Dissociating the Effects of Light at Night from Circadian Misalignment in a Neurodevelopmental Disorder Mouse Model Using Ultradian Light–Dark Cycles
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Groups
4.2. Behavioral Tests
4.3. Cage Conditions and Activity
4.4. Immunofluorescence
4.5. cFos-Positive Cell Counting in the Basolateral Amygdala (BLA)
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| BLA | basolateral amygdala |
| DD | constant darkness |
| DLaN | dim light at night |
| ipRGCs | intrinsically photosensitive retinal ganglion cells |
| KO | knockout |
| LD | light–dark |
| M/P | melanopic to photopic |
| NDDs | neurodevelopmental disorders |
| PIR | passive infrared |
| SCN | suprachiasmatic nucleus |
| WT | wild-type |
References
- Martínez-Cayuelas, E.; Rodríguez-Morilla, B.; Soriano-Guillén, L.; Merino-Andreu, M.; Moreno-Vinués, B.; Gavela-Pérez, T. Sleep Problems and Circadian Functioning in Children and Adolescents With Autism Spectrum Disorder. Pediatr. Neurol. 2022, 126, 57–64. [Google Scholar] [CrossRef]
- Sadikova, E.; Dovgan, K.; Mazurek, M.O. Longitudinal Examination of Sleep Problems and Symptom Severity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023, 53, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Al Lihabi, A. A literature review of sleep problems and neurodevelopment disorders. Front. Psychiatry 2023, 14, 1122344. [Google Scholar] [CrossRef]
- Peters, S.U.; Shelton, A.R.; Malow, B.A.; Neul, J.L. A clinical-translational review of sleep problems in neurodevelopmental disabilities. J. Neurodev. Disord. 2024, 16, 41. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Sohl, K. Sleep and Behavioral Problems in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1906–1915. [Google Scholar] [CrossRef]
- Veatch, O.J.; Sutcliffe, J.S.; Warren, Z.E.; Keenan, B.T.; Potter, M.H.; Malow, B.A. Shorter sleep duration is associated with social impairment and comorbidities in ASD. Autism Res. 2017, 10, 1221–1238. [Google Scholar] [CrossRef]
- Hodge, D.; Hoffman, C.D.; Sweeney, D.P.; Riggs, M.L. Relationship between children’s sleep and mental health in mothers of children with and without autism. J. Autism Dev. Disord. 2013, 43, 956–963. [Google Scholar] [CrossRef]
- Lopez-Wagner, M.C.; Hoffman, C.D.; Sweeney, D.P.; Hodge, D.; Gilliam, J.E. Sleep problems of parents of typically developing children and parents of children with autism. J. Genet. Psychol. 2008, 169, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Engelhardt, C.R.; Hilgard, J.; Sohl, K. Bedtime Electronic Media Use and Sleep in Children with Autism Spectrum Disorder. J. Dev. Behav. Pediatr. 2016, 37, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Y.; Wang, B.; Li, H.H.; Yue, X.J.; Jia, F.Y. Correlation Between Screen Time and Autistic Symptoms as Well as Development Quotients in Children With Autism Spectrum Disorder. Front. Psychiatry 2021, 12, 619994. [Google Scholar] [CrossRef]
- Nagata, J.M.; Cheng, C.M.; Shim, J.; Kiss, O.; Ganson, K.T.; Testa, A.; He, J.; Baker, F.C. Bedtime Screen Use Behaviors and Sleep Outcomes in Early Adolescents: A Prospective Cohort Study. J. Adolesc. Health 2024, 75, 650–655. [Google Scholar] [CrossRef]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Gronli, J.; Byrkjedal, I.K.; Bjorvatn, B.; Nodtvedt, O.; Hamre, B.; Pallesen, S. Reading from an iPad or from a book in bed: The impact on human sleep. A randomized controlled crossover trial. SleepMed 2016, 21, 86–92. [Google Scholar] [CrossRef]
- Didikoglu, A.; Mohammadian, N.; Johnson, S.; van Tongeren, M.; Wright, P.; Casson, A.J.; Brown, T.M.; Lucas, R.J. Associations between light exposure and sleep timing and sleepiness while awake in a sample of UK adults in everyday life. Proc. Natl. Acad. Sci. USA 2023, 120, e2301608120. [Google Scholar] [CrossRef]
- Stefanopoulou, M.; Ruhé, N.; Portengen, L.; van Wel, L.; Vrijkotte, T.G.M.; Vermeulen, R.; Huss, A. Associations of light exposure patterns with sleep among Dutch children: The ABCD cohort study. J. Sleep. Res. 2024, 33, e14184. [Google Scholar] [CrossRef]
- Guindon, G.E.; Murphy, C.A.; Milano, M.E.; Seggio, J.A. Turn off that night light! Light-at-night as a stressor for adolescents. Front. Neurosci. 2024, 18, 1451219. [Google Scholar] [CrossRef]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, J.L.; Trimmer, J.S.; Shrager, P.; Peles, E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef]
- Arking, D.E.; Cutler, D.J.; Brune, C.W.; Teslovich, T.M.; West, K.; Ikeda, M.; Rea, A.; Guy, M.; Lin, S.; Cook, E.H.; et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet. 2008, 82, 160–164. [Google Scholar] [CrossRef]
- Bakkaloglu, B.; O’Roak, B.J.; Louvi, A.; Gupta, A.R.; Abelson, J.F.; Morgan, T.M.; Chawarska, K.; Klin, A.; Ercan-Sencicek, A.G.; Stillman, A.A.; et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am. J. Hum. Genet. 2008, 82, 165–173. [Google Scholar] [CrossRef]
- Nord, A.S.; Roeb, W.; Dickel, D.E.; Walsh, T.; Kusenda, M.; O’Connor, K.L.; Malhotra, D.; McCarthy, S.E.; Stray, S.M.; Taylor, S.M.; et al. Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. Eur. J. Hum. Genet. 2011, 19, 727–731. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Deriziotis, P.; Lee, C.; Vives, L.; Schwartz, J.J.; Girirajan, S.; Karakoc, E.; Mackenzie, A.P.; Ng, S.B.; Baker, C.; et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 2011, 43, 585–589, Erratum in Nat. Gen. 2012, 43, 585–589. [Google Scholar] [CrossRef]
- Strauss, K.A.; Puffenberger, E.G.; Huentelman, M.J.; Gottlieb, S.; Dobrin, S.E.; Parod, J.M.; Stephan, D.A.; Morton, D.H. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med. 2006, 354, 1370–1377. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Choe, K.Y.; Bethlehem, R.A.I.; Safrin, M.; Dong, H.; Salman, E.; Li, Y.; Grinevich, V.; Golshani, P.; DeNardo, L.A.; Peñagarikano, O.; et al. Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse. Neuron 2022, 110, 795–808.e6. [Google Scholar] [CrossRef]
- Angelakos, C.C.; Tudor, J.C.; Ferri, S.L.; Jongens, T.A.; Abel, T. Home-cage hypoactivity in mouse genetic models of autism spectrum disorder. Neurobiol. Learn. Mem. 2019, 165, 107000. [Google Scholar] [CrossRef]
- Wang, H.B.; Tahara, Y.; Luk, S.H.C.; Kim, Y.S.; Hitchcock, O.N.; MacDowell Kaswan, Z.A.; In Kim, Y.; Block, G.D.; Ghiani, C.A.; Loh, D.H.; et al. Melatonin treatment of repetitive behavioral deficits in the Cntnap2 mouse model of autism spectrum disorder. Neurobiol. Dis. 2020, 145, 105064. [Google Scholar] [CrossRef]
- Thomas, A.M.; Schwartz, M.D.; Saxe, M.D.; Kilduff, T.S. Cntnap2 Knockout Rats and Mice Exhibit Epileptiform Activity and Abnormal Sleep-Wake Physiology. Sleep 2017, 40, zsw026. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhou, D.; Luk, S.H.C.; In Cha, H.; Mac, A.; Chae, R.; Matynia, A.; Harrison, B.; Afshari, S.; Block, G.D.; et al. Long wavelength light reduces the negative consequences of dim light at night. Neurobiol. Dis. 2023, 176, 105944. [Google Scholar] [CrossRef]
- LeGates, T.A.; Altimus, C.M.; Wang, H.; Lee, H.K.; Yang, S.; Zhao, H.; Kirkwood, A.; Weber, E.T.; Hattar, S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012, 491, 594–598. [Google Scholar] [CrossRef]
- Duy, P.Q.; Hattar, S. Chronic Circadian Misalignment without Circadian Arrhythmicity or Sleep Deprivation Does Not Impair Adult Hippocampal Neurogenesis. J. Biol. Rhythm. 2017, 32, 621–626. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Fogerson, P.M.; Lazzerini Ospri, L.; Thomsen, M.B.; Layne, R.M.; Severin, D.; Zhan, J.; Singer, J.H.; Kirkwood, A.; Zhao, H.; et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 2018, 175, 71–84.e18. [Google Scholar] [CrossRef]
- Oneda, S.; Cao, S.; Haraguchi, A.; Sasaki, H.; Shibata, S. Wheel-Running Facilitates Phase Advances in Locomotor and Peripheral Circadian Rhythm in Social Jet Lag Model Mice. Front. Physiol. 2022, 13, 821199. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Tye, K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014, 34, 586–595. [Google Scholar] [CrossRef]
- Sun, T.; Song, Z.; Tian, Y.; Tian, W.; Zhu, C.; Ji, G.; Luo, Y.; Chen, S.; Wang, L.; Mao, Y.; et al. Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc. Natl. Acad. Sci. USA 2019, 116, 3799–3804. [Google Scholar] [CrossRef]
- Takumi, T.; Tamada, K.; Hatanaka, F.; Nakai, N.; Bolton, P.F. Behavioral neuroscience of autism. Neurosci. Biobehav. Rev. 2020, 110, 60–76. [Google Scholar] [CrossRef]
- Fuchs, F.; Robin-Choteau, L.; Schneider, A.; Hugueny, L.; Ciocca, D.; Serchov, T.; Bourgin, P. Delaying circadian sleep phase under ultradian light cycle causes time-of-day-dependent alteration of cognition and mood. Sci. Rep. 2023, 13, 20313. [Google Scholar] [CrossRef]
- Altimus, C.M.; Güler, A.D.; Villa, K.L.; McNeill, D.S.; Legates, T.A.; Hattar, S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl. Acad. Sci. USA 2008, 105, 19998–20003. [Google Scholar] [CrossRef]
- Tam, S.K.E.; Brown, L.A.; Wilson, T.S.; Tir, S.; Fisk, A.S.; Pothecary, C.A.; van der Vinne, V.; Foster, R.G.; Vyazovskiy, V.V.; Bannerman, D.M.; et al. Dim light in the evening causes coordinated realignment of circadian rhythms, sleep, and short-term memory. Proc. Natl. Acad. Sci. USA 2021, 118, e2101591118. [Google Scholar] [CrossRef]
- Poliak, S.; Salomon, D.; Elhanany, H.; Sabanay, H.; Kiernan, B.; Pevny, L.; Stewart, C.L.; Xu, X.; Chiu, S.Y.; Shrager, P.; et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003, 162, 1149–1160. [Google Scholar] [CrossRef]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011, Chapter 8, Unit 8.26. [Google Scholar] [CrossRef]
- Lee, F.Y.; Larimore, J.; Faundez, V.; Dell’Angelica, E.C.; Ghiani, C.A. Sex-dimorphic effects of biogenesis of lysosome-related organelles complex-1 deficiency on mouse perinatal brain development. J. Neurosci. Res. 2021, 99, 67–89. [Google Scholar] [CrossRef] [PubMed]

| WT | Cntnap2 KO | Two-Way ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LD | DLaN | DD | T7 | LD | DLaN | DD | T7 | Genotype | Lighting | Interaction | |
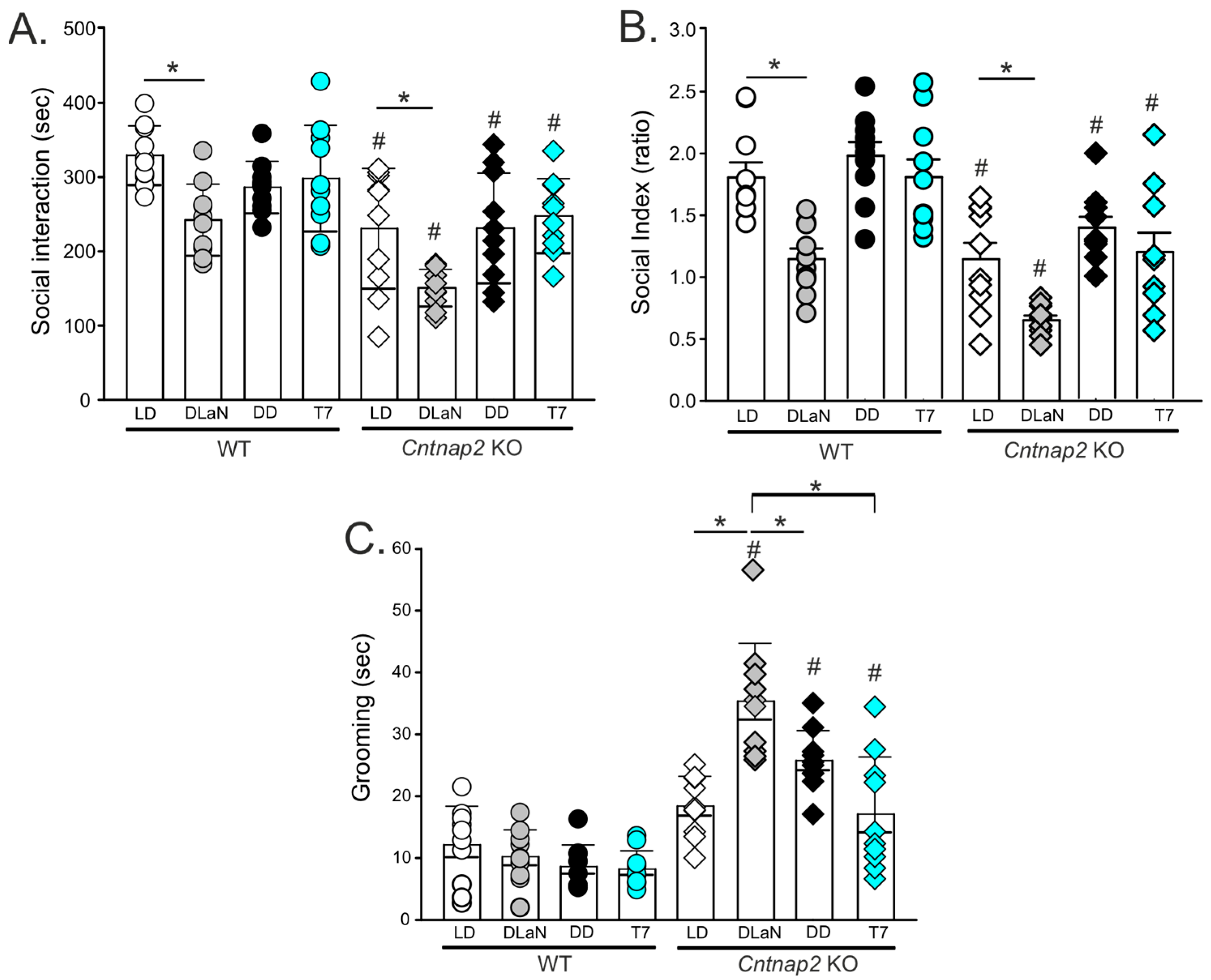
| Social intera-ctions (s) | 329 ± 40 | 242 ± 48 * | 286 ± 35 | 292 ± 51 | 231 ± 81 # | 151 ± 25 *# | 231 ± 74 # | 248 ± 50 # | F(1,79) = 33.628; p < 0.001 | F(3,79) = 8.811; p < 0.001 | F(3,79) = 0.906; p = 0.443 |
| Social pre-ference (ratio) | 1.8 ± 0.4 | 1.1 ± 0.3 * | 1.9 ± 0.3 | 1.8 ± 0.4 | 1.1 ± 0.4 # | 0.6 ± 0.4 *# | 1.3 ± 0.3 # | 1.2 ± 0.5 # | F(1,79) = 51.562; p < 0.001 | F(3,79) = 17.101; p < 0.001 | F(3,79) = 0.181; p = 0.909 |
| Grooming (s) | 12.1 ± 6.2 | 10.2 ± 4.3 | 8.6 ± 3.5 | 8.2 ± 2.9 | 18.4 ± 4.8 | 35.3 ± 9.3 *# | 25.7 ± 4.8 # | 17.1 ± 9.2# | F(1,79) = 66.571; p < 0.001 | F(3,79) = 4.648; p = 0.005 | F(3,79) = 4.512; p = 0.006 |
| WT | Cntnap2 KO | Two-Way ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LD | DLaN | DD | T7 | LD | DLaN | DD | T7 | Genotype | Lighting | Interactions | |
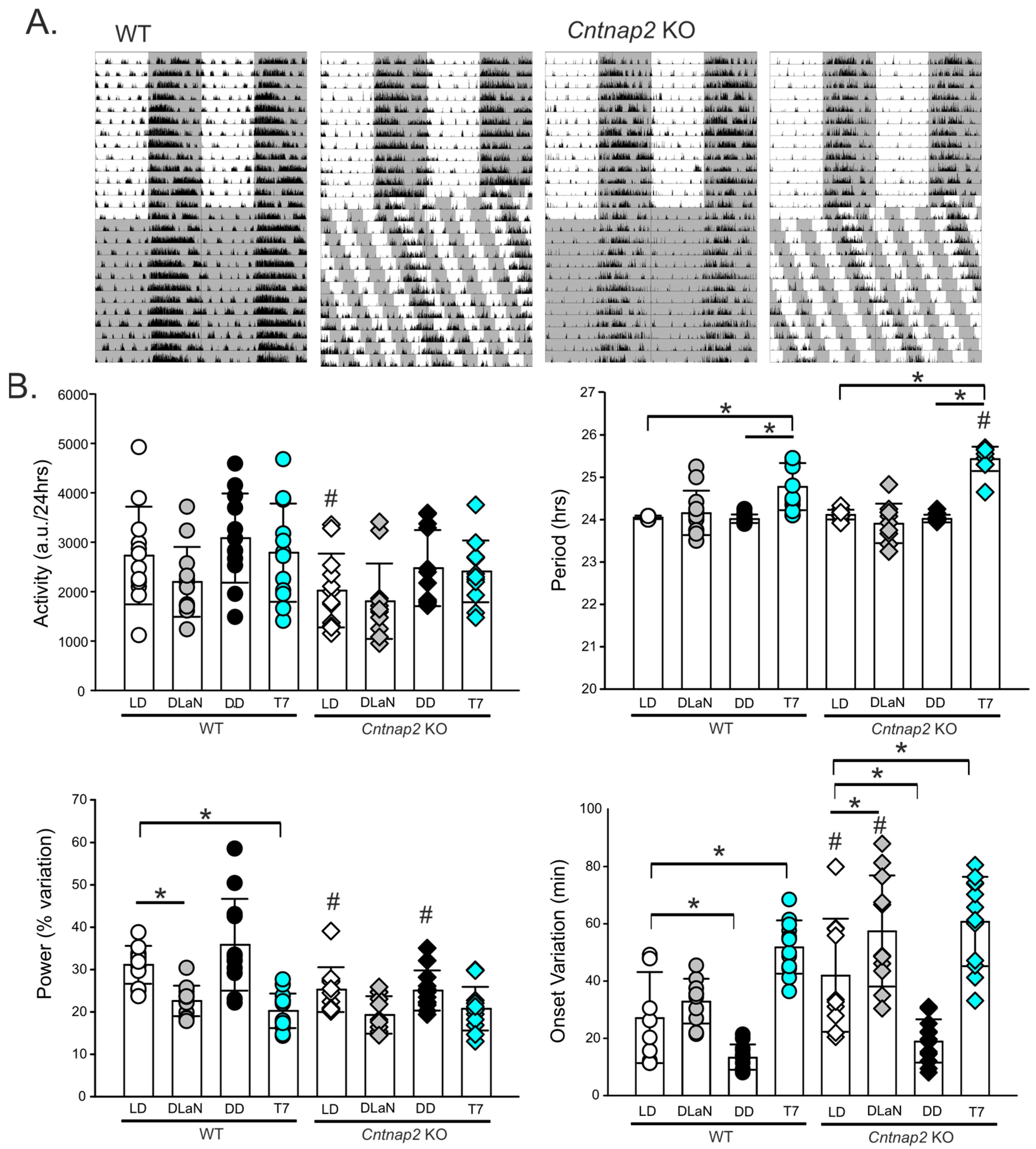
| Total activity (a.u./24 h) | 2730 ± 988 | 2180 ± 707 | 3084 ± 903 | 2788 ± 995 | 2023 ± 747 # | 1806 ± 763 | 2477 ± 769 | 2410 ± 624 | F(1,95) = 9.648; p = 0.003 | F(3, 95) = 3.993; p = 0.010 | F(3,95) = 0.235; p = 0.872 |
| Period (h) | 24.0 ± 0.05 | 24.2 ± 0.52 | 24.0 ± 0.10 | 24.8 ± 0.5 * | 24.1 ± 0.11 | 23.9 ± 0.5 | 24.0 ± 0.09 | 25.4 ± 0.29*# | F(1,95) = 2.937; p = 0.090 | F(3, 95) = 58.80; p < 0.001 | F(3,95) = 7.647; p < 0.001 |
| Power (% variance) | 31.1 ± 4.5 | 22.6 ± 3.6 * | 35.8 ± 6.8 | 20.3 ± 4.1 * | 25.3 ± 5.3 # | 19.3 ± 4.4 | 25.1 ± 4.7 # | 20.8 ± 5.1 | F(1,95) = 17.20; p < 0.001 | F(3, 95) = 18.60; p < 0.001 | F(3,95) = 4.093; p = 0.009 |
| Fragmentation # bouts/24 h | 10.9 ± 4.1 | 11.7 ± 5.7 | 10.2 ± 1.8 | 10.5 ± 1.4 | 10.3 ± 4.8 | 12.4 ± 4.4 | 11.1 ± 2.6 | 11.8 ± 1.7 | F(1,95) = 0.588; p = 0.445 | F(3, 95) = 0.844; p = 0.473 | F(3,95) = 0.281; p = 0.839 |
| Onset variability (min) | 28.0 ± 16.6 | 32.7 ± 8.2 | 13.4 ± 4.4 * | 51.8 ± 9.3 * | 43.0 ± 20 # | 57.5 ± 19.4*# | 19.5 ± 7.6 * | 60.8 ± 15.6 * | F(1,95) = 20.938; p < 0.001 | F(3, 95) = 37.007; p < 0.001 | F(3,95) = 2.105; p = 0.106 |
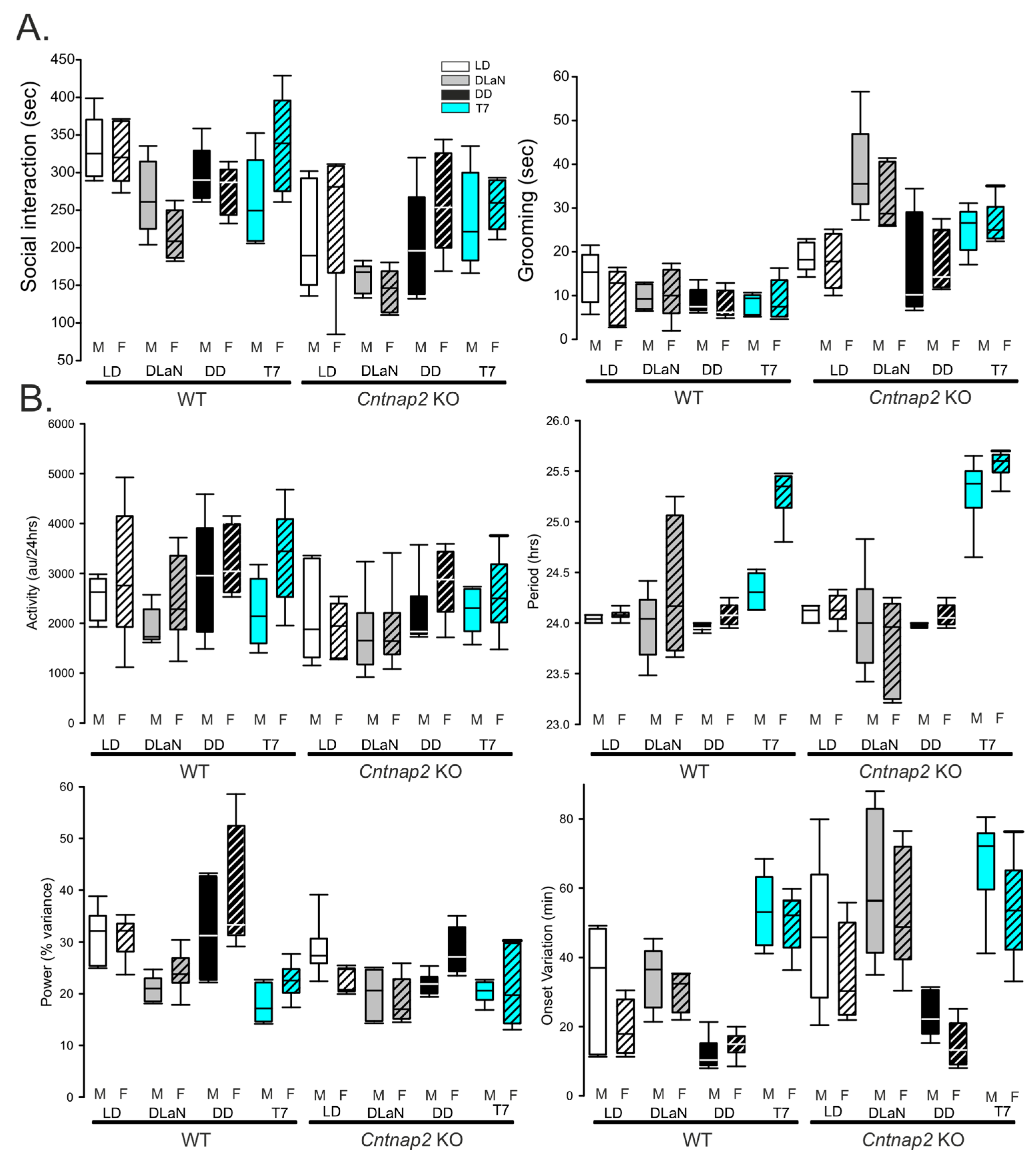
| Sex | Genotype | Lighting | Interactions | |
|---|---|---|---|---|
| Total activity (a.u./24 h) | F(1,95) = 5.853; p = 0.018 | F(1,95) = 10.01; p = 0.002 | F(3,95) = 4.144; p = 0.009 | F(3,95) = 0.648; p = 0.586 |
| Period (h) | F(1.95) = 13.18; p < 0.001 | F(1,95) = 4.239; p = 0.043 | F(3,95) = 84.86; p < 0.001 | F(3,95) = 2.577; p = 0.060 |
| Power (% variance) | F(1 95) = 2.441; p = 0.122 | F(1,95) = 18.94; p < 0.001 | F(3,95) = 20.49; p < 0.001 | F(3,95) = 0.256; p = 0.857 |
| Fragmentation (#bouts/24 h) | F(1,95) = 1.276; p = 0.262 | F(1,95) = 0.585; p = 0.447 | F(3,95) = 0.840; p = 0.476 | F(3,95) = 0.072; p = 0.975 |
| Onset variation (min) | F(1,89) = 8.022; p = 0.006 | F(1 89) = 16.508; p < 0.001 | F(3,89) = 38.041; p < 0.001 | F(3,89) = 0.171; p = 0.915 |
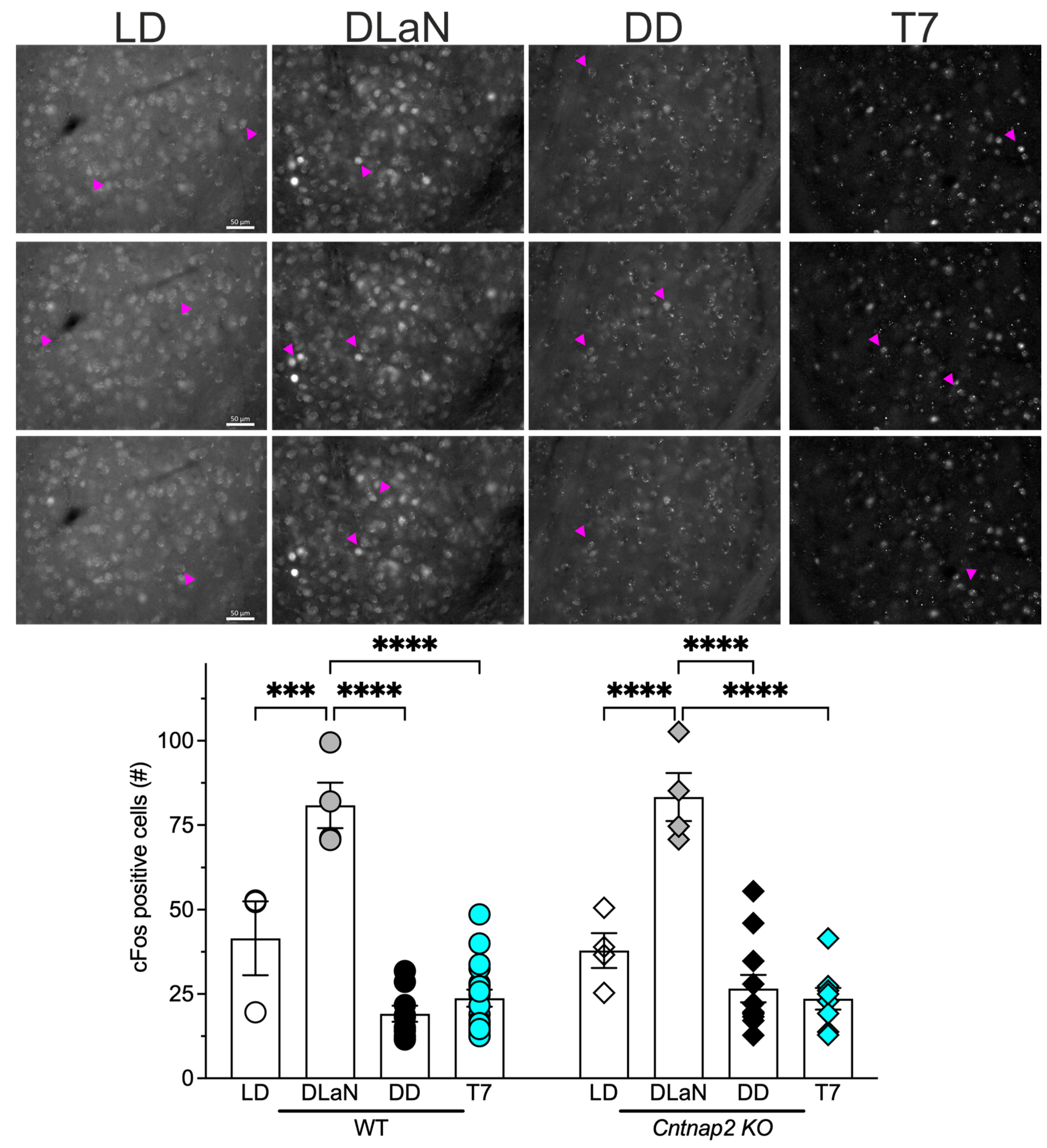
| Genotype | Two-Way ANOVA | ||||
|---|---|---|---|---|---|
| Lighting Cycle | WT | Cntnap2 KO | Genotype | Lighting | Interaction |
| LD | 41.5 ± 18.9 | 37.9 ± 10.3 | F(1,52) = 0.1959; p = 0.6599 | F(3,52) = 60.63; p < 0.0001 | F(3,52) = 0.5808; p = 0.6302 |
| DLaN | 80.8 ± 13.5 *** | 83.3 ± 14.2 *** | |||
| DD | 19.1 ± 7.09 * | 26.6 ± 13.4 | |||
| T7 | 23.8 ± 10.4 | 23.6 ± 9.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva, S.A.M.B.; Wang, H.-B.; Nguyen-Ngo, K.; Chen, C.T.; Stark, G.; Block, G.D.; Ghiani, C.A.; Colwell, C.S. Dissociating the Effects of Light at Night from Circadian Misalignment in a Neurodevelopmental Disorder Mouse Model Using Ultradian Light–Dark Cycles. Clocks & Sleep 2025, 7, 48. https://doi.org/10.3390/clockssleep7030048
Villanueva SAMB, Wang H-B, Nguyen-Ngo K, Chen CT, Stark G, Block GD, Ghiani CA, Colwell CS. Dissociating the Effects of Light at Night from Circadian Misalignment in a Neurodevelopmental Disorder Mouse Model Using Ultradian Light–Dark Cycles. Clocks & Sleep. 2025; 7(3):48. https://doi.org/10.3390/clockssleep7030048
Chicago/Turabian StyleVillanueva, Sophia Anne Marie B., Huei-Bin Wang, Kyle Nguyen-Ngo, Caihan Tony Chen, Gemma Stark, Gene D. Block, Cristina A. Ghiani, and Christopher S. Colwell. 2025. "Dissociating the Effects of Light at Night from Circadian Misalignment in a Neurodevelopmental Disorder Mouse Model Using Ultradian Light–Dark Cycles" Clocks & Sleep 7, no. 3: 48. https://doi.org/10.3390/clockssleep7030048
APA StyleVillanueva, S. A. M. B., Wang, H.-B., Nguyen-Ngo, K., Chen, C. T., Stark, G., Block, G. D., Ghiani, C. A., & Colwell, C. S. (2025). Dissociating the Effects of Light at Night from Circadian Misalignment in a Neurodevelopmental Disorder Mouse Model Using Ultradian Light–Dark Cycles. Clocks & Sleep, 7(3), 48. https://doi.org/10.3390/clockssleep7030048






