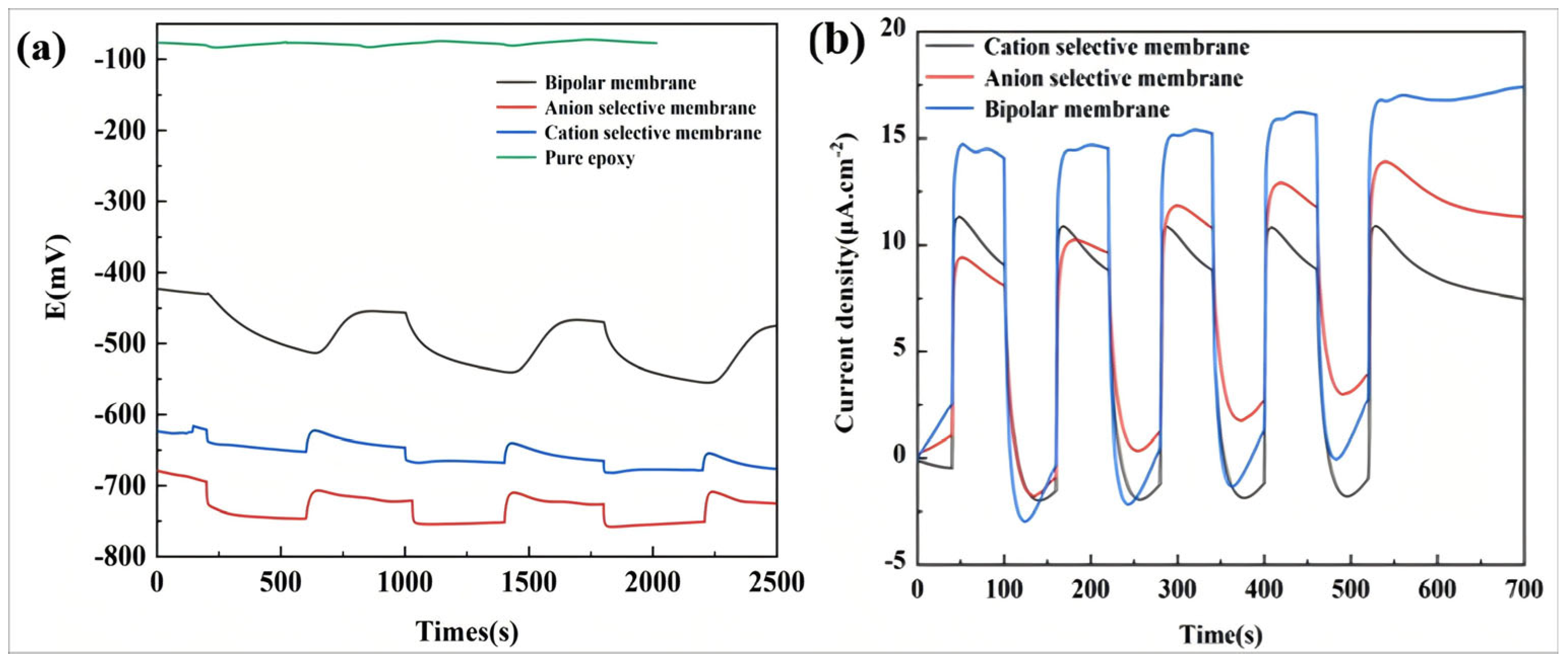
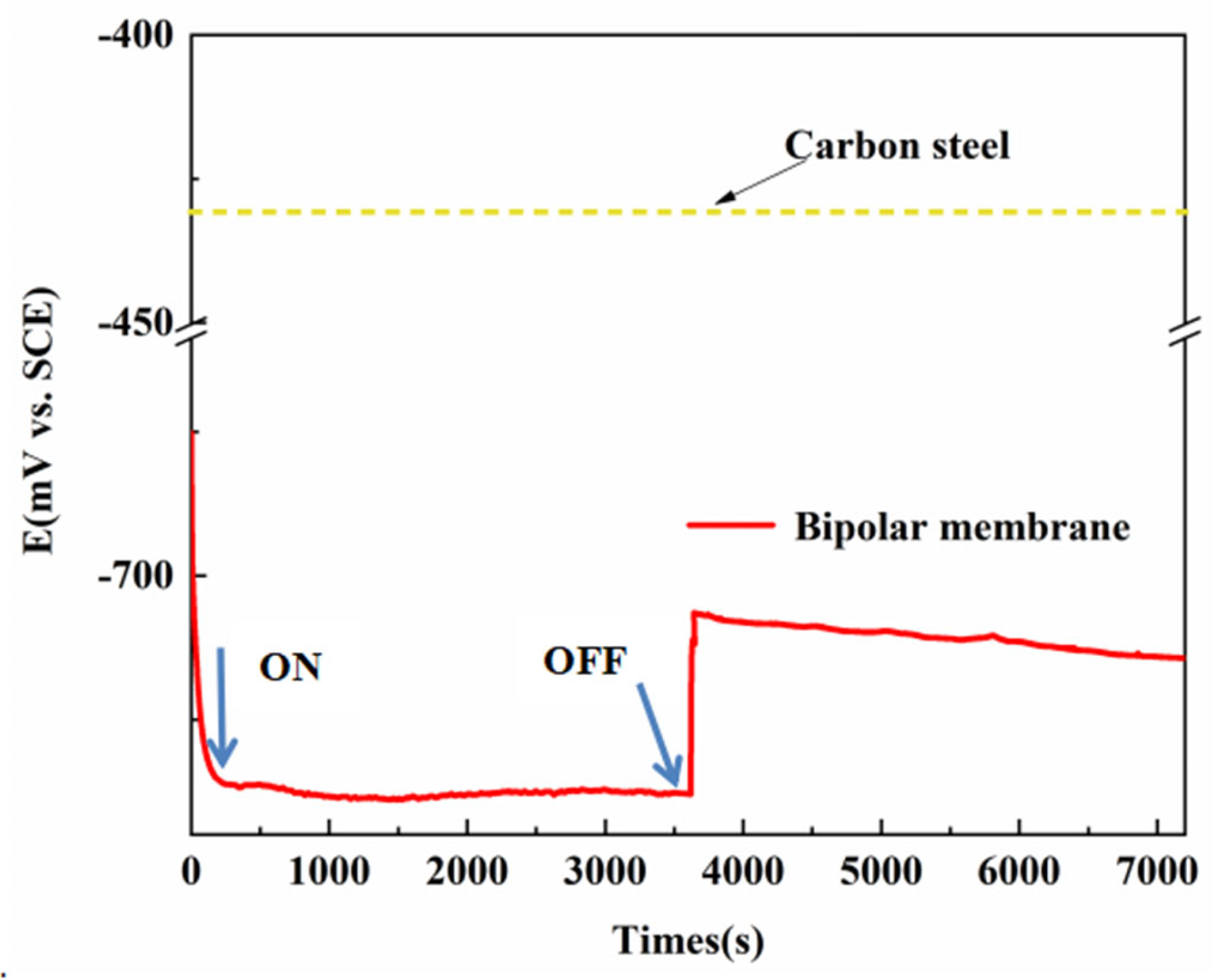
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of corrosion of MnCuP weathering steel submitted to wet/dry cyclic tests in a simulated coastal atmosphere. Corros. Sci. 2012, 58, 175–180. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of atmospheric corrosion of MnCuP weathering steel in a simulated coastal-industrial atmosphere. Corros. Sci. 2012, 59, 270–276. [Google Scholar]
- Wang, Z.; Liu, J.; Wu, L.; Han, R.; Sun, Y. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Oesch, S. The effect of SO2, NO2, NO and O3 on the corrosion of unalloyed carbon steel and weathering steel—The results of laboratory exposures. Corros. Sci. 1996, 38, 1357–1368. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 25, 283–299. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 5, 279–289. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Calvé, P.L.; Favennec, C.; Pautasso, J.P.; Hubert, C. Performance of marine and offshore paint systems: Correlation of accelerated corrosion tests and field exposure on operating ships. Mater. Corros. 2013, 66, 215–225. [Google Scholar] [CrossRef]
- Qiu, P.; Leygraf, C.; Wallinder, I.O. Evolution of corrosion products and metal release from Galvalume coatings on steel during short and long-term atmosphericexposures. Mater. Chem. Phys. 2012, 133, 419–428. [Google Scholar] [CrossRef]
- Nazarov, A.; Bozec, N.L.; Thierry, D.; Calvé, P.L.; Pautasso, J.P. Scanning kelvin probe investigation of corrosion under thick marine paint systems applied on carbon steel. Corrosion 2012, 68, 720–729. [Google Scholar] [CrossRef]
- Almarshad, A.I.; Syed, S. Atmospheric corrosion of galvanized steel and aluminium in marine and marine-industrial environments of Saudi Arabia. Mater. Corros. 2008, 59, 46–51. [Google Scholar] [CrossRef]
- Dhanak, M.; Xiros, N. Principles of Marine Corrosion. In Springer Handbook of Ocean Engineering; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Dahl, M.; Liu, Y.D.; Yin, Y.D. Composite titanium dioxide nanomaterials. Chem. Rev. 2013, 114, 9853–9889. [Google Scholar] [CrossRef]
- Meng, A.Y.; Zhang, J.; Xu, D.F.; Cheng, B.; Yu, J.G. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Xie, X.F.; Chen, S.C.; Tong, S.R.; Lu, G.H.; Pui, D.Y.H.; Sun, J. Cu–Ni nanowire-based TiO2 hybrid for the dynamic photodegradation of acetaldehyde gas pollutant under visible light. Appl. Surf. Sci. 2017, 408, 117–124. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, X.; Guo, H.; Ding, J.; Sun, H.; Zhan, X. Enhanced photoelectrochemical protection of 304 stainless steel induced by the internal electric field effect of a g-C3N4/graphene/TiO2 Z-scheme system. Mater. Res. Bull. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Guan, Z.-C.; Hu, J.; Wang, H.-H.; Shi, H.-Y.; Wang, H.-P.; Wang, X.; Jin, P.; Song, G.-L.; Du, R.-G. Decoration of rutile TiO2 nanorod film with g-C3N4/SrTiO3 for efficient photoelectrochemical cathodic protection. J. Photochem. Photobiol. A Chem. 2023, 443, 114825. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, L.W.; Tian, Y.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Simultaneous nanostructure and heterojunction engineering of graphitic carbon nitride via in situ Ag doping for enhanced photoelectrochemical activity. Appl. Catal. B 2015, 163, 611–622. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, L.; Wang, H.; Zhao, Y.; Zhang, J.; Hu, S. Novel band gap-tunable K-Na co-doped graphitic carbon nitride prepared by molten salt method. Appl. Surf. Sci. 2015, 332, 625–630. [Google Scholar] [CrossRef]
- Zhong, X.; Jin, M.; Dong, H.; Liu, L.; Wang, L.; Yu, H.; Leng, S.; Zhuang, G.; Li, X.; Wang, J.G. TiO2 nanobelts with a uniform coating of g-C3N4 as a highly effective heterostructure for enhanced photocatalytic activities. J. Solid. State Chem. 2014, 220, 54–59. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Xie, H.; Qu, A. Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int. J. Hydrogen Energy 2014, 39, 6354–6363. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.Y.; Lin, T.Q.; Yin, H.; Chen, P.; Wan, D.Y.; Xu, F.F.; Huang, F.Q.; Lin, J.H.; Xie, X.M.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Adv. Funct. Mater. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Lee, D.U.; Jang, S.R.; Vittal, R.; Lee, J.; Kim, K.J. CTAB facilitated spherical rutile TiO2 particles and their advantage in a dye-sensitized solar cell. Sol. Energy 2008, 82, 1042–1048. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Liu, Y.; Chen, F.; Zhang, C.; Liu, B. A review on recent progress in the development of photoelectrodes for photocathodic protection: Design, properties, and prospects. Mater. Des. 2021, 197, 109235. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, S.; Liu, Y.; Sun, X.; Wang, D.; Zhou, Y.; Zhu, M.; Zhang, D.; Zhang, L. Improved photocathodic protection performance of C-vacancy g-C3N4/GO/WO3 for 304 stainless steel. J. Phys. Chem. Solids 2022, 160, 110270–110280. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wu, H.; Cui, Y.; Zheng, X.; Wang, H.; Chen, S.; Wang, Y.; Fu, X. One-step synthesis of 2D/2D-3D NiS/Zn3In2S6 hierarchical structure toward solar-to-chemical energy transformation of biomass-relevant alcohols. Appl. Catal. B Environ. 2020, 266, 118617–118626. [Google Scholar] [CrossRef]


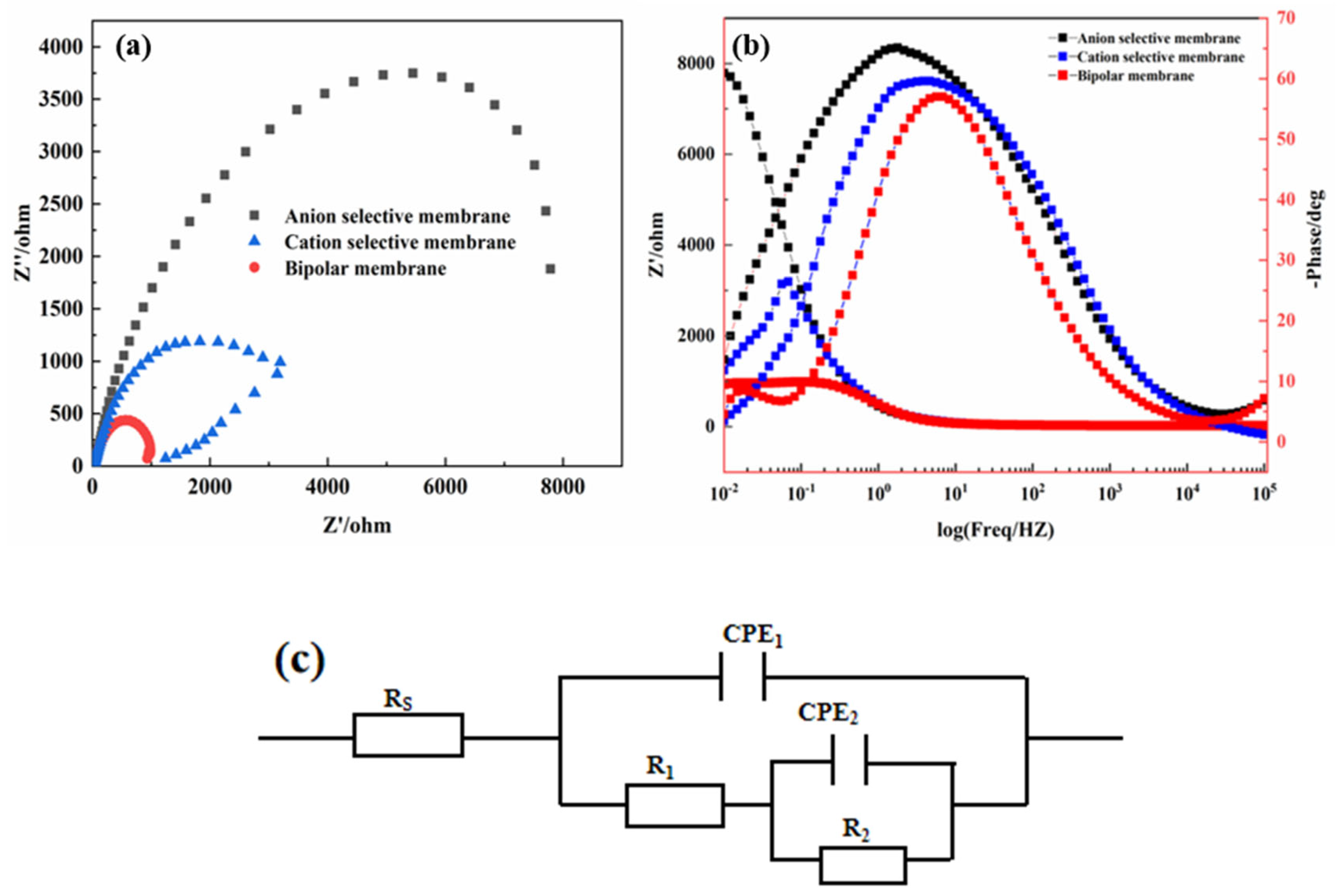

| Conditions | Rs (Ω·cm2) | CPE1 | R1 (Ω·cm2) | CPE2 | R2 (Ω·cm2) | ||
|---|---|---|---|---|---|---|---|
| Y01 × 10−4 (Ω−1·Sn·cm−2) | n1 | Y02 × 10−4 (Ω−1·Sn·cm−2) | n2 | ||||
| Anion selective coating | 17.52 | 1.48 | 0.75 | 33.07 | 9.79 | 0.76 | 994.2 |
| Cation selective coating | 15.84 | 1.46 | 0.79 | 151.3 | 7.99 | 0.83 | 2752 |
| Bipolar coating | 23.06 | 1.13 | 0.83 | 30.28 | 1.23 | 0.86 | 337.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; An, Y.; Wang, X.; Qiu, P. Preparation and Characterization of a Dual-Layer Coating with Synergistic Ionic Selectivity and Photocathodic Protection Property. Corros. Mater. Degrad. 2025, 6, 60. https://doi.org/10.3390/cmd6040060
Cui C, An Y, Wang X, Qiu P. Preparation and Characterization of a Dual-Layer Coating with Synergistic Ionic Selectivity and Photocathodic Protection Property. Corrosion and Materials Degradation. 2025; 6(4):60. https://doi.org/10.3390/cmd6040060
Chicago/Turabian StyleCui, Chuyuan, Yongsheng An, Xiangpeng Wang, and Ping Qiu. 2025. "Preparation and Characterization of a Dual-Layer Coating with Synergistic Ionic Selectivity and Photocathodic Protection Property" Corrosion and Materials Degradation 6, no. 4: 60. https://doi.org/10.3390/cmd6040060
APA StyleCui, C., An, Y., Wang, X., & Qiu, P. (2025). Preparation and Characterization of a Dual-Layer Coating with Synergistic Ionic Selectivity and Photocathodic Protection Property. Corrosion and Materials Degradation, 6(4), 60. https://doi.org/10.3390/cmd6040060






