First-Principles Study of Rare-Earth Doping Effects on Nitrogen Adsorption and Diffusion in Chromium
Abstract
1. Introduction
2. Calculation Details
3. Results and Discussion
3.1. Bulk Properties of Cr Doped with RE
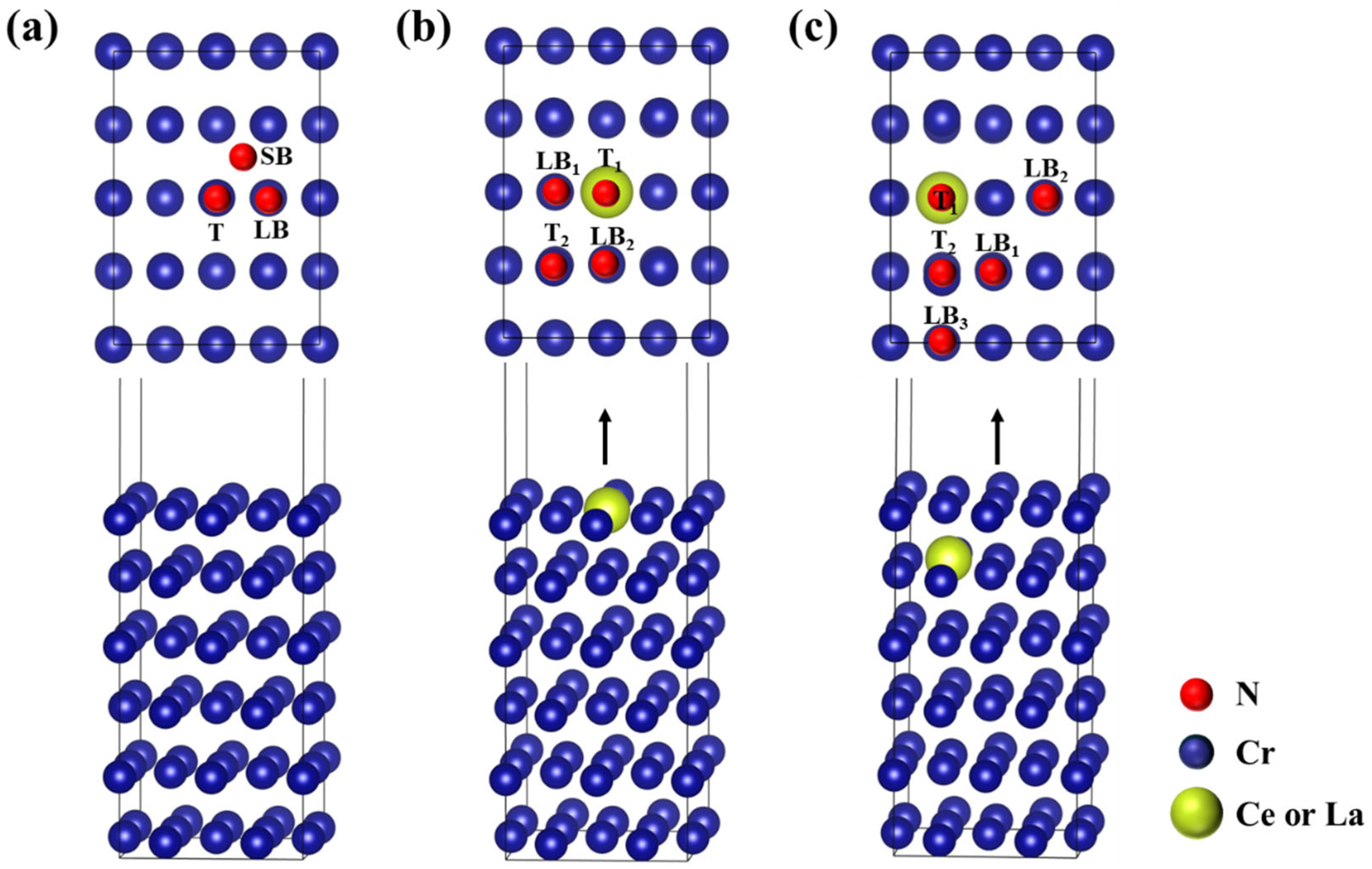
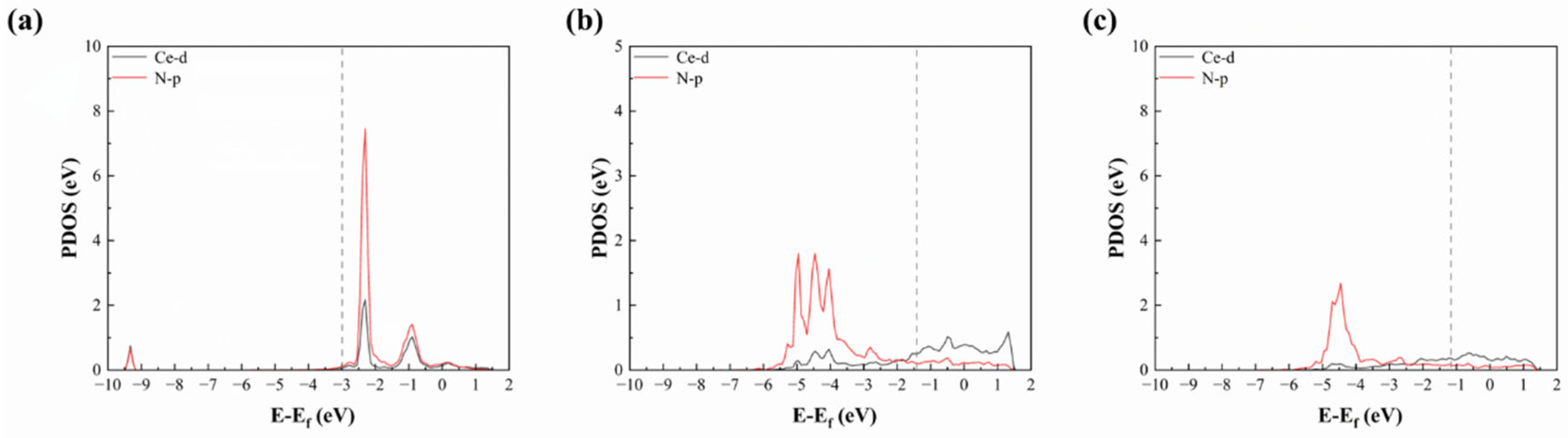
3.2. Adsorption Properties of Nitrogen Atom on Cr(110)
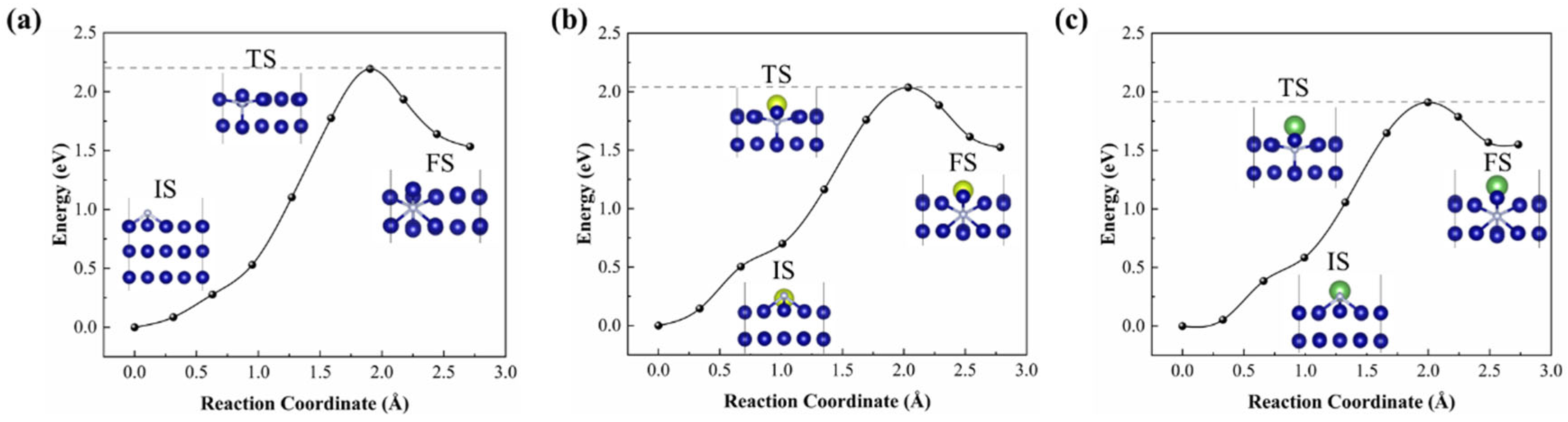
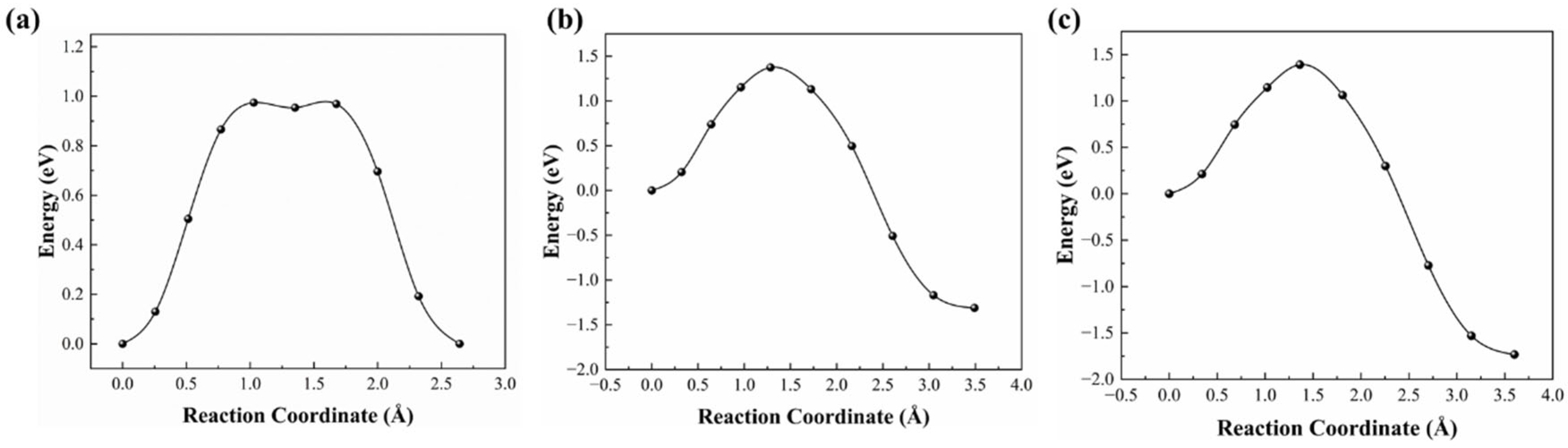
3.3. Penetration Mechanism for Nitrogen Atom with RE Doping
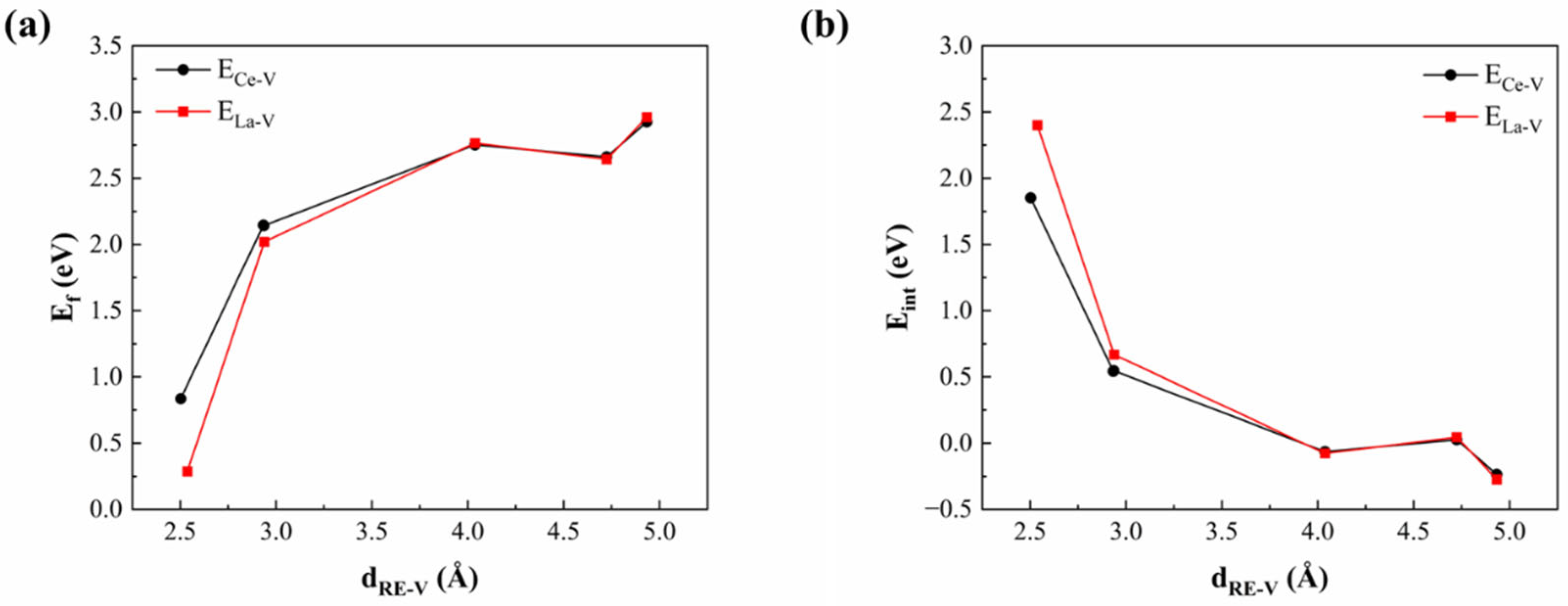
3.4. Influence of RE Doping on Nitrogen and Vacancy Diffusion Behavior in Bulk Cr
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, F.Y.; Zhang, P.; Pang, J.C.; Chen, D.M.; Yang, K.; Zhang, Z.F. Optimizing strength and ductility of austenitic stainless steels through equal-channel angular pressing and adding nitrogen element. Mater. Sci. Eng. 2013, 587, 185–191. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, L. Surface properties of nitrided layer on AISI 316L austenitic stainless steel produced by high temperature plasma nitriding in short time. Appl. Surf. Sci. 2014, 285, 243–250. [Google Scholar] [CrossRef]
- Dong, H.; Qi, P.-Y.; Li, X.; Llewellyn, R. Improving the erosion–corrosion resistance of AISI 316 austenitic stainless steel by low-temperature plasma surface alloying with N and C. Mater. Sci. Eng. A 2006, 431, 137–145. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-wear and intergranular corrosion resistance properties of AISI 304L austenitic stainless steel after low-temperature plasma nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, X. Improving stress corrosion resistance and wear resistance of austenitic hot-stamping die steels via synergistic effects of shot peening and plasma nitriding. Surf. Coat. Technol. 2024, 478, 130448. [Google Scholar] [CrossRef]
- Cao, Z.; Liang, X.; Luo, S.; Song, J.; Pu, C.; Pang, Z.; He, W. Improvement of nitrogen ion implantation on the wear and corrosion resistance of bearing steel in NaCl solution. Vacuum 2024, 222, 112995. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Hao, Z.; Shi, H.; Zhang, H.; Sun, N.; Liu, H.; Tang, F.; Li, H.; Liu, Q. Improvement of corrosion and wear resistances of 300M ultra high strength steel by low temperature cathode assisted plasma nitriding. Surf. Coat. Technol. 2024, 479, 130518. [Google Scholar] [CrossRef]
- You, Y.; Yan, J.; Yan, M. Atomistic diffusion mechanism of rare earth carburizing/nitriding on iron-based alloy. Appl. Surf. Sci. 2019, 484, 710–715. [Google Scholar] [CrossRef]
- Du, C.; Zhang, J.; Zhang, L.; Lian, Y.; Fang, M. Acceleration effects of rare earths on salt bath nitriding: Diffusion kinetics and first-principles calculations. Surf. Eng. 2021, 37, 764–774. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Chen, X.; Chen, H.; Wu, Y.; Wang, Y.; Tang, L.; Cui, G.; Chen, D. Catalytic behavior of LaFeO3 pervoskite oxide during low-pressure gas nitriding. Appl. Surf. Sci. 2020, 506, 145045. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Pan, F.; Ma, Z.; Sa, R.; Zheng, J.; Wang, Q. Effect of rare-earth doping on adsorption of carbon atom on ferrum surface and in ferrum subsurface: A first-principles study. J. Rare Earths 2021, 39, 1144–1150. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Min, Y. Effects of rare earth on nitriding microstructure and properties of a La-Ce micro-alloyed Q235RE steel. Surf. Coat. Technol. 2024, 493, 131240. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, M.; Sun, Z. Experimental and theoretical study on interaction between lanthanum and nitrogen during plasma rare earth nitriding. Appl. Surf. Sci. 2013, 287, 381–388. [Google Scholar] [CrossRef]
- Torchane, L. Influence of rare earths on the gas nitriding kinetics of 32CrMoNiV5 steel at low temperature. Surf. Interfaces 2021, 23, 101016. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Xing, Z.; Wang, H.; Huang, Y.; Guo, W.; Liu, H. Study of the catalytic strengthening of a vacuum carburized layer on alloy steel by rare earth pre-implantation. Materials 2019, 12, 3420. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dai, X.; Yang, X.; Zhang, S.; Li, D. First-principles analysis on the role of rare-earth doping in affecting nitrogen adsorption and diffusion at Fe surface towards clarified catalytic diffusion mechanism in nitriding. Acta Mater. 2020, 196, 347–354. [Google Scholar] [CrossRef]
- Ahlawat, S.; Srinivasu, K.; Biswas, A.; Choudhury, N. First-principle investigation of electronic structures and interactions of foreign interstitial atoms (C, N, B, O) and intrinsic point defects in body-and face-centered cubic iron lattice: A comparative analysis. Comput. Mater. Sci. 2019, 170, 109167. [Google Scholar] [CrossRef]
- Yeo, S.C.; Han, S.S.; Lee, H.M. Adsorption, dissociation, penetration, and diffusion of N 2 on and in bcc Fe: First-principles calculations. Phys. Chem. Chem. Phys. 2013, 15, 5186–5192. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wang, H. Calculated elastic constants and electronic and magnetic properties of bcc, fcc, and hcp Cr crystals and thin films. Phys. Rev. B 2000, 62, 5136. [Google Scholar] [CrossRef]
- Boda, A.; Bajania, S.; Ali, S.M.; Shenoy, K.; Mohan, S. Chemisorption, diffusion and permeation of hydrogen isotopes in bcc bulk cr and cr (100) surface: First-principles dft simulations. J. Nucl. Mater. 2021, 543, 152538. [Google Scholar] [CrossRef]
- You, Y.; Yan, J.; Yan, M.; Zhang, C.; Chen, H.; Wang, Y.; Zhang, Y.; Wang, C. La interactions with C and N in bcc Fe from first principles. J. Alloys Compd. 2016, 688, 261–269. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Li, L.; Lu, H.; Li, D.; Yan, M. Carbon adsorption on doped cementite surfaces for effective catalytic growth of diamond-like carbon: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 32341–32348. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Jónsson, H. Long time scale kinetic Monte Carlo simulations without lattice approximation and predefined event table. J. Chem. Phys. 2001, 115, 9657–9666. [Google Scholar] [CrossRef]
- Bolef, D.t.; De Klerk, J. Anomalies in the elastic constants and thermal expansion of chromium single crystals. Phys. Rev. 1963, 129, 1063. [Google Scholar] [CrossRef]
- Razumovskiy, V.I.; Ruban, A.V.; Korzhavyi, P.A. First-principles study of elastic properties of Cr-and Fe-rich Fe-Cr alloys. Phys. Rev. B—Condens. Matter Mater. Phys. 2011, 84, 024106. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, F.; Jiang, L.; Wang, G. The correlation between yielding behavior and precipitation in ultra purified ferritic stainless steels. Mater. Sci. Eng. A 2010, 527, 3800–3806. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Lin, J.; Carter, J.T.; Sachdev, A.K. Spatio-temporal characteristics of propagative plastic instabilities in a rare earth containing magnesium alloy. Int. J. Plast. 2014, 57, 52–76. [Google Scholar] [CrossRef]


| Type | /Å | C11/GPa | C12/GPa | C44/GPa | B/GPa | G/GPa | E/GPa |
|---|---|---|---|---|---|---|---|
| Cr | 2.85 | 483.78 | 146.64 | 84.73 | 258.82 | 111.94 | 293.50 |
| Crcalc [28] | 2.85 | 484 | 140 | 105 | 255 | - | - |
| [27] | 2.88 | 391.00 | 91.00 | 103.00 | 191.00 | - | - |
| Cr53Ce | 2.86 | 510.34 | 139.15 | 108.29 | 262.88 | 134.57 | 344.87 |
| Cr53La | 2.86 | 508.86 | 139.85 | 111.16 | 262.85 | 136.34 | 348.72 |
| Initial Site | Ead (eV/Atom) | hN-S (Å) | Final Site | |
|---|---|---|---|---|
| Cr(110) | T | −0.921 | 1.604 | T |
| LB | −3.230 | 1.027 | LB | |
| SB | −2.490 | 1.207 | SB | |
| (110) | T1 | 1.285 | 1.753 | T1 |
| T2 | −3.559 | 1.100 | LB2 | |
| LB1 | −2.597 | 0.924 | LB1 | |
| LB2 | −3.559 | 1.100 | LB2 | |
| (110) | T1 | −3.021 | 0.755 | T1 |
| T2 | −3.448 | 0.842 | LB3 | |
| LB1 | −3.226 | 1.036 | LB1 | |
| LB2 | −2.881 | 1.074 | LB2 | |
| LB3 | −3.448 | 0.842 | LB3 | |
| (110) | T1 | 2.151 | 4.296 | T1 |
| T2 | −3.645 | 1.117 | LB2 | |
| LB1 | −3.177 | 0.765 | LB1 | |
| LB2 | −3.645 | 1.117 | LB2 | |
| (110) | T1 | −3.333 | 1.106 | T1 |
| T2 | −3.440 | 0.983 | LB3 | |
| LB1 | −3.248 | 1.053 | LB1 | |
| LB2 | −2.999 | 0.738 | LB2 | |
| LB3 | −3.440 | 0.983 | LB3 |
| Cr(110) | (110) | (110) | ||||
|---|---|---|---|---|---|---|
| Site | O | T | O | T | O | T |
| Ead (eV/atom) | −1.697 | −0.352 | −2.035 | −0.909 | −2.095 | −0.584 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Chen, B.; Liu, M.; Liu, J.; Li, G.; Jin, Y. First-Principles Study of Rare-Earth Doping Effects on Nitrogen Adsorption and Diffusion in Chromium. Corros. Mater. Degrad. 2025, 6, 57. https://doi.org/10.3390/cmd6040057
Chen S, Chen B, Liu M, Liu J, Li G, Jin Y. First-Principles Study of Rare-Earth Doping Effects on Nitrogen Adsorption and Diffusion in Chromium. Corrosion and Materials Degradation. 2025; 6(4):57. https://doi.org/10.3390/cmd6040057
Chicago/Turabian StyleChen, Shuhui, Bao Chen, Min Liu, Ji Liu, Gen Li, and Ying Jin. 2025. "First-Principles Study of Rare-Earth Doping Effects on Nitrogen Adsorption and Diffusion in Chromium" Corrosion and Materials Degradation 6, no. 4: 57. https://doi.org/10.3390/cmd6040057
APA StyleChen, S., Chen, B., Liu, M., Liu, J., Li, G., & Jin, Y. (2025). First-Principles Study of Rare-Earth Doping Effects on Nitrogen Adsorption and Diffusion in Chromium. Corrosion and Materials Degradation, 6(4), 57. https://doi.org/10.3390/cmd6040057






