Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys
Abstract
1. Introduction
2. Principles of Main AFM Based Techniques
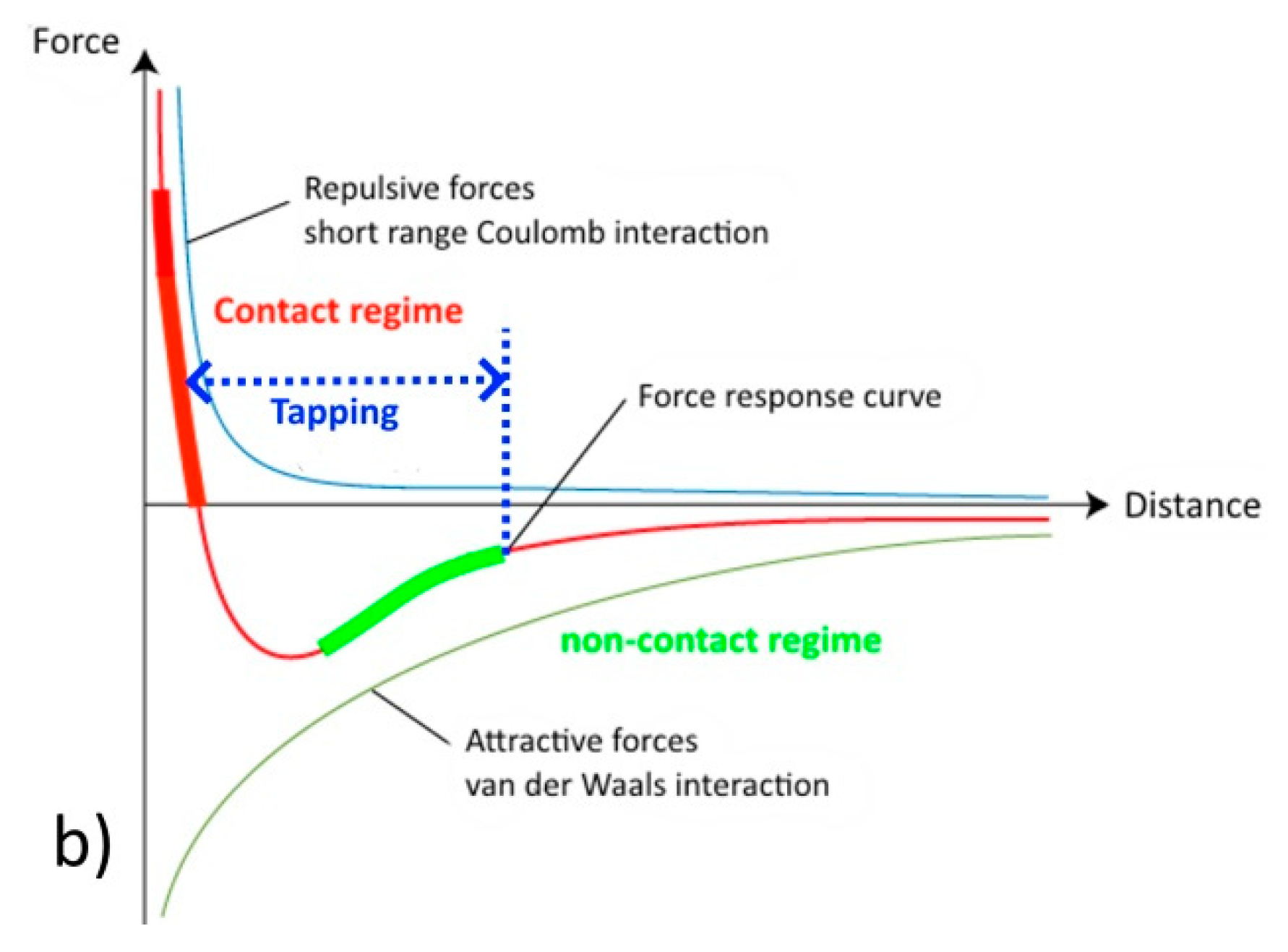
2.1. AFM
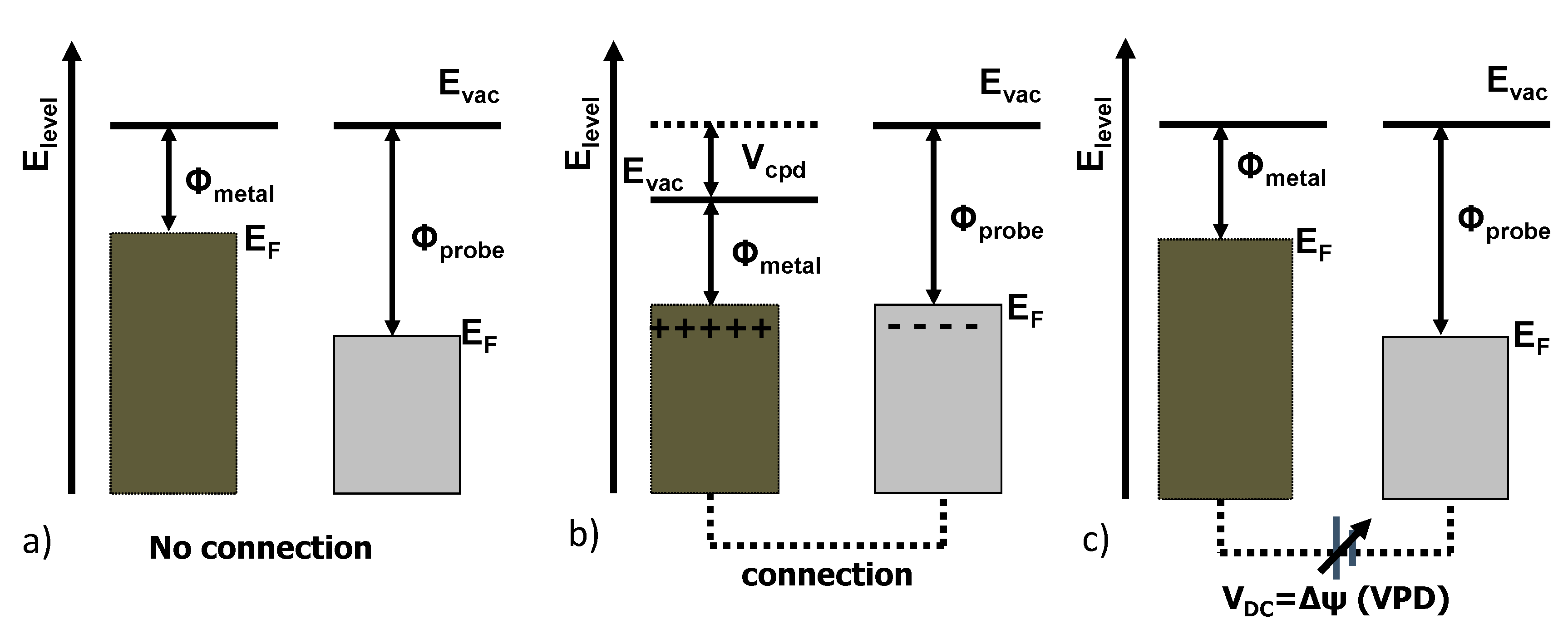
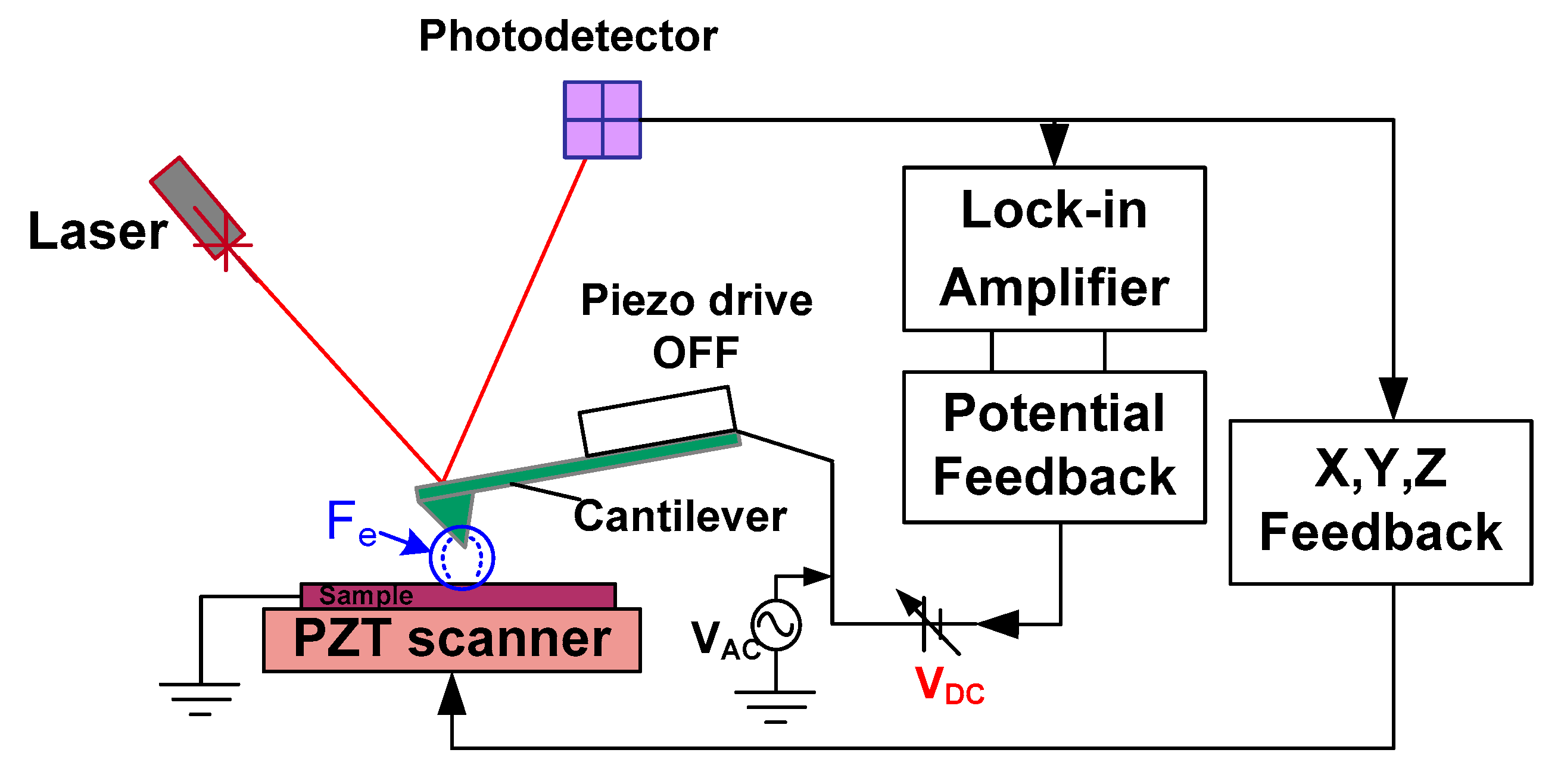
2.2. SKPFM
2.3. EC-AFM
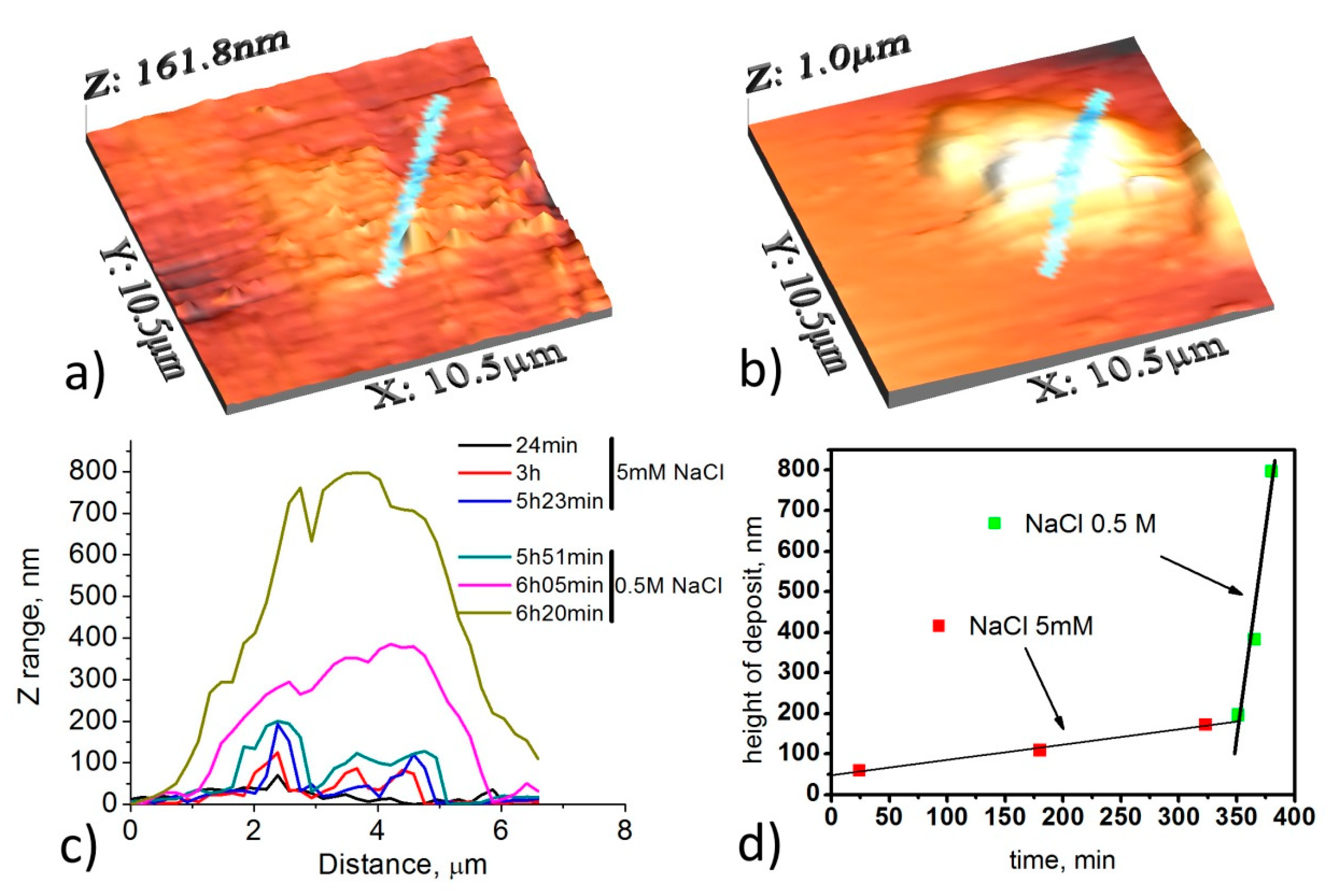
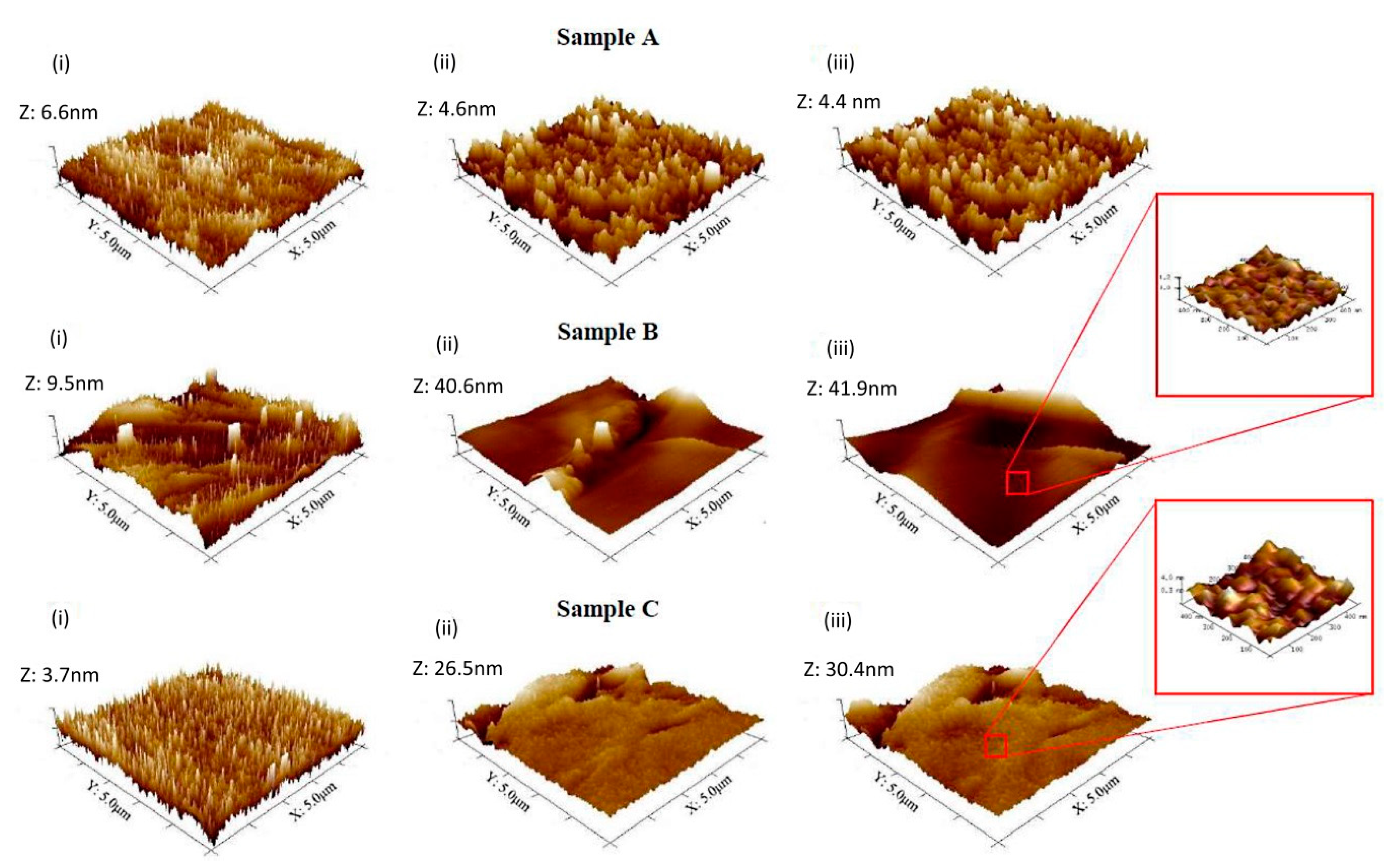
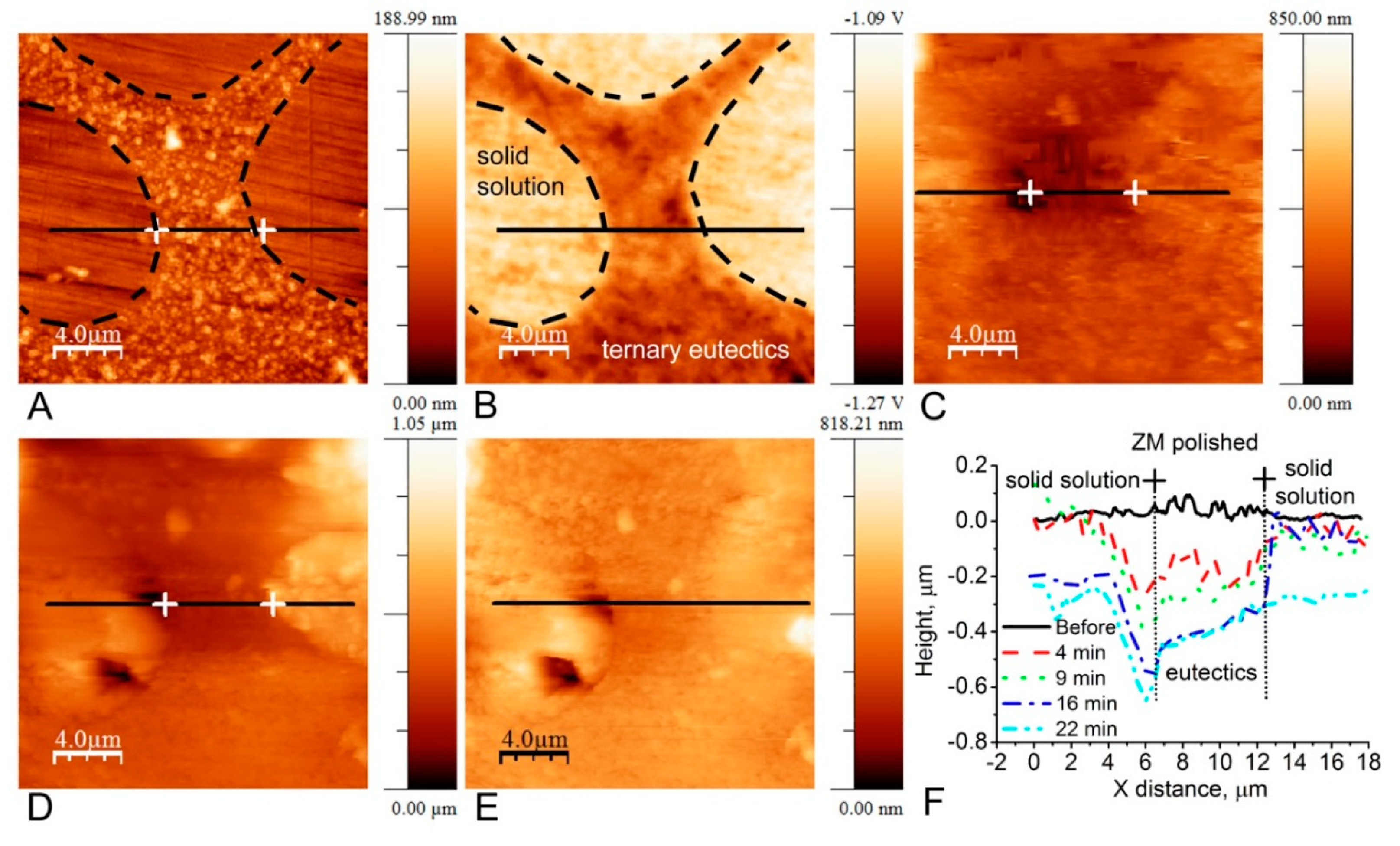
3. In Situ AFM Investigation of Metallic Surfaces
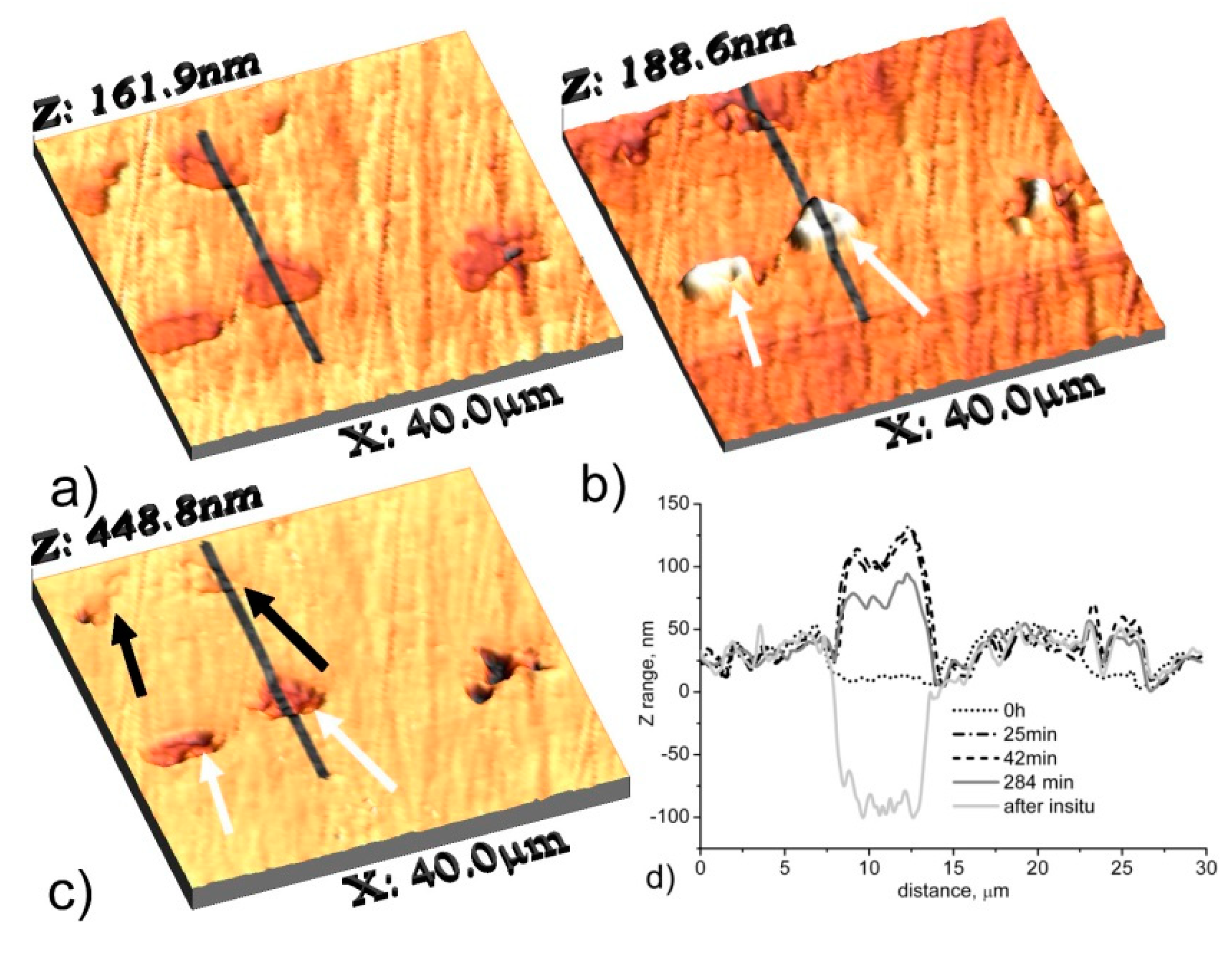
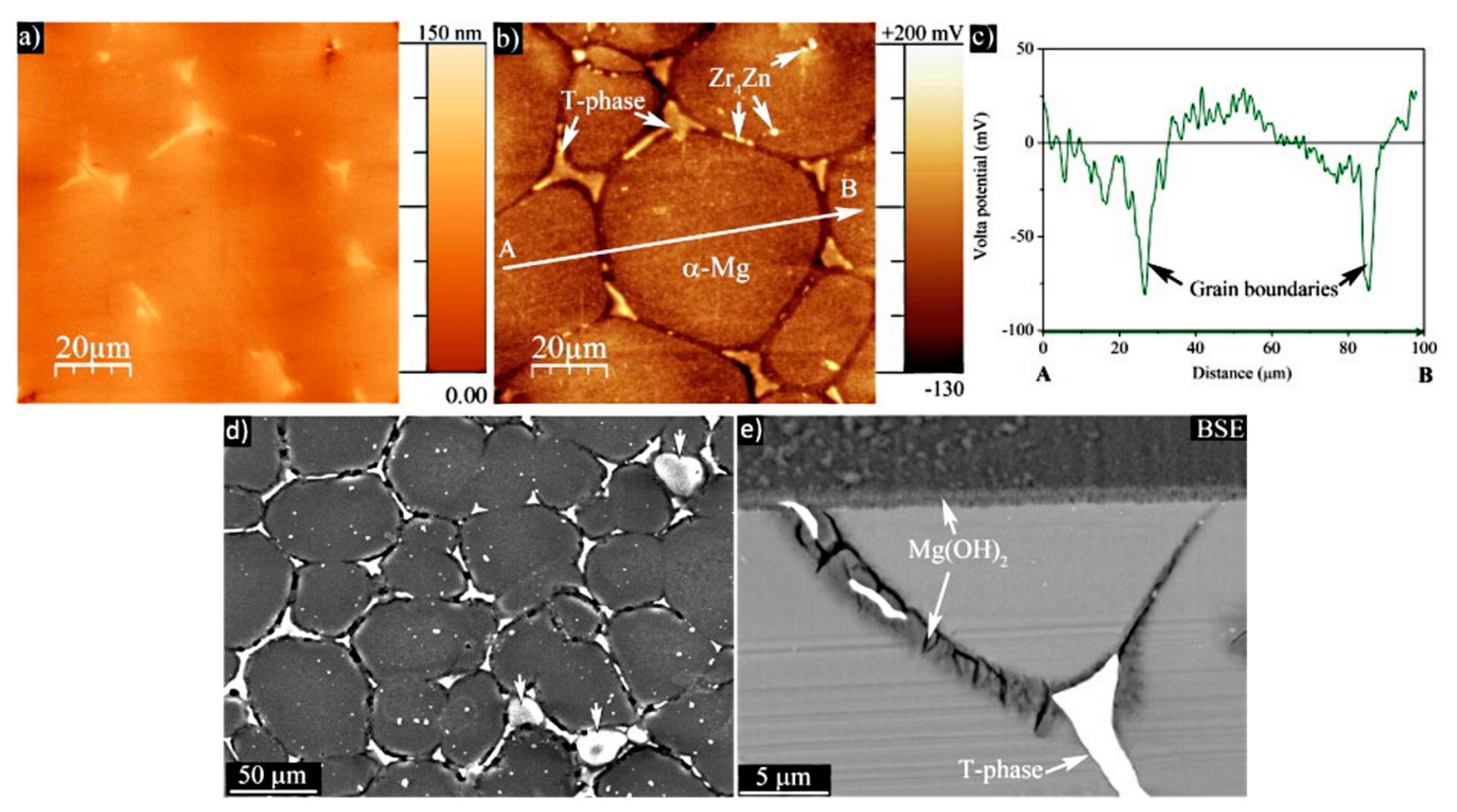
4. Application of SKPFM for Studying Localized Corrosion Susceptibility
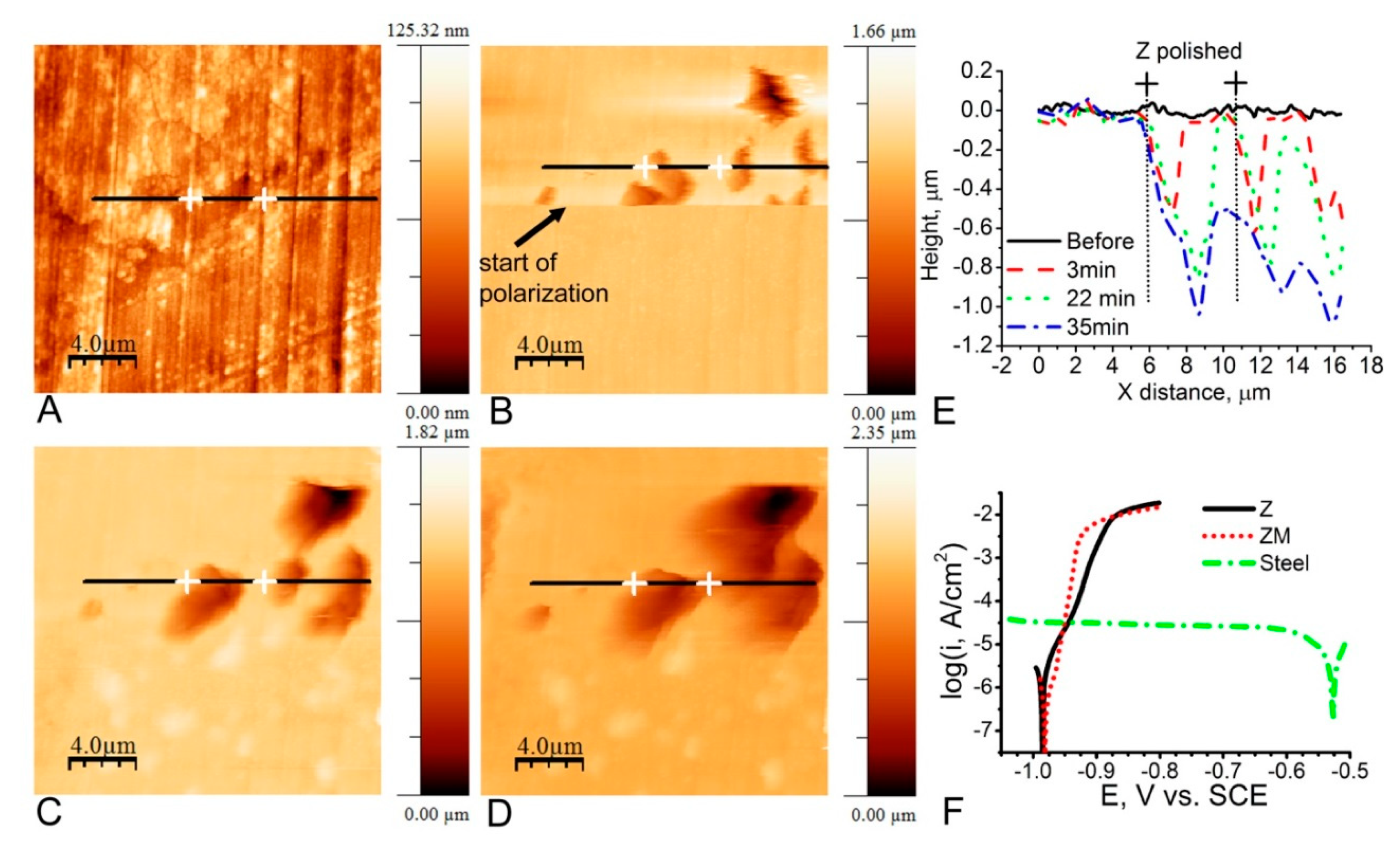
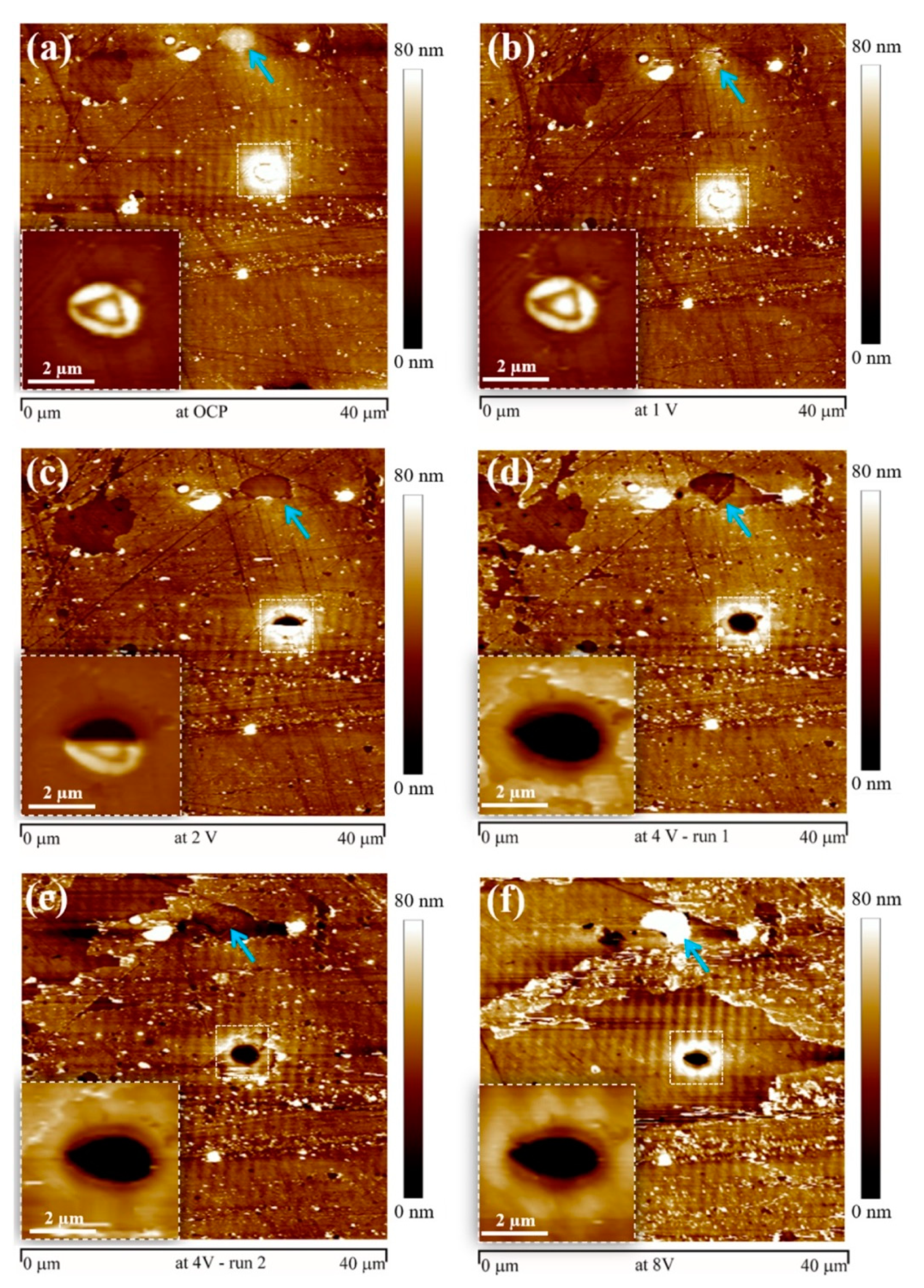
5. In Situ Corrosion Investigation by EC-AFM
6. Conclusions
Funding
Conflicts of Interest
References
- The NACE International IMPACT Study. Available online: http://impact.nace.org/economic-impact.aspx (accessed on 27 September 2020).
- Shreir, L.L.; Burstein, G.T.; Jarman, R.A. Corrosion, 3rd ed.; Volume 1 Metal/Environment Reactions; Butterworth-Heinemann: Linacn House, Jordan Hill, Oxford, UK, 1994; pp. 2:3–2:163. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Totten, G.E.; MacKenzie, D.S. Handbook of Aluminum: Volume 1: Physical Metallurgy and Processes; Marcel Dekker, Inc.: Basel, Switzerland, 2003. [Google Scholar]
- EU. The Energy Efficiency Directive 2012/27/EU. Off. J. Eur. Union 2012, L 315/1. [Google Scholar]
- Lightweight alloys for aerospace application. In Proceedings of the Symposium Sponsored by the Non-Ferrous Metals Committee of the Structural Materials Division (SMD) of TMS (The Minerals, Metals & Materials Society), the TMS Annual Meeting, New Orleans, LA, USA, 12–14 February 2001; TMS: Warrendale, PA, USA, 2001.
- Kainer, K.U.; Kaiser, F.; Materialkunde, D.G.f. Magnesium Alloys and Technology; DGM Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Uijl, N.J.D.; Carless, L.J. 3—Advanced metal-forming technologies for automotive applications. In Advanced Materials in Automotive Engineering; Rowe, J., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 28–56. [Google Scholar]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef] [PubMed]
- Boag, A.; Taylor, R.J.; Muster, T.H.; Goodman, N.; McCulloch, D.; Ryan, C.; Rout, B.; Jamieson, D.; Hughes, A.E. Stable pit formation on AA2024-T3 in a NaCl environment. Corros. Sci. 2010, 52, 90–103. [Google Scholar] [CrossRef]
- Hughes, A.; Muster, T.H.; Boag, A.; Glenn, A.M.; Luo, C.; Zhou, X. Thompson, G.E.; McCulloch, D. Co-operative corrosion phenomena. Corros. Sci. 2010, 52, 665–668. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Meng, Q.; Frankel, G.S. Effect of Cu Content on Corrosion Behavior of 7xxx Series Aluminum Alloys. J. Electrochem. Soc. 2004, 151, B271. [Google Scholar] [CrossRef]
- Buchheit, R.G. A Compilation of Corrosion Potentials Reported for Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 1995, 142, 3994–3996. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef]
- Jönsson, M.; Thierry, D.; LeBozec, N. The influence of microstructure on the corrosion behaviour of AZ91D studied by scanning Kelvin probe force microscopy and scanning Kelvin probe. Corros. Sci. 2006, 48, 1193–1208. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Li, C.-F.; Yu, B.-L. Characterization and Improvement in the Corrosion Performance of a Hot-Chamber Diecast Mg Alloy Thin Plate by the Removal of Interdendritic Phases at the Die Chill Layer. Metall. Mater. Trans. A 2008, 39, 703–715. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Yu, J.; Shan, D.; Han, H. Characterization of Filiform Corrosion of Mg–3Zn Mg Alloy. J. Electrochem. Soc. 2017, 164, C574–C580. [Google Scholar] [CrossRef]
- Li, J.; Birbilis, N.; Buchheit, R.G. Electrochemical assessment of interfacial characteristics of intermetallic phases present in aluminium alloy 2024-T3. Corros. Sci. 2015, 101, 155–164. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Garves, J.; Bland, L.G.; Locke, J.; Allison, J.; Frankel, G.S. Micro- and nano-scale intermetallic phases in AA2070-T8 and their corrosion behavior. Electrochim. Acta 2019, 319, 634–648. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.R.; Grütter, P.; Horne, D.; Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 1991, 69, 668–673. [Google Scholar] [CrossRef]
- Seifert, J.; Rheinlaender, J.; Novak, P.; Korchev, Y.E.; Schäffer, T.E. Comparison of Atomic Force Microscopy and Scanning Ion Conductance Microscopy for Live Cell Imaging. Langmuir 2015, 31, 6807–6813. [Google Scholar] [CrossRef]
- Checco, A.; Cai, Y.; Gang, O.; Ocko, B.M. High resolution non-contact AFM imaging of liquids condensed onto chemically nanopatterned surfaces. Ultramicroscopy 2006, 106, 703–708. [Google Scholar] [CrossRef]
- Setvín, M.; Wagner, M.; Schmid, M.; Parkinson, G.S.; Diebold, U. Surface point defects on bulk oxides: Atomically-resolved scanning probe microscopy. Chem. Soc. Rev. 2017, 46, 1772–1784. [Google Scholar] [CrossRef]
- Breakspear, S.; Smith, J.R.; Campbell, S.A. AFM in Surface Finishing: Part III. Lateral Force Microscopy and Friction Measurements. Trans. IMF 2003, 81, B68–B70. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Rohwerder, M.; Turcu, F. High-resolution Kelvin probe microscopy in corrosion science: Scanning Kelvin probe force microscopy (SKPFM) versus classical scanning Kelvin probe (SKP). Electrochim. Acta 2007, 53, 290–299. [Google Scholar] [CrossRef]
- Jacobs, H.O.; Leuchtmann, P.; Homan, O.J.; Stemmer, A. Resolution and contrast in Kelvin probe force microscopy. J. Appl. Phys. 1998, 84, 1168–1173. [Google Scholar] [CrossRef]
- Hudlet, S.; Jean, M.S.; Roulet, B.; Berger, J.; Guthmann, C. Electrostatic forces between metallic tip and semiconductor surfaces. J. Appl. Phys. 1995, 77, 3308–3314. [Google Scholar] [CrossRef]
- Baumgart, C.; Helm, M.; Schmidt, H. Quantitative dopant profiling in semiconductors: A Kelvin probe force microscopy model. Phys. Rev. B 2009, 80, 085305. [Google Scholar] [CrossRef]
- Jacobs, H.O.; Knapp, H.F.; Müller, S.; Stemmer, A. Surface potential mapping: A qualitative material contrast in SPM. Ultramicroscopy 1997, 69, 39–49. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Höche, D.; Lamaka, S.L.; Ferreira, M.G.S.; Zheludkevich, M.L. Kelvin microprobe analytics on iron-enriched corroded magnesium surface. Corrosion 2017, 73, 583–595. [Google Scholar] [CrossRef]
- Koley, G.; Spencer, M.G.; Bhangale, H.R. Cantilever effects on the measurement of electrostatic potentials by scanning Kelvin probe microscopy. Appl. Phys. Lett. 2001, 79, 545–547. [Google Scholar] [CrossRef]
- Elias, G.; Glatzel, T.; Meyer, E.; Schwarzman, A.; Boag, A.; Rosenwaks, Y. The role of the cantilever in Kelvin probe force microscopy measurements. Beilstein J. Nanotechnol. 2011, 2, 252–260. [Google Scholar] [CrossRef]
- Cherniavskaya, O.; Chen, L.; Weng, V.; Yuditsky, L.; Brus, L.E. Quantitative Noncontact Electrostatic Force Imaging of Nanocrystal Polarizability. J. Phys. Chem. B 2003, 107, 1525–1531. [Google Scholar] [CrossRef]
- Bruker Application Note #140: PeakForce Kelvin Probe Force Microscopy. Available online: https://www.bruker.com/fileadmin/user_upload/8-PDF-Docs/SurfaceAnalysis/AFM/ApplicationNotes/AN140-RevA1-PeakForce_KPFM-AppNote.pdf (accessed on 25 September 2020).
- Manne, S.; Massie, J.; Elings, V.B.; Hansma, P.K.; Gewirth, A.A. Electrochemistry on a gold surface observed with the atomic force microscope. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1991, 9, 950–954. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; He, M.; Liu, Y.; Wu, Z. Application of Electrochemical Atomic Force Microscopy (EC-AFM) in the Corrosion Study of Metallic Materials. Materials 2020, 13, 668. [Google Scholar] [CrossRef]
- Wu, H.; Feng, X.; Kieviet, B.D.; Zhang, K.; Zandvliet, H.J.W.; Canters, G.W.; Schön, P.M.; Vancso, G.J. Electrochemical atomic force microscopy reveals potential stimulated height changes of redox responsive Cu-azurin on gold. Eur. Polym. J. 2016, 83, 529–537. [Google Scholar] [CrossRef]
- Xu, K.; Sun, W.; Shao, Y.; Wei, F.; Zhang, X.; Wang, W.; Li, P. Recent development of PeakForce Tapping mode atomic force microscopy and its applications on nanoscience. Nanotechnol. Rev. 2018, 7, 605. [Google Scholar] [CrossRef]
- Mudali, U.K.; Padhy, N. Electrochemical scanning probe microscope (EC-SPM) for the in situ corrosion study of materials: An overview with examples. Corros. Rev. 2011, 29, 73. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Corrosion Study of AA2024-T3 by Scanning Kelvin Probe Force Microscopy and In Situ Atomic Force Microscopy Scratching. J. Electrochem. Soc. 1998, 145, 2295–2306. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. Inhibition of Aluminum Alloy 2024 Corrosion by Vanadates: An In Situ Atomic Force Microscopy Scratching Investigation. Corrosion 2007, 63, 672–688. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Kaya, S. Anti-corrosion investigation of pyrimidine derivatives as green and sustainable corrosion inhibitor for N80 steel in highly corrosive environment: Experimental and AFM/XPS study. Sustain. Chem. Pharm. 2020, 16, 100257. [Google Scholar] [CrossRef]
- Singh, A.K.; Chugh, B.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.-M.; Pal, S.; Prakash, R. Green approach of synthesis of thiazolyl imines and their impeding behavior against corrosion of mild steel in acid medium. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124824. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Mazumder, M.A.J.; Singh, A. Chitosan Schiff base: An environmentally benign biological macromolecule as a new corrosion inhibitor for oil & gas industries. Int. J. Biol. Macromol. 2020, 144, 305–315. [Google Scholar] [CrossRef]
- Sliem, M.H.; Radwan, A.B.; Mohamed, F.S.; Alnuaimi, N.A.; Abdullah, A.M. An efficient green ionic liquid for the corrosion inhibition of reinforcement steel in neutral and alkaline highly saline simulated concrete pore solutions. Sci. Rep. 2020, 10, 14565. [Google Scholar] [CrossRef]
- Benaioun, N.E.; Maafa, I.; Florentin, A.; Denys, E.; Hakiki, N.E.; Moulayat, N.; Bubendorff, J.L. Time dependence of the natural passivation process on AISI 304 in an alkaline medium: Atomic force microscopy and scanning Kelvin probe force microscopy as additional tools to electrochemical impedance spectroscopy. Appl. Surf. Sci. 2018, 436, 646–652. [Google Scholar] [CrossRef]
- Handoko, W.; Pahlevani, F.; Sahajwalla, V. The Effect of Low-Quantity Cr Addition on the Corrosion Behaviour of Dual-Phase High Carbon Steel. Metals 2018, 8, 199. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by Scanning Kelvin Probe Force Microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Örnek, C.; Leygraf, C.; Pan, J. On the Volta potential measured by SKPFM–fundamental and practical aspects with relevance to corrosion science. Corros. Eng. Sci. Technol. 2019, 54, 185–198. [Google Scholar] [CrossRef]
- Cook, A.B.; Barrett, Z.; Lyon, S.B.; McMurray, H.N.; Walton, J.; Williams, G. Calibration of the scanning Kelvin probe force microscope under controlled environmental conditions. Electrochim. Acta 2012, 66, 100–105. [Google Scholar] [CrossRef]
- Efaw, C.; da Silva, T.; Davis, P.; Li, L.; Graugnard, E.; Hurley, M. Improving the Relative Calculations of Volta Potential Differences Acquired from Scanning Kelvin Probe Force Microscopy (SKPFM) from Comparing an Inert Material to First-Principle Calculations. ECS Trans. 2018, 85, 701–713. [Google Scholar] [CrossRef]
- Beerbom, M.M.; Lägel, B.; Cascio, A.J.; Doran, B.V.; Schlaf, R. Direct comparison of photoemission spectroscopy and in situ Kelvin probe work function measurements on indium tin oxide films. J. Electron. Spectrosc. Relat. Phenom. 2006, 152, 12–17. [Google Scholar] [CrossRef]
- Kalb, H.; Rzany, A.; Hensel, B. Impact of microgalvanic corrosion on the degradation morphology of WE43 and pure magnesium under exposure to simulated body fluid. Corros. Sci. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of rare-earth-magnesium alloys to micro-galvanic corrosion. Corros. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Yasakau, K.A.; Arrabal, R.; Matykina, E.; Mingo, B.; Scharnagl, N.; Ferreira, M.G.S.; Zheludkevich, M.L. Characterization and corrosion behavior of binary Mg-Ga alloys. Mater. Charact. 2017, 128, 85–99. [Google Scholar] [CrossRef]
- Woo, S.K.; Blawert, C.; Yasakau, K.A.; Yi, S.; Scharnagl, N.; Suh, B.-C.; Kim, Y.M.; Sun You, B.; Dong Yim, C. Effects of combined addition of Ca and Y on the corrosion behaviours of die-cast AZ91D magnesium alloy. Corros. Sci. 2020, 166, 108451. [Google Scholar] [CrossRef]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Yasakau, K.A.; Wang, L.; Laipple, D.; Schieda, M.; Di, S.; Zheludkevich, M.L. Corrosion inhibition of pure Mg containing a high level of iron impurity in pH neutral NaCl solution. Corros. Sci. 2018, 142, 222–237. [Google Scholar] [CrossRef]
- Hurley, M.F.; Efaw, C.M.; Davis, P.H.; Croteau, J.R.; Graugnard, E.; Birbilis, N. Volta potentials measured by scanning kelvin probe force microscopy as relevant to corrosion of magnesium alloys. Corrosion 2015, 71, 160–170. [Google Scholar] [CrossRef]
- de Wit, J.H.W. Local potential measurements with the SKPFM on aluminium alloys. Electrochim. Acta 2004, 49, 2841–2850. [Google Scholar] [CrossRef]
- Senöz, C.; Rohwerder, M. Scanning Kelvin probe force microscopy for the in situ observation of the direct interaction between active head and intermetallic particles in filiform corrosion on aluminium alloy. Electrochim. Acta 2011, 56, 9588–9595. [Google Scholar] [CrossRef]
- Lafouresse, M.C.; Charvillat, C.; Blanc, C.; de Bonfils-Lahovary, M.-L.; Laffont, L. Role of hydrogen in intergranular corrosion of 2024 aluminum alloy: An SKPFM Study. In CORROSION 2017; NACE International: New Orleans, LA, USA, 2017; p. 11. [Google Scholar]
- Mallinson, C.F.; Yates, P.M.; Baker, M.A.; Castle, J.E.; Harvey, A.; Watts, J.F. The localised corrosion associated with individual second phase particles in AA7075-T6: A study by SEM, EDX, AES, SKPFM and FIB-SEM. Mater. Corros. 2017, 68, 748–763. [Google Scholar] [CrossRef]
- Esfahani, Z.; Rahimi, E.; Sarvghad, M.; Rafsanjani-Abbasi, A.; Davoodi, A. Correlation between the histogram and power spectral density analysis of AFM and SKPFM images in an AA7023/AA5083 FSW joint. J. Alloy. Compd. 2018, 744, 174–181. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Martinez, M.A.; Montes, L.P. Evidence for Cu Ion Formation by Dissolution and Dealloying the Al2CuMg Intermetallic Compound in Rotating Ring-Disk Collection Experiments. J. Electrochem. Soc. 2000, 147, 119. [Google Scholar] [CrossRef]
- Rohwerder, M.; Hornung, E.; Stratmann, M. Microscopic aspects of electrochemical delamination: An SKPFM study. Electrochim. Acta 2003, 48, 1235–1243. [Google Scholar] [CrossRef]
- Guo, L.Q.; Li, M.; Shi, X.L.; Yan, Y.; Li, X.Y.; Qiao, L.J. Effect of annealing temperature on the corrosion behavior of duplex stainless steel studied by in situ techniques. Corros. Sci. 2011, 53, 3733–3741. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Application of Surface Science to Corrosion. Surf. Interface Sci. 2020, 799–825. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhou, D.; Zhao, W.; Qian, X.; Yang, Q.; Sun, T.; Shen, C. Ultra-low Au–Pt Co-decorated TiO2 nanotube arrays: Construction and its improved visible-light-induced photocatalytic properties. Sol. Energy Mater. Sol. Cells 2019, 201, 110065. [Google Scholar] [CrossRef]
- Brummel, O.; Waidhas, F.; Khalakhan, I.; Vorokhta, M.; Dubau, M.; Kovács, G.; Aleksandrov, H.A.; Neyman, K.M.; Matolín, V.; Libuda, J. Structural transformations and adsorption properties of PtNi nanoalloy thin film electrocatalysts prepared by magnetron co-sputtering. Electrochim. Acta 2017, 251, 427–441. [Google Scholar] [CrossRef]
- Li, J.; Ecco, L.; Ahniyaz, A.; Pan, J. Probing electrochemical mechanism of polyaniline and CeO2 nanoparticles in alkyd coating with in-situ electrochemical-AFM and IRAS. Prog. Org. Coat. 2019, 132, 399–408. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Fielden, M.; Pan, J.; Ecco, L.; Schellbach, C.; Delmas, G.; Claesson, P.M. Towards the mechanism of electrochemical activity and self-healing of 1 wt% PTSA doped polyaniline in alkyd composite polymer coating: Combined AFM-based studies. RSC Adv. 2016, 6, 19111–19127. [Google Scholar] [CrossRef]
- Khalakhan, I.; Choukourov, A.; Vorokhta, M.; Kúš, P.; Matolínová, I.; Matolín, V. In situ electrochemical AFM monitoring of the potential-dependent deterioration of platinum catalyst during potentiodynamic cycling. Ultramicroscopy 2018, 187, 64–70. [Google Scholar] [CrossRef]
- Kreta, A.; Rodošek, M.; Slemenik Perše, L.; Orel, B.; Gaberšček, M.; Šurca Vuk, A. In situ electrochemical AFM, ex situ IR reflection–absorption and confocal Raman studies of corrosion processes of AA 2024-T3. Corros. Sci. 2016, 104, 290–309. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Balke, N.; Liaw, P.K.; Yang, B. In-situ electrochemical-AFM study of localized corrosion of AlxCoCrFeNi high-entropy alloys in chloride solution. Appl. Surf. Sci. 2018, 439, 533–544. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kallip, S.; Lisenkov, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Initial stages of localized corrosion at cut-edges of adhesively bonded Zn and Zn-Al-Mg galvanized steel. Electrochim. Acta 2016, 211, 126–141. [Google Scholar] [CrossRef]
- Moore, S.; Burrows, R.; Picco, L.; Martin, T.L.; Greenwell, S.J.; Scott, T.B.; Payton, O.D. A study of dynamic nanoscale corrosion initiation events using HS-AFM. Faraday Discuss. 2018, 210, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Nilsson, J.-O.; Pan, J. In Situ and Operando AFM and EIS Studies of Anodization of Al 6060: Influence of Intermetallic Particles. J. Electrochem. Soc. 2016, 163, C609–C618. [Google Scholar] [CrossRef]
- Zhang, F.; Örnek, C.; Nilsson, J.-O.; Pan, J. Anodisation of aluminium alloy AA7075—Influence of intermetallic particles on anodic oxide growth. Corros. Sci. 2020, 164, 108319. [Google Scholar] [CrossRef]
- Zhao, W.; Song, W.; Cheong, L.-Z.; Wang, D.; Li, H.; Besenbacher, F.; Huang, F.; Shen, C. Beyond imaging: Applications of atomic force microscopy for the study of Lithium-ion batteries. Ultramicroscopy 2019, 204, 34–48. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, K. Studying the Localized Electrochemical Phenomena in Rechargeable Li-Ion Batteries by Scanning Probe Microscopy Techniques. In Nanotechnology for Sustainable Energy; Hu, Y.H., Burghaus, U., Eds.; American Chemical Society: Washington, DC, USA, 2013; Volume 1140, pp. 23–53. [Google Scholar]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef]
- Aurbach, D.; Cohen, Y. The Application of Atomic Force Microscopy for the Study of Li Deposition Processes. J. Electrochem. Soc. 1996, 143, 3525–3532. [Google Scholar] [CrossRef]
- Vidu, R.; Quinlan, F.T.; Stroeve, P. Use of in Situ Electrochemical Atomic Force Microscopy (EC-AFM) to Monitor Cathode Surface Reaction in Organic Electrolyte. Ind. Eng. Chem. Res. 2002, 41, 6546–6554. [Google Scholar] [CrossRef]
- Shen, C.; Hu, G.; Cheong, L.-Z.; Huang, S.; Zhang, J.-G.; Wang, D. Direct Observation of the Growth of Lithium Dendrites on Graphite Anodes by Operando EC-AFM. Small Methods 2018, 2, 1700298. [Google Scholar] [CrossRef]
- Alliata, D.; Kötz, R.; Novák, P.; Siegenthaler, H. Electrochemical SPM investigation of the solid electrolyte interphase film formed on HOPG electrodes. Electrochem. Commun. 2000, 2, 436–440. [Google Scholar] [CrossRef]
- Ramdon, S.; Bhushan, B.; Nagpure, S.C. In situ electrochemical studies of lithium-ion battery cathodes using atomic force microscopy. J. Power Sources 2014, 249, 373–384. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Wan, L. Progress of electrode/electrolyte interfacial investigation of Li-ion batteries via in situ scanning probe microscopy. Sci. Bull. 2015, 60, 839–849. [Google Scholar] [CrossRef]
- Shi, X.; Qing, W.; Marhaba, T.; Zhang, W. Atomic force microscopy—Scanning electrochemical microscopy (AFM-SECM) for nanoscale topographical and electrochemical characterization: Principles, applications and perspectives. Electrochim. Acta 2020, 332, 135472. [Google Scholar] [CrossRef]
- Izquierdo, J.; Fernández-Pérez, B.M.; Eifert, A.; Souto, R.M.; Kranz, C. Simultaneous atomic force—scanning electrochemical microscopy (AFM-SECM) imaging of copper dissolution. Electrochim. Acta 2016, 201, 320–332. [Google Scholar] [CrossRef]
- Izquierdo, J.; Eifert, A.; Kranz, C.; Souto, R.M. In situ investigation of copper corrosion in acidic chloride solution using atomic force—Scanning electrochemical microscopy. Electrochim. Acta 2017, 247, 588–599. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasakau, K. Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys. Corros. Mater. Degrad. 2020, 1, 345-372. https://doi.org/10.3390/cmd1030017
Yasakau K. Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys. Corrosion and Materials Degradation. 2020; 1(3):345-372. https://doi.org/10.3390/cmd1030017
Chicago/Turabian StyleYasakau, Kiryl. 2020. "Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys" Corrosion and Materials Degradation 1, no. 3: 345-372. https://doi.org/10.3390/cmd1030017
APA StyleYasakau, K. (2020). Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys. Corrosion and Materials Degradation, 1(3), 345-372. https://doi.org/10.3390/cmd1030017




