Optimizing Anti-Corrosive Properties of Polyester Powder Coatings Through Montmorillonite-Based Nanoclay Additive and Film Thickness
Abstract
1. Introduction
2. Experimental
2.1. Statistical Method of Mixture Design
2.2. Materials and Coating Formulations
2.3. Preparation of Powder-Coated Panels
2.4. Coating Performance Evaluations
3. Results and Discussion
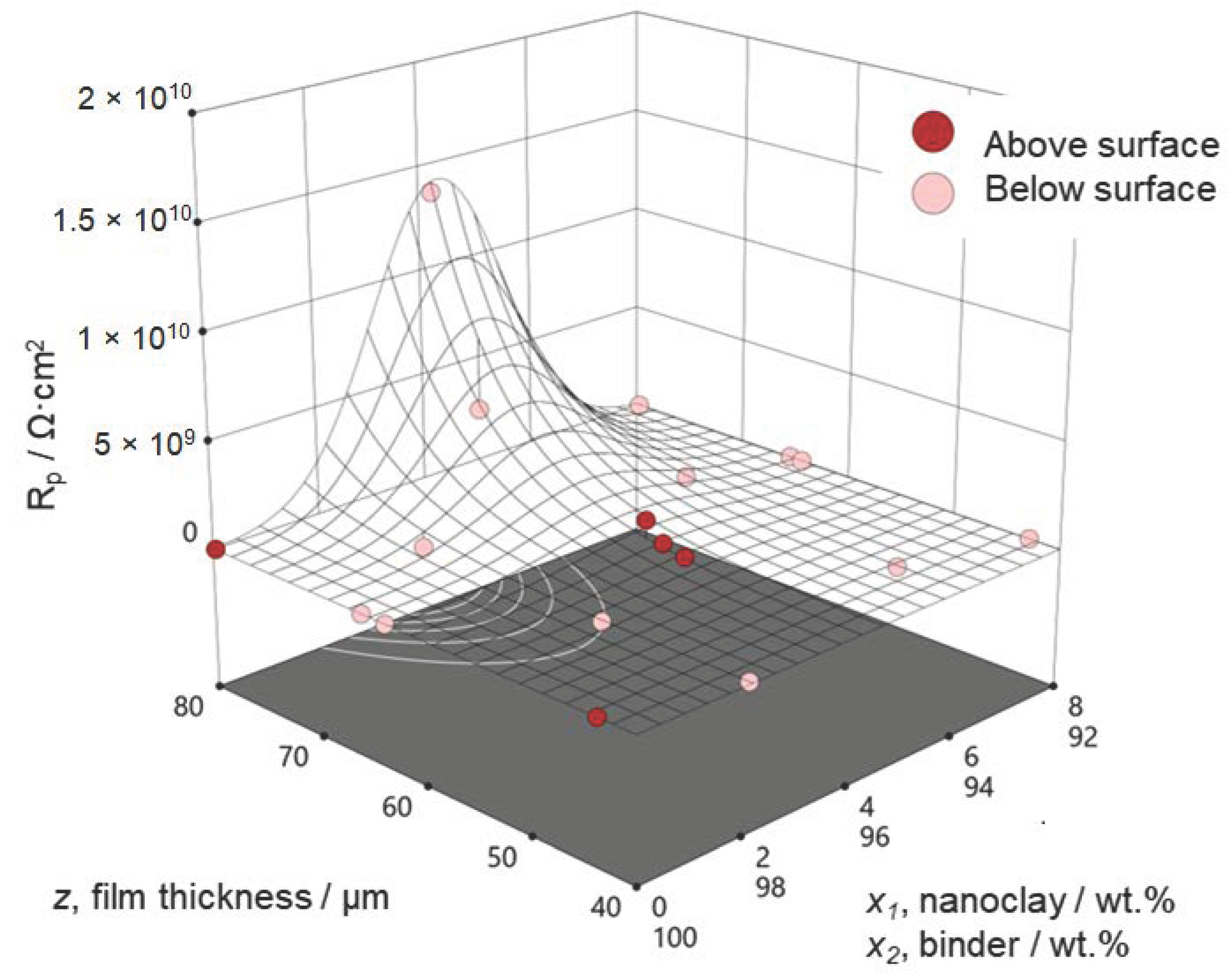
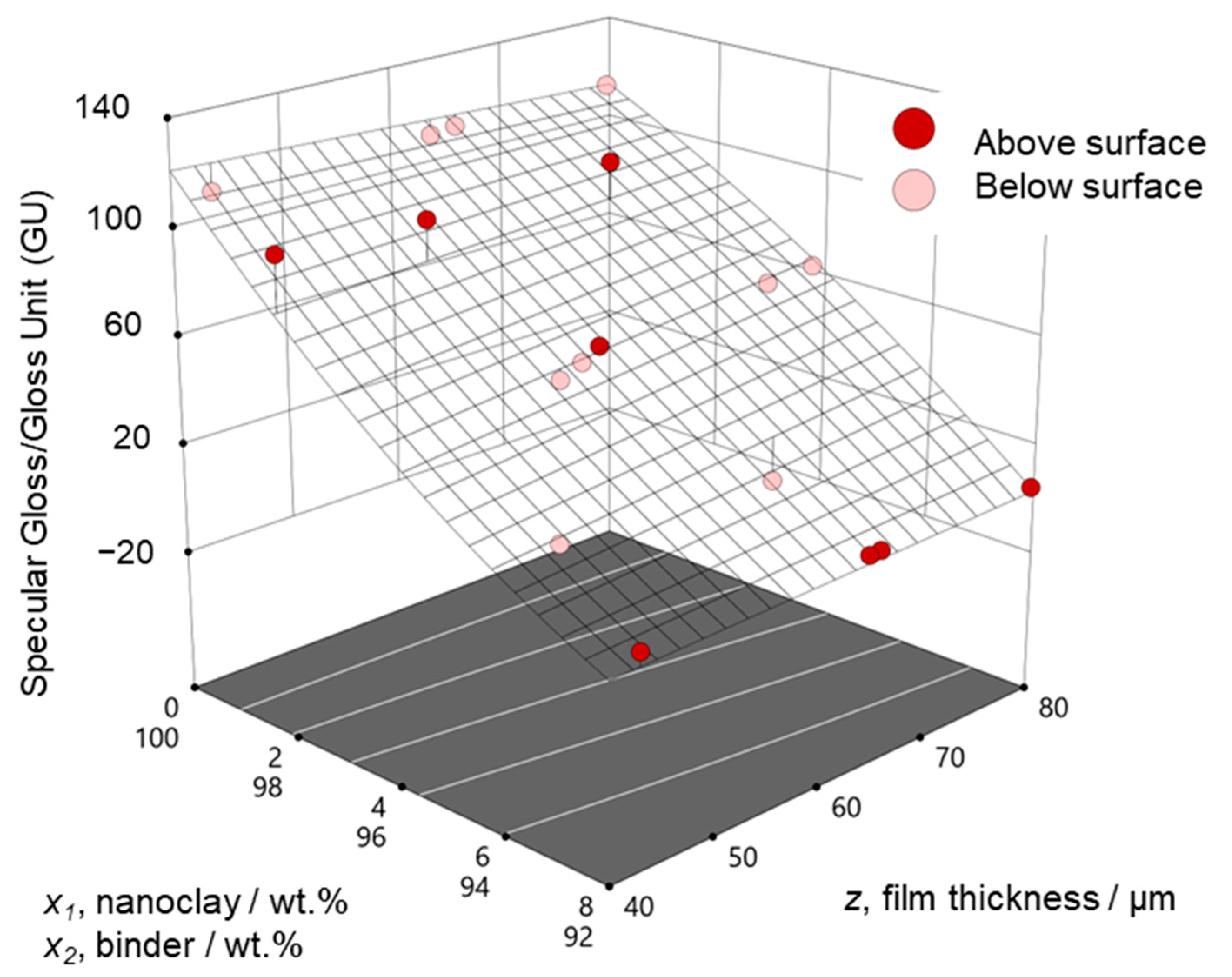
3.1. Measured Values and Resulting Regression Models
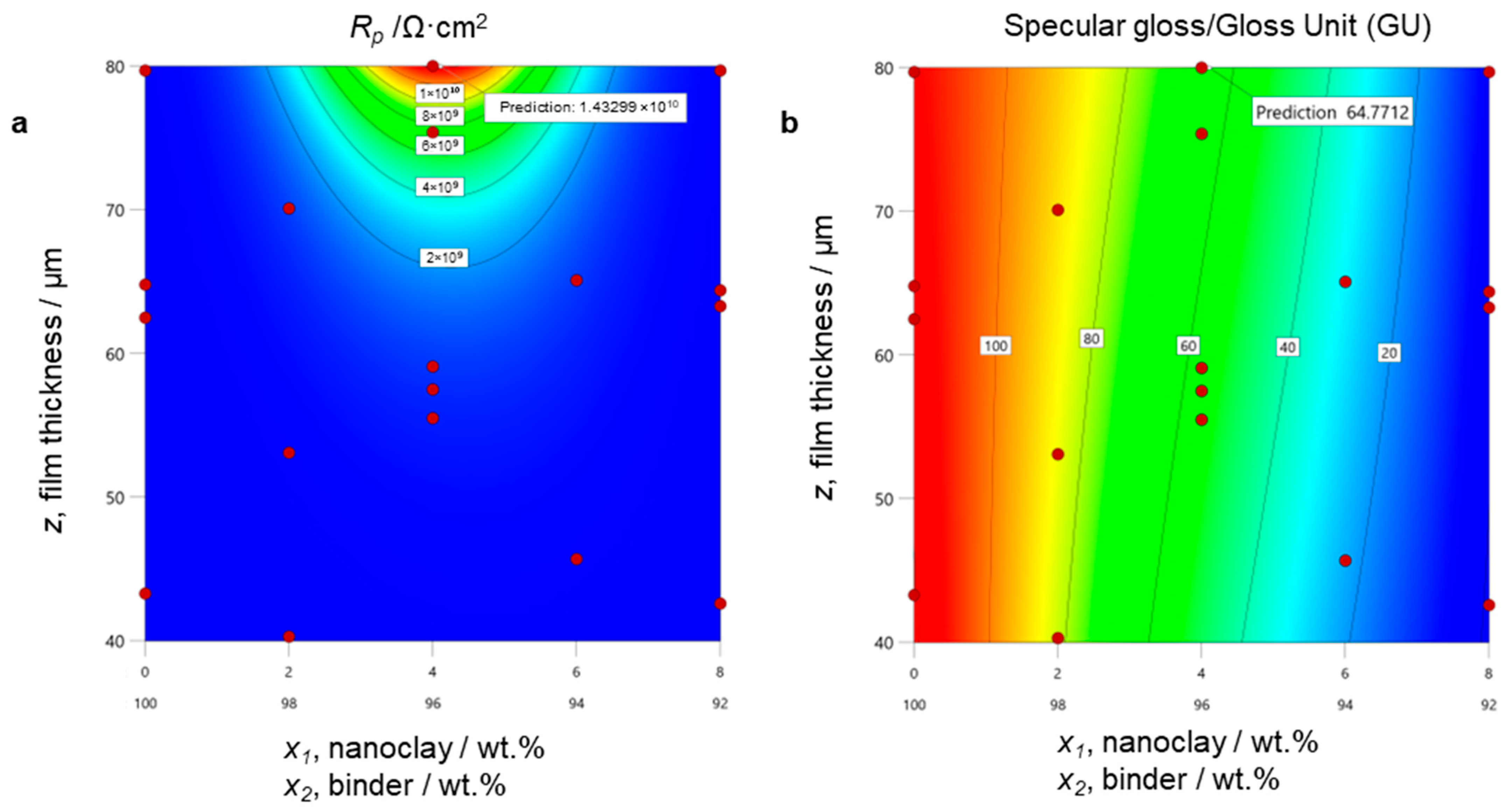
3.2. Optimization of Coating Performance
3.3. Model Validation
3.4. Characterization Findings and Detailed Electrochemical Analysis
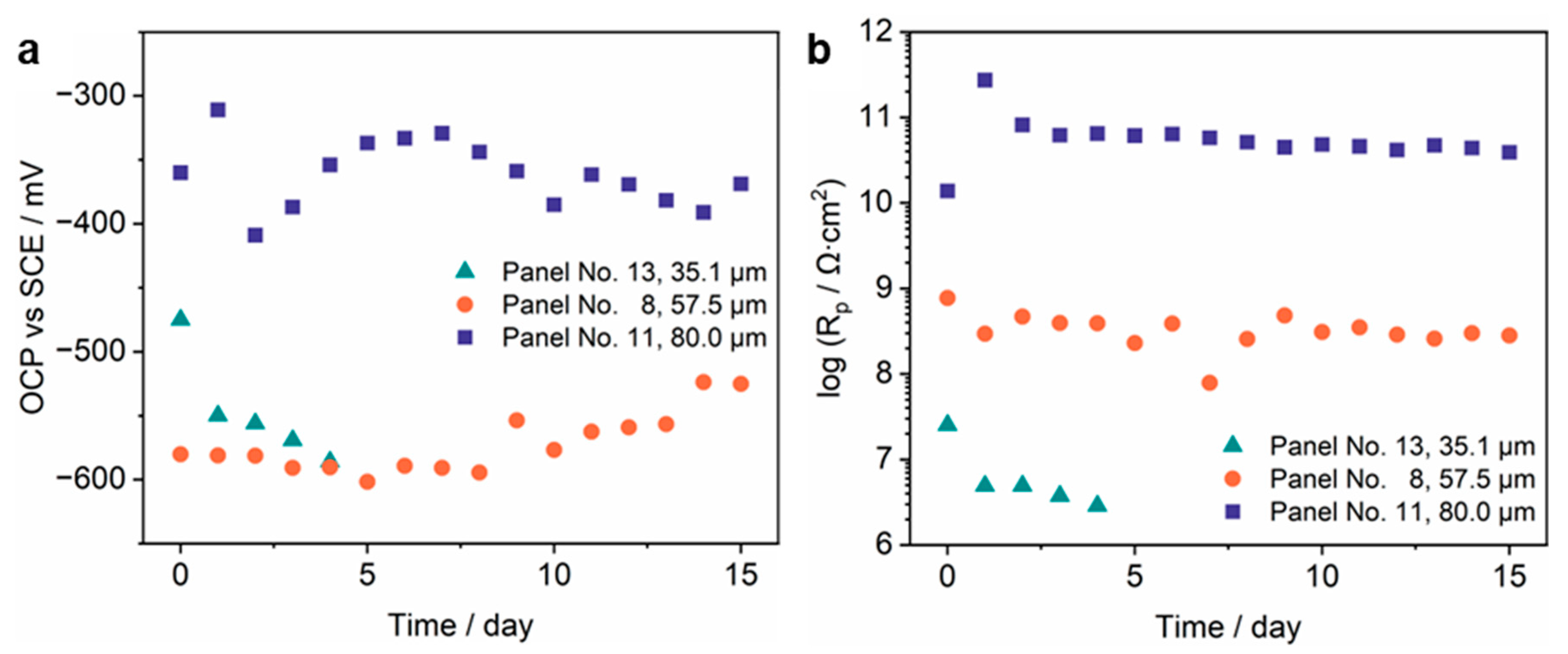
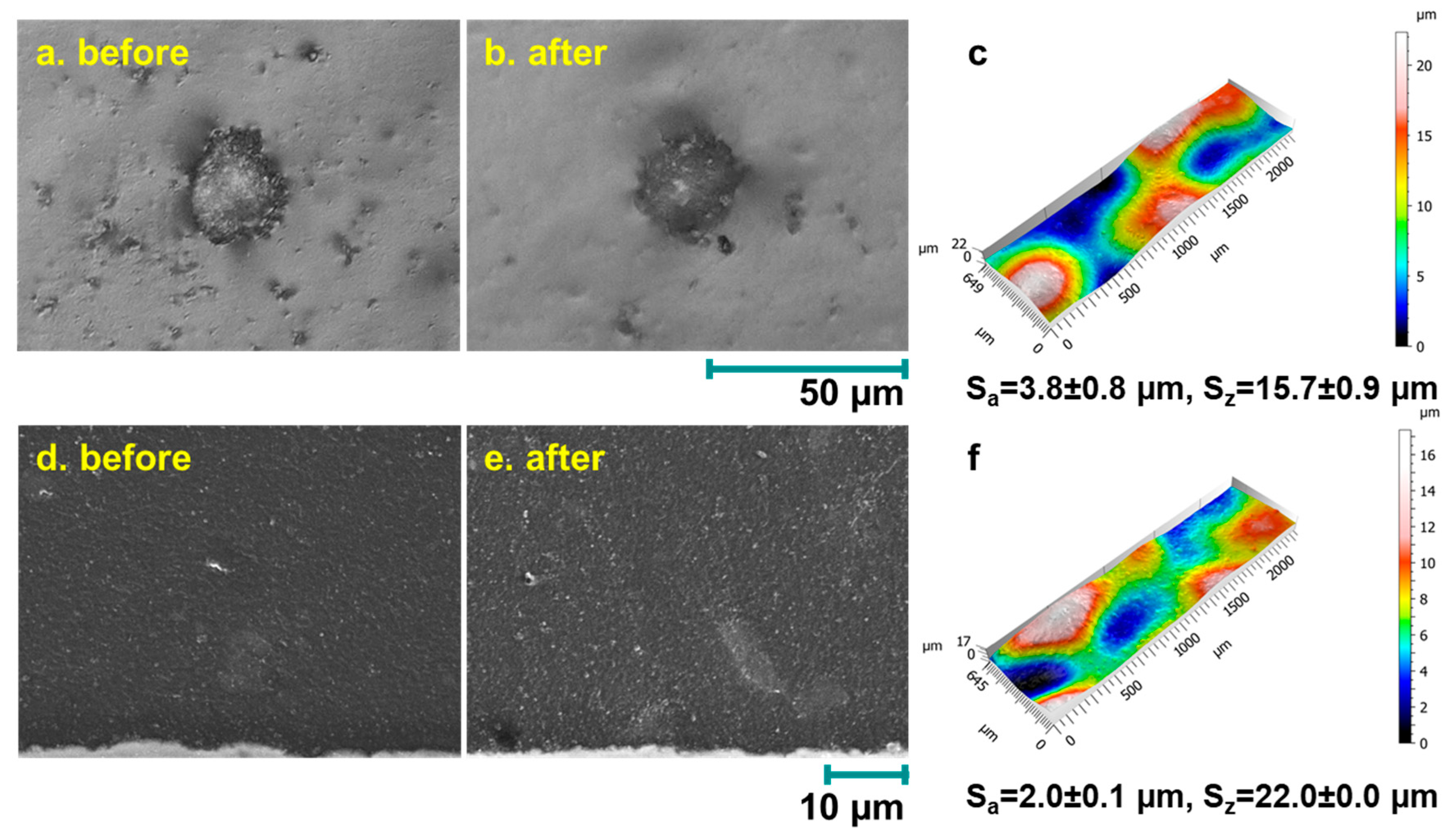
3.4.1. Coating Morphologies, OCP and Rp Measurement Results
3.4.2. EIS Spectra Analysis
3.5. Further Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement
Correction Statement
References
- Crapper, G. Powder Coatings. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 541–566. ISBN 9780080878621. [Google Scholar]
- Turner, S.; Baskir, J.; Nunez, C. Powder Coatings: A Technology Review. Pollut. Prev. Rev. 1999, 9, 7–21. [Google Scholar]
- Fu, J.; Krantz, M.; Zhang, H.; Zhu, J.; Kuo, H.; Wang, Y.M.; Lis, K. Investigation of the Recyclability of Powder Coatings. Powder Technol. 2011, 211, 38–45. [Google Scholar] [CrossRef]
- Du, Z.; Wen, S.; Wang, J.; Yin, C.; Yu, D.; Luo, J. The Review of Powder Coatings. J. Mater. Sci. Chem. Eng. 2016, 4, 54–59. [Google Scholar] [CrossRef]
- Perera, D.Y. Effect of Pigmentation on Organic Coating Characteristics. Prog. Org. Coat. 2004, 50, 247–262. [Google Scholar] [CrossRef]
- Métayer, M.; Labbé, M.; Marais, S.; Langevin, D.; Chappey, C.; Dreux, F.; Brainville, M.; Belliard, P. Diffusion of Water through Various Polymer Films: A New High Performance Method of Characterization. Polym. Test. 1999, 18, 533–549. [Google Scholar] [CrossRef]
- Uribe-Padilla, J.; Graells, M.; Salgado-Valle, J.; López Serrano, J. A Viscosity-Mediated Model for Relating Gloss and Film Thickness of Coatings. Prog. Org. Coat. 2019, 136, 105195. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Nabey, B.A.A.E.; Khamis, E.; Abdelattef, O.A.; Aglan, H.; Ludwick, A. Influence of Natural Inhibitor, Pigment and Extender on Corrosion of Polymer Coated Steel. Prog. Org. Coat. 2010, 69, 402–409. [Google Scholar] [CrossRef]
- Muizebelt, W.J.; Heuvelsland, W.J.M. Permeabilities of Model Coatings: Effect of Cross-Link Density and Polarity. In Polymeric Materials for Corrosion Control; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1986; pp. 110–114. [Google Scholar] [CrossRef]
- Biris, A.S.; Mazumder, M.K.; Yurteri, C.U.; Sims, R.A.; Snodgrass, J.; De, S. Gloss and Texture Control of Powder Coated Films. Part. Sci. Technol. 2011, 19, 199–217. [Google Scholar] [CrossRef]
- Spyrou, E. Powder Coatings: Chemistry and Technology, 3rd ed.; Vincentz Network GmbH & Co KG: Hannover, Germany, 2012; ISBN 978-3-86630-824-4. [Google Scholar]
- Bello, L.H.A.D.; Vieira, A.F.D.C. Tutorial for mixture-process experiments with an industrial application. Pesquisa Operacional 2011, 31, 543–564. [Google Scholar] [CrossRef]
- Lin, S.M.; Wen, T.C. A Mixture Design Approach to the Service Life and the Oxygen Evolving Catalytic Activity of Ru-Sn-Ti Ternary Oxide Coated Electrodes. J. Appl. Electrochem. 1993, 23, 487–494. [Google Scholar] [CrossRef]
- Carrado, K.A. Synthetic Organo-and Polymer-Clays: Preparation, Characterization, and Materials Applications. Appl. Clay Sci. 2000, 17, 1–23. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn-Al Layered Double Hydroxides as Chloride Nanotraps in Active Protective Coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Cai, Y.; Yin, Y.; Zhang, Y.; Yang, F.; Zhang, C.; Wang, W. Nanoclay-Enabled Advanced Metal Protection: Emerging Barrier Engineering and Smart Corrosion Inhibition Strategies. Coord. Chem. Rev. 2025, 545, 217014. [Google Scholar] [CrossRef]
- Haghi, M.; Kazemian, H.; Ranjbar, Z. Improvement of the Corrosion Resistance of Acrylic Electrocoating in the Presence of Acid-Modified Montmorillonite Nano Clay. Prog. Org. Coat. 2023, 182, 107689. [Google Scholar] [CrossRef]
- Affaf, N.; Alias, J.; Alang, N. Montmorillonite (MMT) Nanoclay in Smart Coatings for Corrosion Protection of Metal Alloy: A Brief Review. J. Phys. Conf. Ser. 2024, 2688, 012012. [Google Scholar] [CrossRef]
- Yang, M.S.; Huang, J.; Zhang, H.; Noël, J.J.; Hedberg, Y.S.; Chen, J.; Eduok, U.; Barker, I.; Henderson, J.D.; Xian, C.; et al. Study on the Self-Repairing Effect of Nanoclay in Powder Coatings for Corrosion Protection. Coatings 2023, 13, 1220. [Google Scholar] [CrossRef]
- Goos, P.; Jones, B.; Syafitri, U. I-Optimal Design of Mixture Experiments. J. Am. Stat. Assoc. 2016, 111, 899–911. [Google Scholar] [CrossRef]
- Design-Expert®, version 13.0.9.0; Stat-Ease, Inc.: Minneapolis, MN, USA, 2024.
- ASTM D609; Standard Practice for Preparation of Cold-Rolled Steel Panels for Testing Paint, Varnish, Conversion Coatings, and Related Coating Products. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–3.
- ASTM D7091; Practice for Nondestructive Measurement of Dry Film Thickness of Nonmagnetic Coatings Applied to Ferrous Metals and Nonmagnetic, Nonconductive Coatings Applied to Non-Ferrous Metals. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D523; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–5.
- ASTM D610; Standard Practice for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–6.
- Walter, G.W. A Critical Review of d.c. Electrochemical Tests for Painted Metals. Corros. Sci. 1986, 26, 39–47. [Google Scholar] [CrossRef]
- Huang, J.; Yang, M.S.; Xian, C.; Noël, J.J.; Hedberg, Y.S.; Chen, J.; Eduok, U.; Barker, I.; Henderson, J.D.; Zhang, H.; et al. Synergistic Effect of Nanoclay and Barium Sulfate Fillers on the Corrosion Resistance of Polyester Powder Coatings. Coatings 2023, 13, 1680. [Google Scholar] [CrossRef]
- Neath, A.A.; Cavanaugh, J.E. The Bayesian Information Criterion: Background, Derivation, and Applications. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 199–203. [Google Scholar] [CrossRef]
- Bosma, M.; Brinkhuis, R.; Rensen, E.; Watson, R. A New Method for the Quantitative Determination and Prediction of Sag and Levelling in Powder Coatings. Prog. Org. Coat. 2011, 72, 26–33. [Google Scholar] [CrossRef]
- Perron, S.; Tully, C.S.; Gupta, S.; Fox, M.S.; Zagidulin, D.; Noël, J.J.; Ouriadov, A. Implementation of the X-Centric Pulse Sequence at Low Field for MRI of Water Penetration in Clay. J. Magn. Reson. 2025, 373, 107852. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile Preparation of Water-Dispersible Graphene Sheets Stabilized by Carboxylated Oligoanilines and Their Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17648. [Google Scholar] [CrossRef]
- Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental Evaluation of Polyester and Epoxy-Polyester Powder Coatings in Aggressive Media. Coatings 2018, 8, 98. [Google Scholar] [CrossRef]
- Golgoon, A.; Aliofkhazraei, M.; Toorani, M.; Moradi, M.H.; Rouhaghdam, A.S. Corrosion and Wear Properties of Nanoclay-Polyester Nanocomposite Coatings Fabricated by Electrostatic Method. Procedia Mater. Sci. 2015, 11, 536–541. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Vaganov, G.V.; Yudin, V.E.; Vuorinen, J. Characterization and Corrosion Protection Properties of Epoxy Powder Coatings Containing Nanoclays. Prog. Org. Coat. 2013, 76, 757–767. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer-Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Rashmi; Renukappa, N.M.; Chikkakuntappa, R.; Kunigal, N.S. Montmorillonite Nanoclay Filler Effects on Electrical Conductivity, Thermal and Mechanical Properties of Epoxy-Based Nanocomposites. Polym. Eng. Sci. 2011, 51, 1827–1836. [Google Scholar] [CrossRef]

| Panel No. | Experiment No. | x1, Nanoclay/wt.% | x2, Binder/wt.% | z, Film Thickness/µm |
|---|---|---|---|---|
| 1 | 7 | 0 | 100 | 40 |
| 2 | 11 | 0 | 100 | 80 |
| 3 | 17 | 0 | 100 | 60 |
| 4 | 18 | 0 | 100 | 60 |
| 5 | 1 | 2 | 98 | 70 |
| 6 | 3 | 2 | 98 | 40 |
| 7 | 10 | 2 | 98 | 50 |
| 8 | 4 | 4 | 96 | 60 |
| 9 | 8 | 4 | 96 | 80 |
| 10 | 9 | 4 | 96 | 60 |
| 11 | 13 | 4 | 96 | 80 |
| 12 | 14 | 4 | 96 | 60 |
| 13 | 16 | 4 | 96 | 40 |
| 14 | 6 | 6 | 94 | 70 |
| 15 | 15 | 6 | 94 | 50 |
| 16 | 2 | 8 | 92 | 80 |
| 17 | 5 | 8 | 92 | 60 |
| 18 | 12 | 8 | 92 | 60 |
| 19 | 19 | 8 | 92 | 40 |
| Panel No. | Experiment No. | Actual Coating Film Thickness/µm | Response 1, Rp/Ω∙cm2 | Response 2, Specular Gloss/Gloss Unit (GU) |
|---|---|---|---|---|
| 1 | 7 | 43.3 | 2.32 × 106 | 110.3 |
| 2 | 11 | 79.7 | 6.77 × 107 | 113.5 |
| 3 | 17 | 62.5 | 4.62 × 106 | 111.1 |
| 4 | 18 | 64.8 | 9.94 × 106 | 112.2 |
| 5 | 1 | 70.1 | 8.38 × 107 | 103.4 |
| 6 | 3 | 40.3 | 1.07 × 107 | 102.6 |
| 7 | 10 | 53.1 | 1.23 × 107 | 100.4 |
| 8 | 4 | 57.5 | 7.70 × 108 | 55.0 |
| 9 | 8 | 75.4 | 3.98 × 109 | 62.3 |
| 10 | 9 | 59.1 | 1.57 × 109 | 59.0 |
| 11 | 13 | 80.0 | 1.38 × 1010 | 63.6 |
| 12 | 14 | 55.5 | 5.07 × 108 | 51.1 |
| 13 | 16 | 35.1 | 2.53 × 107 | 31.0 |
| 14 | 6 | 65.1 | 7.08 × 108 | 14.5 |
| 15 | 15 | 45.7 | 1.17 × 107 | 20.6 |
| 16 | 2 | 79.7 | 3.55 × 107 | 5.2 |
| 17 | 5 | 64.4 | 3.16 × 107 | 4.8 |
| 18 | 12 | 63.3 | 2.73 × 107 | 4.7 |
| 19 | 19 | 42.6 | 1.05 × 107 | 4.3 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 95.50 | 5 | 19.10 | 19.68 | <0.0001 |
| Linear Mixture | 3.63 | 1 | 3.63 | 3.73 | 0.0754 |
| x1 × x2 | 55.55 | 1 | 55.55 | 57.22 | <0.0001 |
| x1 × z | 1.76 | 1 | 1.76 | 1.82 | 0.2008 |
| x2 × z | 5.52 | 1 | 5.52 | 5.68 | 0.0331 |
| x1 × x2 × z | 3.83 | 1 | 3.83 | 3.95 | 0.0685 |
| Residual | 12.62 | 13 | 0.9708 | ||
| Corrected Total | 108.12 | 18 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 31,244.21 | 5 | 6248.84 | 50.83 | <0.0001 |
| Linear Mixture | 30,867.18 | 1 | 30,867.18 | 251.08 | <0.0001 |
| x1 × x2 | 14.87 | 1 | 14.87 | 0.1209 | 0.7336 |
| x1 × z | 9.15 | 1 | 9.15 | 0.0744 | 0.7893 |
| x2 × z | 26.88 | 1 | 26.88 | 0.2186 | 0.6478 |
| x1 × x2 × z | 165.96 | 1 | 165.96 | 1.35 | 0.2662 |
| Residual | 1598.19 | 13 | 122.94 | ||
| Corrected Total | 32,842.40 | 18 |
| Time | CPEcoat | Rpore | CPEdl | Rct | Ws-RD | Ws-TD | Ws-P | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sn | αcoat | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sn | αdl | Ω∙cm2 | Ω∙cm2∙sP | s | ||
| 0 | 4.99 × 10−11 | 0.981 | 6.36 × 108 | 6.96 × 10−12 | 0.634 | 1.44 × 1011 | 3.44 × 10−4 | |||
| 1 | 4.40 × 10−11 | 0.992 | 6.11 × 108 | 2.31 × 10−11 | 0.633 | 4.58 × 1011 | 1.26 × 10−4 | |||
| 2 | 4.37 × 10−11 | 0.993 | 4.35 × 109 | 9.44 × 10−11 | 0.510 | 1.53 × 1011 | 3.78 × 10−4 | |||
| 3 | 4.30 × 10−11 | 0.994 | 5.55 × 109 | 1.28 × 10−10 | 0.506 | 3.71 × 1011 | 1.09 × 10−4 | |||
| 4 | 4.35 × 10−11 | 0.993 | 6.40 × 109 | 1.72 × 10−10 | 0.619 | 1.60 × 1011 | 1.25 × 10−4 | |||
| 5 | 4.30 × 10−11 | 0.994 | 6.16 × 109 | 1.52 × 10−10 | 0.559 | 4.47 × 1010 | 3.61 × 1011 | 1.04 × 103 | 0.727 | 1.18 × 10−4 |
| 10 | 4.21 × 10−11 | 0.994 | 5.24 × 109 | 1.51 × 10−10 | 0.583 | 7.67 × 109 | 7.08 × 1010 | 1.95 × 103 | 0.550 | 1.08 × 10−4 |
| 15 | 4.26 × 10−11 | 0.994 | 4.52 × 109 | 1.30 × 10−10 | 0.539 | 1.08 × 1010 | 5.76 × 1010 | 2.52 × 103 | 0.538 | 1.02 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.S.; Xian, C.; Chen, J.; Hedberg, Y.S.; Noël, J.J. Optimizing Anti-Corrosive Properties of Polyester Powder Coatings Through Montmorillonite-Based Nanoclay Additive and Film Thickness. Corros. Mater. Degrad. 2025, 6, 39. https://doi.org/10.3390/cmd6030039
Yang MS, Xian C, Chen J, Hedberg YS, Noël JJ. Optimizing Anti-Corrosive Properties of Polyester Powder Coatings Through Montmorillonite-Based Nanoclay Additive and Film Thickness. Corrosion and Materials Degradation. 2025; 6(3):39. https://doi.org/10.3390/cmd6030039
Chicago/Turabian StyleYang, Marshall Shuai, Chengqian Xian, Jian Chen, Yolanda Susanne Hedberg, and James Joseph Noël. 2025. "Optimizing Anti-Corrosive Properties of Polyester Powder Coatings Through Montmorillonite-Based Nanoclay Additive and Film Thickness" Corrosion and Materials Degradation 6, no. 3: 39. https://doi.org/10.3390/cmd6030039
APA StyleYang, M. S., Xian, C., Chen, J., Hedberg, Y. S., & Noël, J. J. (2025). Optimizing Anti-Corrosive Properties of Polyester Powder Coatings Through Montmorillonite-Based Nanoclay Additive and Film Thickness. Corrosion and Materials Degradation, 6(3), 39. https://doi.org/10.3390/cmd6030039









