Preliminary Assessment of Cooling Water Chemistry for Fusion Power Plants
Abstract
:1. Introduction
2. Materials and Methods for Experimental Activities
2.1. Demo Wcll Water Chemistry Optimization
- EUROFER 97 coupons (Type A),
- AISI 316L coupons (Type B),
- Heterogeneous welded joints constituted by EUROFER 97 and AISI 316L with a central welded zone (Type AB), and
- Homogeneous welded joints constituted by EUROFER 97 with a central welded zone (Type AA).
- A primary production phase of Vacuum Induction Melting (VIM),
- A Vacuum Arc Remelting (VAR) for the second phase, and
- A final melting in a prismatic ingot of 80 kg.
- Scanning Electron Miscroscope (SEM) Model Zeiss EVO MA-15;
- Transmission Electron Microscopy (TEM), Model JEOL JEM-3200FS-HR, for the characterization of the oxide scale forms; and
- Focussed Ion Beam milling combined with Scanning Electron Microscopy (FIB-SEM) for the extraction of the TEM lamella from the sample top surface.
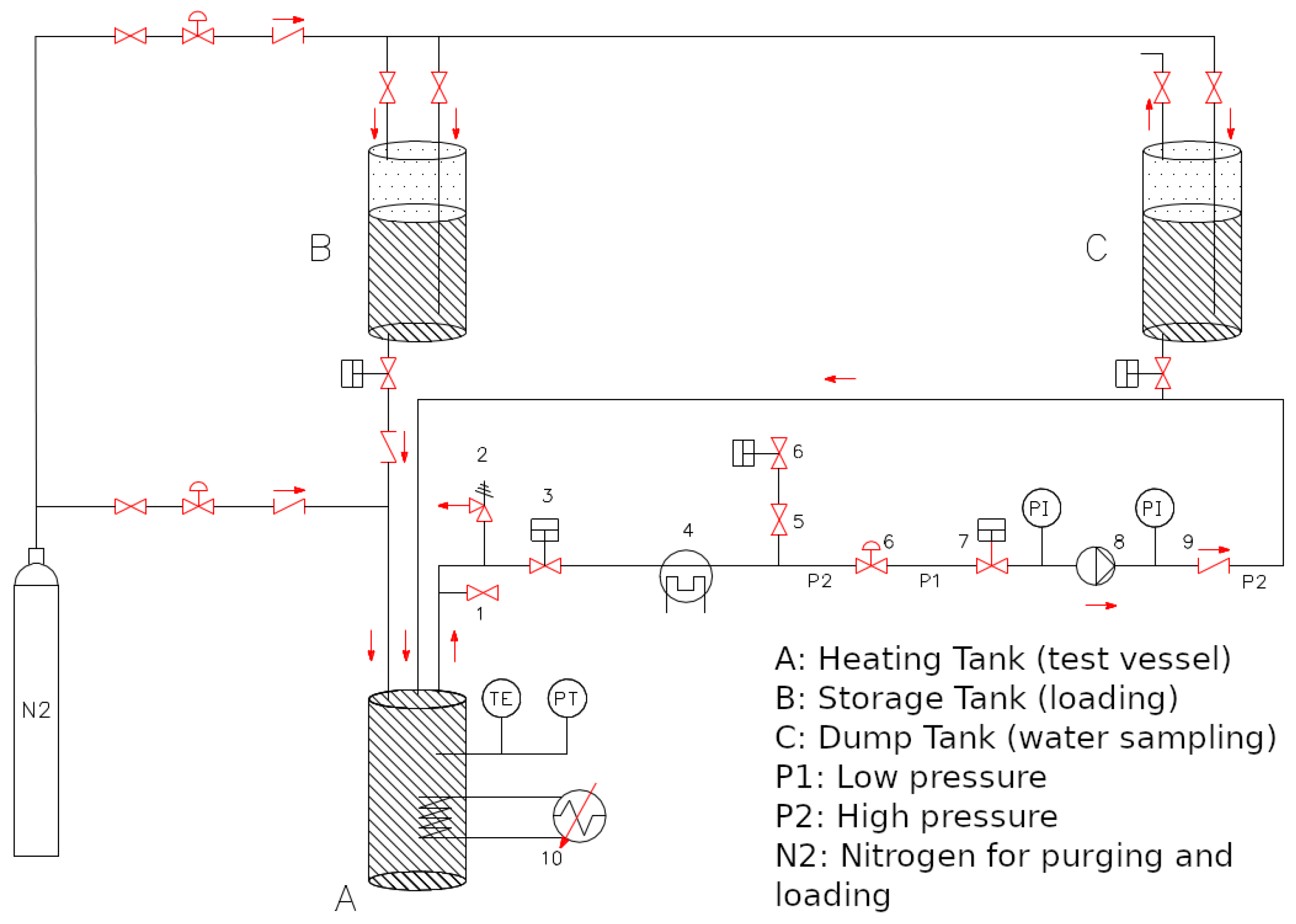
2.2. DTT VV Water Cooling Circuit Chemistry
3. Results
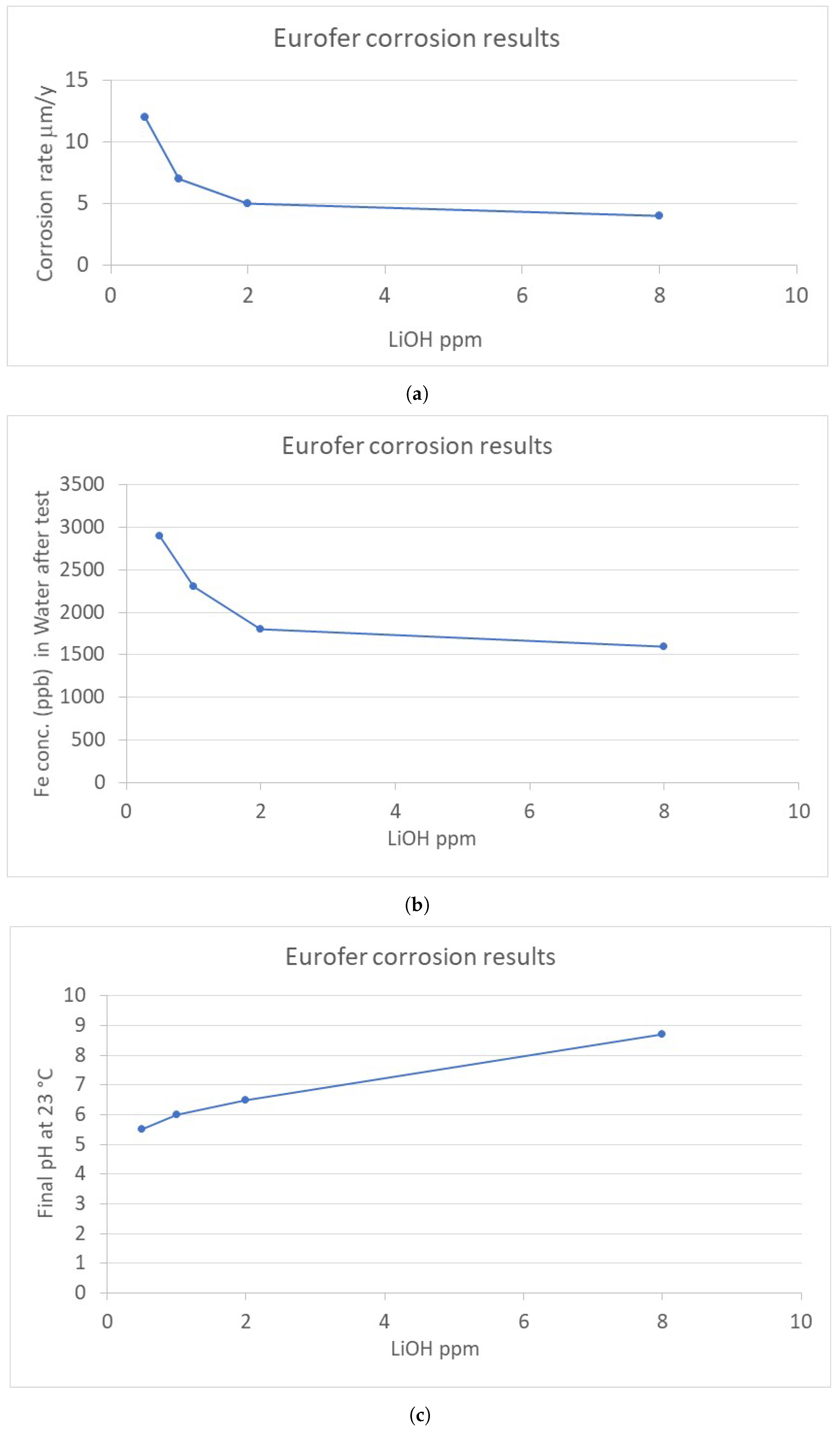
3.1. Results of Experimental Activities for DEMO WCLL BB Water Chemistry
- The form of the corrosion (uniform or localized) in both EUROFER 97 and welded specimens was determined using a 3D optical microscope (OM);
- The presence of cracks originated by Stress Corrosion Cracking (SCC) phenomena was investigated through OM observations on cross-sectioned U-bended speciemens (only on AISI 316L and Ni alloy 625 in autoclave);
- SEM and Energy Dispersion Spectroscopy (EDS) analyses on cross-sectioned specimens; and
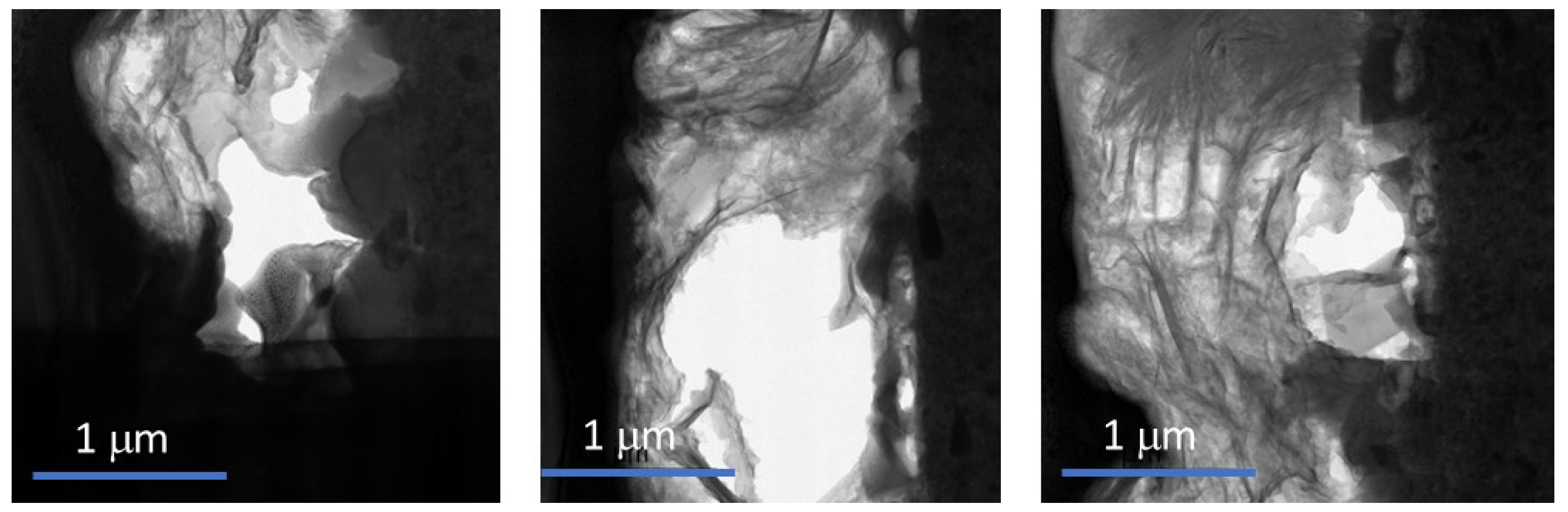
- TEM and EDS analyses on cross sectioned specimens to evaluate the oxide layer composition, structure, and porosity.
- The first one considers the initial and the final weights of the coupons, without any corrosion product removal from the surfaces.
- The second approach compares the speciemens weight after a gentle chemical pickling with specific acid solutions in order to evalute oxide scale formation during exposure. The removal was performed in accordance with the ASTM G1 procedure.
3.2. Preliminary Activities for DTT VV Water Cooling Circuit Chemistry
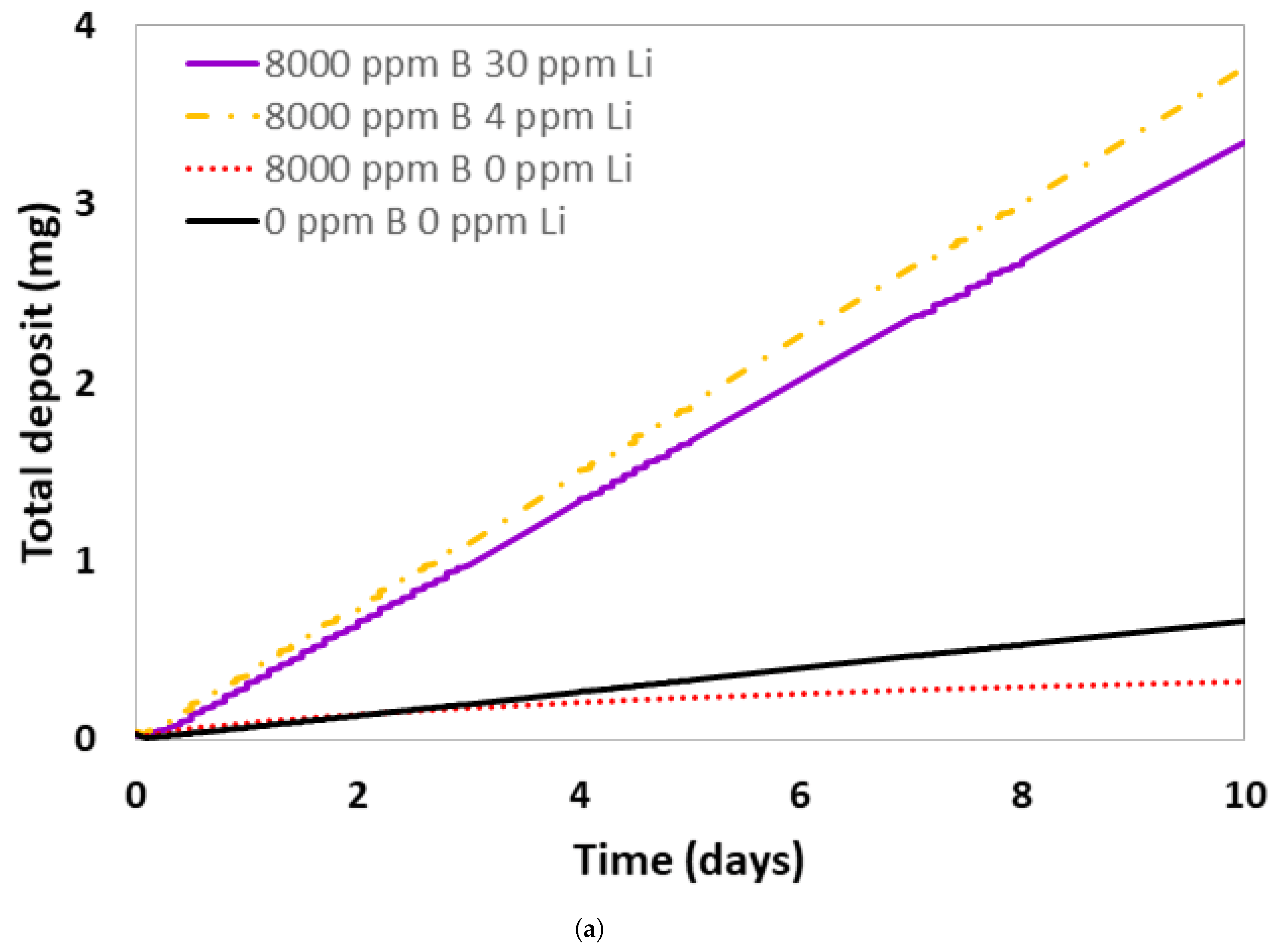
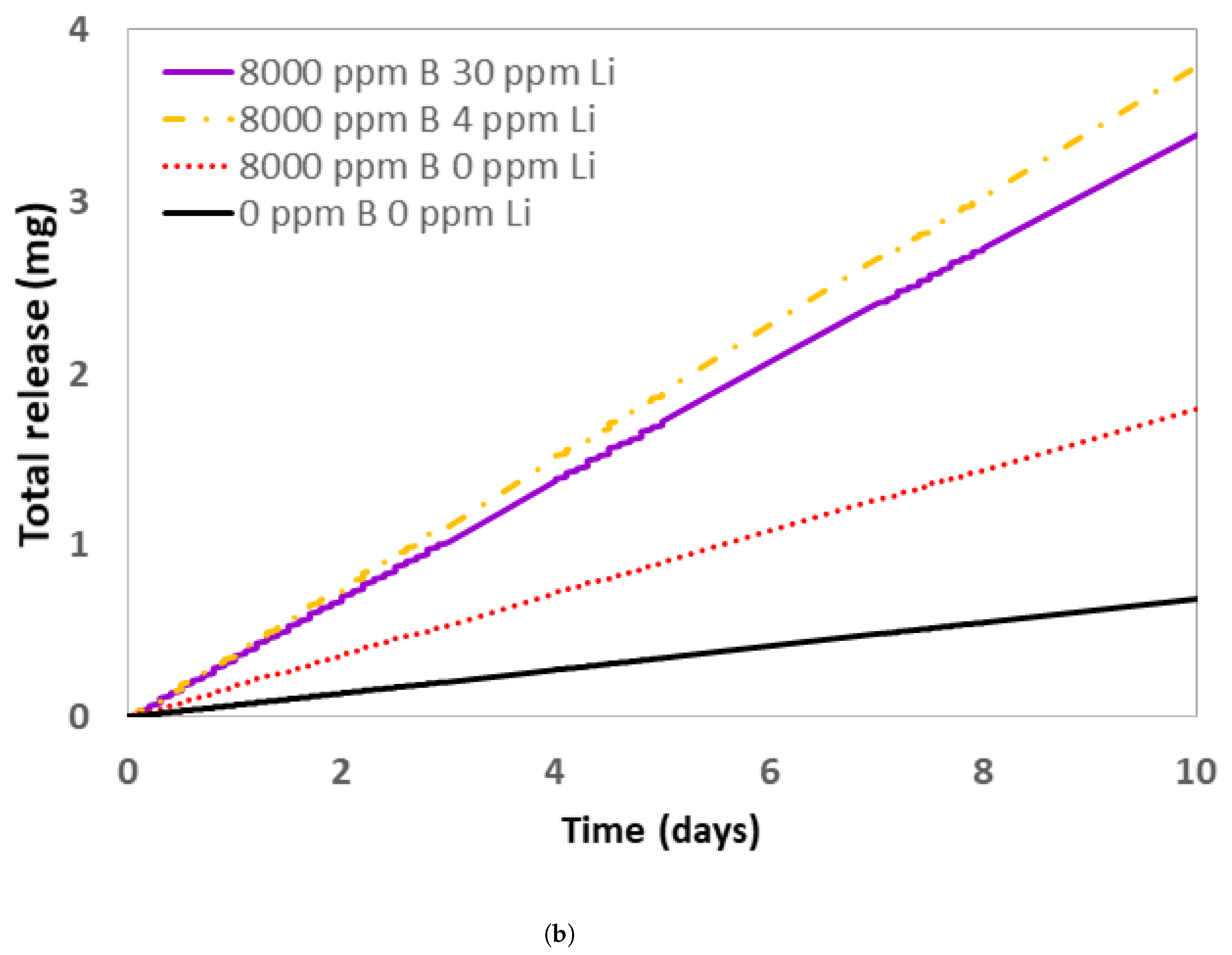
3.3. Comparison of Corrosion Experiments Results with Pactiter V2.1 Code Corrosion Rates Predictions
- To use validated input data, especially for EUROFER 97, for simulations runs;
- To validate not only corrosion and release models but also other main phenomena in ACP assessment by computer codes, transport, diffusion etc.
4. Discussion
4.1. Experimental Activities on DEMO WCLL BB Corrosion
4.2. Experimental Activities on DTT VV Water Cooling Circuit Chemistry
4.3. Overall Discussion
5. Conclusions
- The corrosion testing in water chemistries with LiOH addition showed, in general, low corrosion rates for EUROFER and no cracking for AISI 316L, both on base materials and welded joint samples. Considering the corrosion behavior of a Ni-Alloy UNS625 as reference, similar results were obtained in the case of ammonia chemistries.
- EUROFER was shown to be more affected by uniform corrosion for the effect of welding process. In this regard, corrosion rates detected in the case of EUROFER welded joint specimens were 30–50% higher than those of unwelded samples.
- The beneficial effect of a higher pH condition was observed for the corrosion susceptibility of EUROFER, and no SCC phenomena was detected for both AISI 316 and Ni-Alloy UNS625. These results are very promising for enlarging the operative range of pH for LiOH chemistry, simplifying the chemistry control of the coolant for CVCS units.
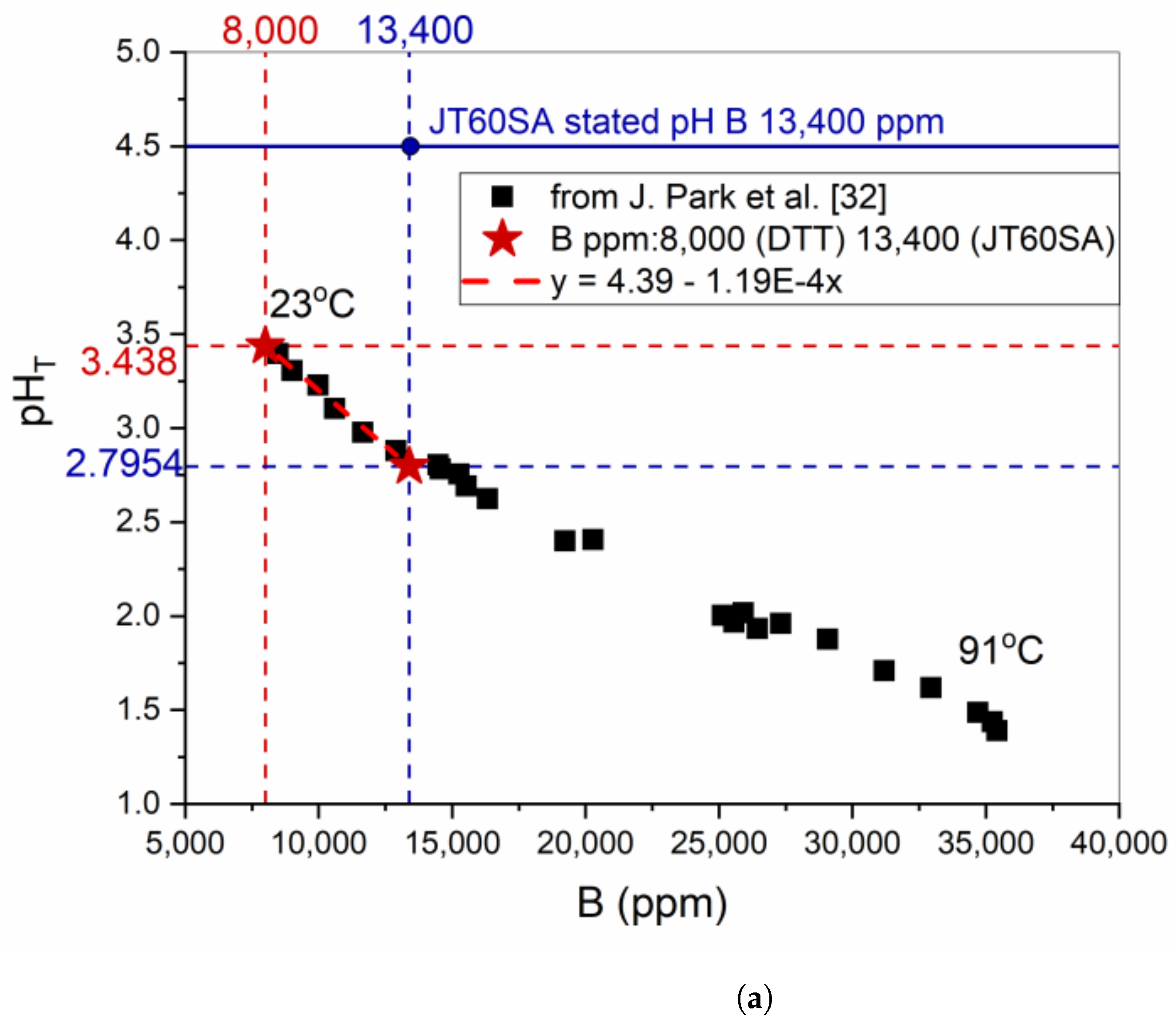
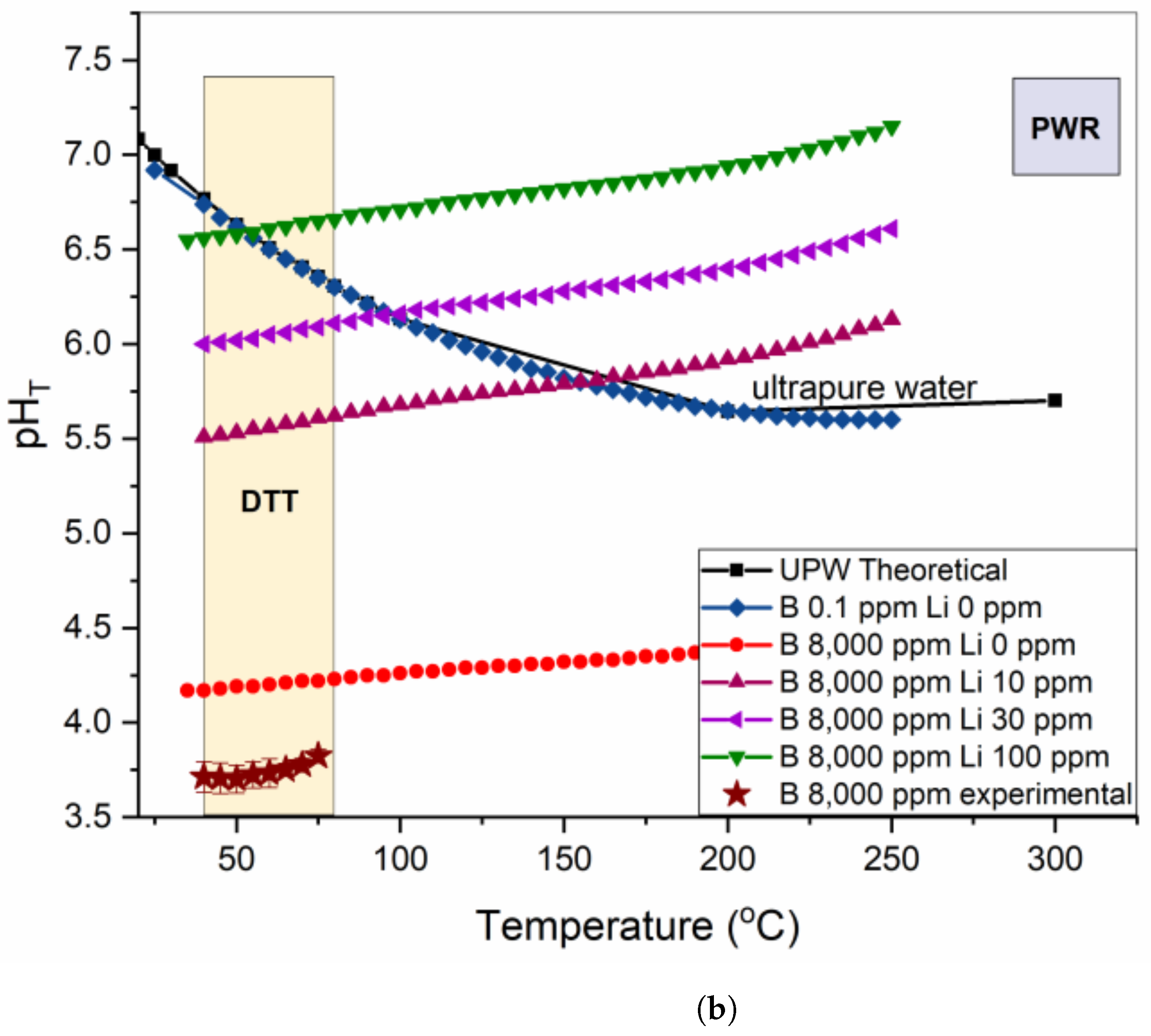
- DTT VV exploited a highly enriched borated water (8000 ppm B) solution alternated to UPW as a coolant. The choice of adding a base, LiOH, to neutralize the borated water pH was discussed here, but contradictory results from simulations were obtained.
- Experimental tests showed that the ACP codes developed for PWR water chemistry regimes (pH25°C = 6.2–7.3 with Li varying from 0.5 to 4 ppm and B varying from 0 to 2400 ppm) overestimated the pHT of 8000 ppm B borated water solution needed for DTT.
- The choice of adding LiOH to a DTT VV borated water solution needs to be validated by experimental tests to ensure that the code that will be used to assess DTT ACPs is representative of the real situation as well as to minimize corrosion in the circuit.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belous, V.; Kalinin, G.; Lorenzetto, P.; Velikopolskiy, S. Assessment of the corrosion behaviour of structural materials in the water coolant of ITER. J. Nucl. Mater. 1998, 258–263, 351–356. [Google Scholar] [CrossRef]
- Harrington, C.; Baron-Wiechec, A.; Burrows, R.; Holmes, R.; Clark, R.; Walters, S.; Martin, T.; Springell, R.; Öijerholm, J.; Becker, R.; et al. Chemistry and corrosion research and development for the water cooling circuits of European DEMO. Fusion Eng. Des. 2019, 146, 478–481. [Google Scholar] [CrossRef]
- Cavallini, C.; Dalla Palma, M.; Fellin, F.; Gasparrini, C.; Tinti, P.; Zamengo, A.; Zaupa, M. Investigation of corrosion-erosion phenomena in the primary cooling system of SPIDER. Fusion Eng. Des. 2021, 166, 112271. [Google Scholar] [CrossRef]
- Bigot, B. ITER assembly phase: Progress toward first plasma. Fusion Eng. Des. 2021, 164, 112207. [Google Scholar] [CrossRef]
- Crisanti, F.; Martone, R.; Mazzitelli, G.; Pizzuto, A. The DTT device: Guidelines of the operating program. Fusion Eng. Des. 2017, 122, 382–386. [Google Scholar] [CrossRef]
- Ishida, S.; Barabaschi, P.; Kamada, Y. Overview of the JT-60SA project. Nucl. Fusion 2011, 51, 094018. [Google Scholar] [CrossRef]
- Federici, G.; Bachmann, C.; Barucca, L.; Biel, W.; Boccaccini, L.; Brown, R.; Bustreo, C.; Ciattaglia, S.; Cismondi, F.; Coleman, M.; et al. DEMO design activity in Europe: Progress and updates. Fusion Eng. Des. 2018, 136, 729–741. [Google Scholar] [CrossRef]
- Karditsas, P.J. Water radiolysis in fusion neutron environments. Fusion Eng. Des. 2011, 86, 2701–2704. [Google Scholar] [CrossRef]
- Kawamura, H.; Hirano, H.; Katsumura, Y.; Uchida, S.; Mizuno, T.; Kitajima, H.; Tsuzuki, Y.; Terachi, T.; Nagase, M.; Usui, N.; et al. BWR water chemistry guidelines and PWR primary water chemistry guidelines in Japan—Purpose and technical background. Nucl. Eng. Des. 2016, 309, 161–174. [Google Scholar] [CrossRef]
- Di Pace, L.; Tarabelli, D.; You, D. Development of the PACTITER code and its application to the assessment of the ITER divertor cooling loop corrosion products. Fusion Technol. 1998, 34, 733–737. [Google Scholar] [CrossRef]
- Kot, A. Water and Water Treatment in Nuclear Power Plants; IPST: Jerusalem, Israel, 1964. [Google Scholar]
- Castelli, R. Nuclear Corrosion Modelling. The Nature of CRUD; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Li, L.; Zhang, J.; Song, W.; Fu, Y.; Xu, X.; Chen, Y. CATE: A code for activated corrosion products evaluation of water-cooled fusion reactor. Fusion Eng. Des. 2015, 100, 340–344. [Google Scholar] [CrossRef]
- Terranova, N.; Di Pace, L. DEMO WCLL primary heat transfer system loops activated corrosion products assessment. Fusion Eng. Des. 2021, 170, 112456. [Google Scholar] [CrossRef]
- Karditsas, P.; Caloutsis, A. Corrosion and activation in fusion cooling loops–TRACT. Fusion Eng. Des. 2007, 82, 2729–2733. [Google Scholar] [CrossRef]
- Di Pace, L.; Dacquait, F.; Schindler, P.; Blet, V.; Nguyen, F.; Philibert, Y.; Larat, B. Development of the PACTITER code and its application to safety analyses of ITER Primary Cooling Water System. Fusion Eng. Des. 2007, 82, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Genin, J.; Marteau, H.; Dacquait, F. The OSCAR code package: A unique tool for simulating PWR contamination. In Proceedings of the Nuclear Power Plant Conference 2010 (NPC 2010): International Conference on Water Chemistry of Nuclear Reactor Systems and 8th International Radiolysis, Electrochemistry and Materials Performance Workshop, Quebec City, QC, Canada, 3–8 October 2010. [Google Scholar]
- OECD/NEA. Occupational Exposures at Nuclear Power Plants. In Twenty-Eighth Annual Report of the ISOE Programme; Technical Report, No. 7536; NEA: Boulogne-Billancourt, France, 2021. [Google Scholar]
- ITER. Preliminary Safety Report (RPrS); Technical Report, IDM UID 3ZR2NC; ITER: Saint-Paul-lez-Durance, France, 2010. [Google Scholar]
- Di Pace, L.; Quintieri, L. Assessment of activated corrosion products for the DEMO WCLL. Fusion Eng. Des. 2018, 136, 1168–1172. [Google Scholar] [CrossRef]
- Welding and Allied Processes. Types of Joint Preparation. Part 1: Manual Metal arc Welding, Gas-Shielded Metal Arc Welding, Gas Welding, TIG Welding and Beam Welding of Steels; EN ISO 9692-1:2013; The International Organization for Standardization: Geneva, Switzerland, 2013.
- Christensen, H. Remodeling of the Oxidant Species During Radiolysis of High-Temperature Water in a Pressurized Water Reactor. Nucl. Technol. 1995, 109, 373–382. [Google Scholar] [CrossRef]
- Aaltonen, P.; Hanninen, H. Water Chemistry and Behavior of Materials in PWRs and BWRs; Technical Report, IAEA-TECDOC-965; IAEA: Vienna, Austria, 1997. [Google Scholar]
- EPRI. PWR Primary Water Chemistry Guidelines; Technical Report; EPRI: Palo Alto, CA, USA, 2014. [Google Scholar]
- Bevilacqua, R.; Hambsch, F.J.; Vidali, M.; Ruskov, I.; Lamia, L. 10B(n, α)7Li and 10B(n,α1γ)7Li cross section data up to 3 MeV incident neutron energy. EPJ Web Conf. 2017, 146, 11010. [Google Scholar] [CrossRef] [Green Version]
- Beslu, P. PACTOLE: A Calculation Code for Predicting Corrosion Product Behavior and Activation in PWR Primary Coolant Systems; Technical Report, CEA Technical Note SCOS-LCC-90-096; CEA: Saint-Paul-lez-Durance, France, 1990. [Google Scholar]
- Martone, R.; Albanese, R.; Crisanti, F.; Pizzuto, A.; Martin, P. Divertor Tokamak Test Facility Interim Design Report. Available online: https://www.dtt-dms.enea.it/share/s/avvglhVQT2aSkSgV9vuEtw (accessed on 22 June 2019).
- Light, T.S. Temperature dependence and measurement of resistivity of pure water. Anal. Chem. 1984, 56, 1138–1142. [Google Scholar] [CrossRef]
- Torella, R.; Lo Piccolo, E. Water Chemistry Control: Validation of Buffered Water Solutions for WCLL BB System by Corrosion Testing; Technical Report, WPBB 3.3.1 T005-D001 EFDA_D_2P5N82; EUROfusion: Garching, Germany, 2019. [Google Scholar]
- Colangeli, A.; Villari, R.; Luis, R.; Moro, F.; Sandri, S.; Fonnesu, N.; Flammini, D.; Mariano, G.; Crisanti, F.; Ramogida, G.; et al. Neutronics study for DTT tokamak building. Fusion Eng. Des. 2019, 146, 2581–2585. [Google Scholar] [CrossRef]
- Villari, R.; Barabaschi, P.; Cucchiaro, A.; della Corte, A.; Di Zenobio, A.; Dolgetta, N.; Lacroix, B.; Moro, F.; Muzzi, L.; Nicollet, S.; et al. Neutronic analysis of the JT-60SA toroidal magnets. Fusion Eng. Des. 2009, 84, 1947–1952. [Google Scholar] [CrossRef]
- Park, J.H. Boric Acid Corrosion of Light Water Reactor Pressure Vessel Materials; Technical Report, NUREG/CR-6875; ANL: Washington, DC, USA, 2005. [Google Scholar]
- IAEA. Good Practices for Water Quality Management in Research Reactors and Spent Fuel Storage Facilities; Technical Report, Nuclear Energy Series No. NP-T-5.2; IAEA: Vienna, Austria, 2011. [Google Scholar]
- IAEA. Coolant Technology of Water Cooled Reactors: An Overview; Technical Report Series No. 347; IAEA: Vienna, Austria, 1993. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; CEBELCOR: Brussels, Belgium, 1974. [Google Scholar]
- EPRI. PWR Primary Water Chemistry Guidelines; Technical Report, TR-105714-V1R4; EPRI: Palo Alto, CA, USA, 1999. [Google Scholar]
- Burrows, R.; Harrington, C.; Baron-Wiechec, A.; Warren, A. Development of Conceptual Water Chemistry Guidelines for Water Coolant Circuits of a Demonstration Fusion Power Reactor. In Proceedings of the 20th Nuclear Plant Chemistry Conference, Brighton, UK, 2–7 October 2016. [Google Scholar]
- Burrows, R.; Holmes, R.; Walters, S. WPBB 3.3.2-T001-D002 EFDA_D_2NBJRL; Technical Report; EUROfusion: Garching, Germany, 2018. [Google Scholar]
- Villari, R.; Angelone, M.; Caiffi, B.; Colangeli, A.; Crisanti, F.; Flammini, D.; Fonnesu, N.; Luis, R.; Mariano, G.; Marocco, D.; et al. Nuclear design of Divertor Tokamak Test (DTT) facility. Fusion Eng. Des. 2020, 155, 111551. [Google Scholar] [CrossRef]
- Wanner, M. Project Plant Integration Document (PID) V4.2; Technical Report, JT-60 SA, BA_D_222UJY; JT-60 SA: Naka, Japan, 2020. [Google Scholar]
- Saji, G. Degradation of aged plants by corrosion: “Long cell action” in unresolved corrosion issues. Nucl. Eng. Des. 2009, 239, 1591–1613. [Google Scholar] [CrossRef]
- Mesmer, R.E.; Baes, C.F.; Sweeton, F.H. Acidity measurements at elevated temperatures. IV. Apparent dissociation product of water in 1 m potassium chloride up to 292. deg. J. Phys. Chem. 1970, 74, 1937–1942. [Google Scholar] [CrossRef]
- Palmer, D.; Benezeth, P.; Wesolowski, D.J. Boric acid hydrolysis: A new look at the available data. Power Plant Chemistry. Power Plant Chem. 2000, 2, 261–264. [Google Scholar]
- Provens, H. Primary Circuit Contamination in Nuclear Power Plants: Contribution to Occupational Exposure; Technical Report, Nucléaire; Institut de Radioprotection et de Sûreté: Fontenay aux Roses, France, 2002. [Google Scholar]
- Garrone, E.; Caramello, M.; Paoletti, F.; Pierantoni, V. Preliminary Concept Design Study of Cvcs for Demo Wcll Bb Phts&Bop Indirect Coupling Plan; Technical Report; EUROfusion: Garching, Germany, 2020. [Google Scholar]



| Cr | Ni | Mn | Ti | V | Al | Ta | W | Mo |
| 8.89 | 0.01 | 0.51 | 0.005 | 0.34 | 0.01 | 0.10 | 0.92 | 0.01 |
| C | Si | P | Sn | Sb | N | S | Co | Nb |
| 0.11 | 0.05 | 0.005 | 0.001 | 0.001 | 0.21 | 0.003 | 0.06 | 0.01 |
| NH3 | EUROFER | AISI 316L |
|---|---|---|
| Concentration (ppm) | Corrosion Rate (μm yr−1) | Corrosion Rate (μm yr−1) |
| 500 | 5 | 1 |
| 750 | 4 | 1 |
| Zone | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total Ave. |
|---|---|---|---|---|---|---|---|---|---|
| % | 1.4 | 43.0 | 6.3 | 8.6 | 1.8 | 2.8 | 7.9 | 11.1 | 10.3 |
| Solution pH | Specimen/Mat. | Corr. Rate μm yr−1 | Weight Loss mgm−1 |
|---|---|---|---|
| 6.8 (1 ppm of LiOH) | Type A EUR_3 | 10.5 | 9.42 |
| Type AA EUR_3 | 18.0 | 16.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Piccolo, E.; Torella, R.; Terranova, N.; Di Pace, L.; Gasparrini, C.; Dalla Palma, M. Preliminary Assessment of Cooling Water Chemistry for Fusion Power Plants. Corros. Mater. Degrad. 2021, 2, 512-530. https://doi.org/10.3390/cmd2030027
Lo Piccolo E, Torella R, Terranova N, Di Pace L, Gasparrini C, Dalla Palma M. Preliminary Assessment of Cooling Water Chemistry for Fusion Power Plants. Corrosion and Materials Degradation. 2021; 2(3):512-530. https://doi.org/10.3390/cmd2030027
Chicago/Turabian StyleLo Piccolo, Eugenio, Raffaele Torella, Nicholas Terranova, Luigi Di Pace, Claudia Gasparrini, and Mauro Dalla Palma. 2021. "Preliminary Assessment of Cooling Water Chemistry for Fusion Power Plants" Corrosion and Materials Degradation 2, no. 3: 512-530. https://doi.org/10.3390/cmd2030027
APA StyleLo Piccolo, E., Torella, R., Terranova, N., Di Pace, L., Gasparrini, C., & Dalla Palma, M. (2021). Preliminary Assessment of Cooling Water Chemistry for Fusion Power Plants. Corrosion and Materials Degradation, 2(3), 512-530. https://doi.org/10.3390/cmd2030027






