Sleep, Physical Activity, and Executive Functions in Students: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- The study population included students of different age groups (primary school, middle school, high school, and university);
- The variables taken into consideration were executive functions (working memory, cognitive flexibility, and inhibition), sleep (sleep quality and, sleep duration), and physical activity (acute or, chronic;, aerobic or, anaerobic;, high, medium, or low intensity).
- The articles were published in English.
- Reviews or meta-analyses;
- Conference papers or editorials;
- Duplicated studies;
- Animal studies.
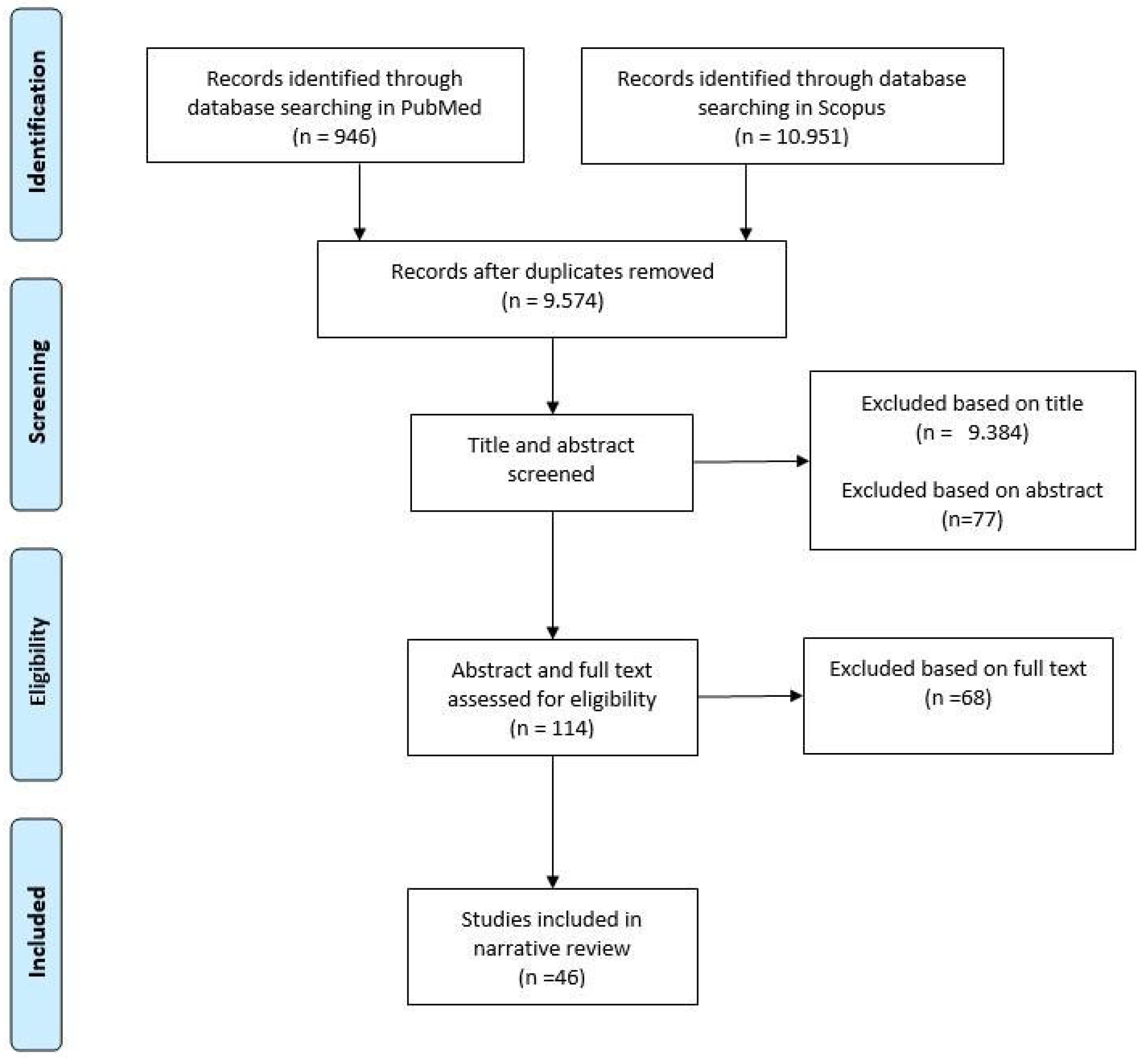
2.3. Study Selection
3. Results
4. Executive Functions
5. Sleep
Sleep and Cognitive Development
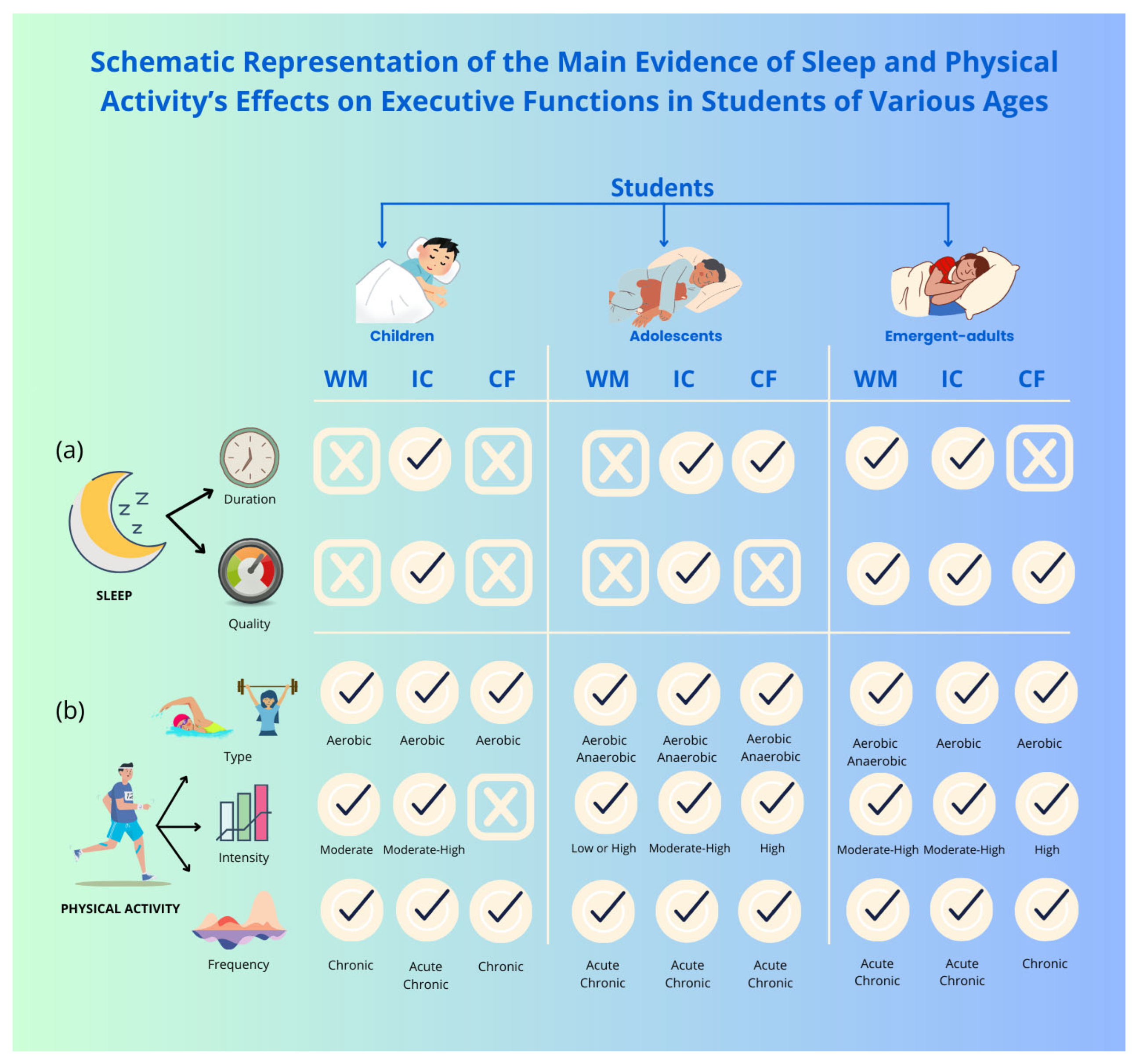
6. Sleep and Executive Functions in Student Populations
6.1. Effects of Sleep Duration on Executive Functions
6.2. Effects of Sleep Quality on Executive Functions
7. Physical Activity
Physical Activity and Cognitive Development
8. Physical Activity and Executive Functions in Student Populations
8.1. Effect of Aerobic and Anaerobic Activity on Executive Functions
8.2. Effect of Frequency and Intensity of Exercise on Executive Functions
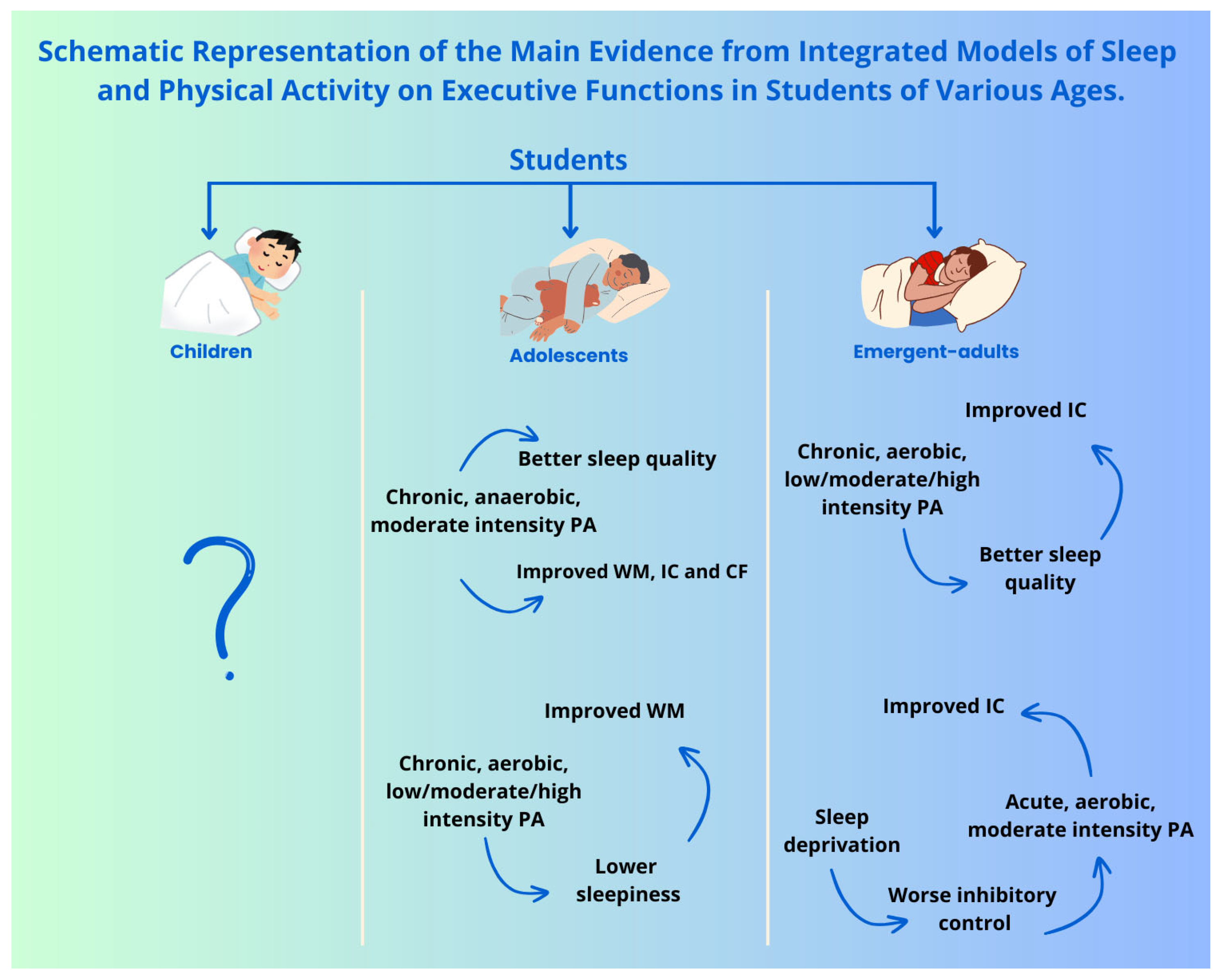
9. Sleep, Physical Activity, and Executive Functions in Student Populations
10. Discussion
Overall, Sleep Has a Positive Effect on All Domains of EFs
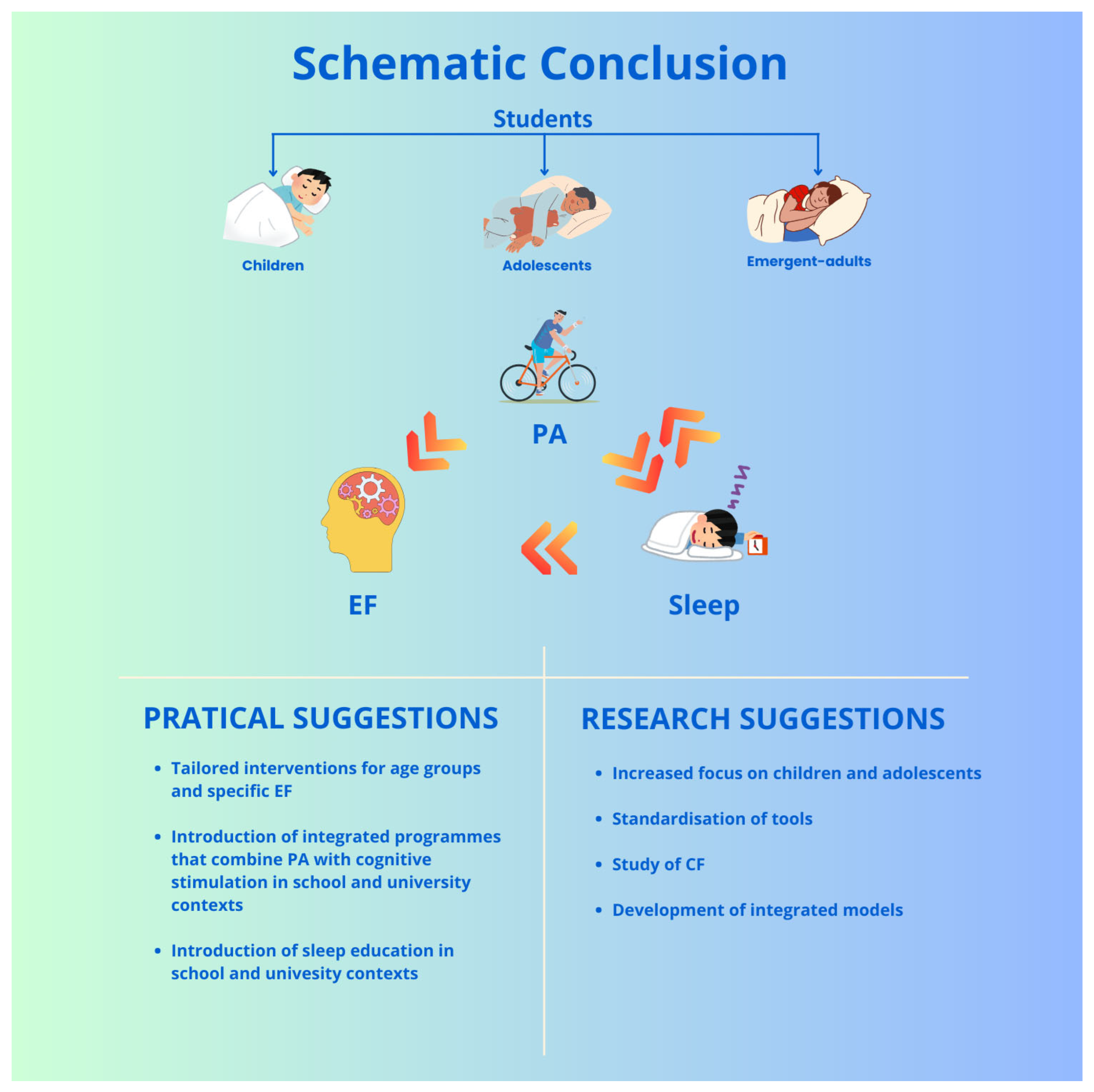
11. Conclusions
- An increased focus on children and adolescents: Research focuses mainly on university students; studies involving younger populations are essential for early interventions.
- The standardisation of tools: The lack of validated tools for assessing SQ in younger individuals limits the comparability of results and the accuracy of interpretations.
- The study of CF: This executive domain remains underexplored, particularly in studies combining sleep and PA. Given its relevance for academic adjustment, it is necessary to systematically include it in experimental protocols.
- The development of integrated models: The few studies that simultaneously analyse sleep, PA, and EFs indicate a complex interaction among these variables. Integrated and longitudinal experimental paradigms able to measure direct, mediated, and moderated effects over time are essential for constructing ecologically valid models that are useful for educational and clinical practice.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EF | Executive function |
| PA | Physical activity |
| WM | Working memory |
| IC | Inhibitory control |
| CF | Cognitive flexibility |
| dlPFC | Dorsolateral Prefrontal Cortex |
| vlPFC | Ventrolateral Prefrontal Cortex |
| mPFC | Medial Prefrontal Cortex |
| oFC | Orbitofrontal Cortex |
| FAT | Fronto-Aslant Tract |
| NREM | Non-rapid eye movement |
| REM | Rapid Eye Movement |
| SWS | Slow-wave sleep |
| SD | Sleep deprivation |
| TSD | Total sleep deprivation |
| ERP | Event-related potential |
| SJL | Social jet lag |
| IS | Insufficient sleep |
| SS | Sufficient sleep |
| SQ | Sleep quality |
| MET | Metabolic equivalent task |
| ATP | Adenosine triphosphate |
| HIIT | High-intensity interval training |
| OA | Obese adolescent |
| NWA | Normal-weight adolescent |
References
- Jokela, M. Why is cognitive ability associated with psychological distress and wellbeing? Exploring psychological, biological, and social mechanisms. Personal. Individ. Differ. 2022, 192, 111592. [Google Scholar] [CrossRef]
- Tikhomirova, T.; Malykh, A.; Malykh, S. Predicting Academic Achievement with Cognitive Abilities: Cross-Sectional Study across School Education. Behav. Sci. 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Reidy, N. Assessing Executive Function in Preschoolers. Neuropsychol. Rev. 2012, 22, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.A.; AlAmodi, A.A.; Al-Khateeb, A.A.; Abudan, Z.; Alkhani, M.A.; Zebian, S.I.; Qannita, A.S.; Tabrizi, M.J. The interplay between academic performance and quality of life among preclinical students. BMC Med. Educ. 2015, 15, 193. [Google Scholar] [CrossRef]
- Cushman, G.K.; West, K.B.; Davis, M.; LaMotte, J.; Eaton, C.K.; Gutierrez-Colina, A.M.; Suveg, C.; Blount, R.L. The role of executive functioning, healthcare management, and self-efficacy in college students’ health-related quality of life. J. Am. Coll. Health 2021, 70, 2356–2364. [Google Scholar] [CrossRef]
- Jirout, J.; LoCasale-Crouch, J.; Turnbull, K.; Gu, Y.; Cubides, M.; Garzione, S.; Evans, T.M.; Weltman, A.L.; Kranz, S. How Lifestyle Factors Affect Cognitive and Executive Function and the Ability to Learn in Children. Nutrients 2019, 11, 1953. [Google Scholar] [CrossRef]
- Maniaci, G.; La Cascia, C.; Giammanco, A.; Ferraro, L.; Palummo, A.; Saia, G.; Pinetti, G.; Zarbo, M.; La Barbera, D. The impact of healthy lifestyles on academic achievement among Italian adolescents. Curr. Psychol. 2023, 42, 5055–5061. [Google Scholar] [CrossRef]
- Ruedl, G.; Niedermeier, M.; Posch, M.; Kirschner, W.; Wirnitzer, K.; Cocca, A.; Greier, K. Association of modifiable factors with the development of physical fitness of Austrian primary school children: A 4-year longitudinal study. J. Sports Sci. 2022, 40, 920–927. [Google Scholar] [CrossRef]
- Mei, S.; Zheng, C.; Liang, L.; Kiyum, M.; Yuan, T.; Fei, J.; Liu, K.; Li, H.; Lin, X. The developmental trajectories and modifiable factors of adolescents’ subjective well-being from late adolescence to early adulthood. Child Adolesc. Psychiatry Ment. Health 2025, 19, 21. [Google Scholar] [CrossRef]
- Corney, K.B.; Stuart, A.L.; Mohebbi, M.; Pasco, J.A.; Kavanagh, B.E.; Sui, S.X.; Williams, L.J.; Cumming, P. Modifiable Lifestyle Factors and Cognitive Function: A Population-Based Study Amongst Nondemented Men. Acta Neurol. Scand. 2024, 2024, 1935091. [Google Scholar] [CrossRef]
- Balsamo, F.; Meneo, D.; Berretta, E.; Baglioni, C.; Gelfo, F. Could sleep be a brain/cognitive/neural reserve-builder factor? A systematic review on the cognitive effects of sleep modulation in animal models. Neurosci. Biobehav. Rev. 2025, 169, 106015. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Iwamoto, K.; Kawano, N.; Noda, Y.; Ozaki, N.; Noda, A. Differential effects of physical activity and sleep duration on cognitive function in young adults. J. Sport Health Sci. 2018, 7, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.T. Sueño, memoria y aprendizaje [Sleep, memory and learning]. Medicina 2019, 79 (Suppl. 3), 29–32. [Google Scholar] [PubMed]
- Kekäläinen, T.; Luchetti, M.; Terracciano, A.; Gamaldo, A.; Mogle, J.; Lovett, H.H.; Brown, J.; Rantalainen, T.; Sliwinski, M.J.; Sutin, A.R. Physical activity and cognitive function: Moment-to-moment and day-to-day associations. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 137. [Google Scholar] [CrossRef]
- Alnawwar, M.A.; Alraddadi, M.I.; Algethmi, R.A.; Salem, G.A.; Salem, M.A.; Alharbi, A.A. The Effect of Physical Activity on Sleep Quality and Sleep Disorder: A Systematic Review. Cureus 2023, 15, e43595. [Google Scholar] [CrossRef]
- James, J.; Pringle, A.; Mourton, S.; Roscoe, C.M.P. The Effects of Physical Activity on Academic Performance in School-Aged Children: A Systematic Review. Children 2023, 10, 1019. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, S.; Sun, H.; Wen, X.; Xiang, P. Physical Activity in Children’s Health and Cognition. BioMed. Res. Int. 2018, 2018, 8542403. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Carlson, S.M. Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Dev. Perspect 2012, 6, 354–360. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory: The Interface between Memory and Cognition. J. Cogn. Neurosci. 1992, 4, 281–288. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory. Curr. Biol. 2010, 20, R136–R140. [Google Scholar] [CrossRef]
- Fiske, A.; Holmboe, K. Neural substrates of early executive function development. Dev. Rev. 2019, 52, 42–62. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Pickering, S.J.; Ambridge, B.; Wearing, H. The Structure of Working Memory from 4 to 15 Years of Age. Dev. Psychol. 2004, 40, 177–190. [Google Scholar] [CrossRef]
- Sankalaite, S.; Huizinga, M.; Warreyn, P.; Dewandeleer, J.; Baeyens, D. The association between working memory, teacher-student relationship, and academic performance in primary school children. Front. Psychol. 2023, 14, 1240741. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Pérez, D.M.; Otero-Ojeda, G.A.; Pliego-Rivero, F.B.; Ferreira-Martínez, A.A. Relationship of working memory and eeg to academic performance: A study among high school students. Int. J. Neurosci. 2007, 117, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.; Canny, B.J.; Reser, D.H.; Rajan, R. Poorer verbal working memory for a second language selectively impacts academic achievement in university medical students. PeerJ 2013, 1, e22. [Google Scholar] [CrossRef] [PubMed]
- Kormos, J.; Sáfár, A. Phonological short-term memory, working memory and foreign language performance in intensive language learning. Biling. Lang. Cogn. 2008, 11, 261–271. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Buttelmann, F.; Karbach, J. Development and Plasticity of Cognitive Flexibility in Early and Middle Childhood. Front. Psychol. 2017, 8, 1040. [Google Scholar] [CrossRef]
- Feng, X.; Perceval, G.J.; Feng, W.; Feng, C. High Cognitive Flexibility Learners Perform Better in Probabilistic Rule Learning. Front. Psychol. 2020, 11, 415. [Google Scholar] [CrossRef]
- Zheng, W.; Akaliyski, P.; Ma, C.; Xu, Y. Cognitive flexibility and academic performance: Individual and cross-national patterns among adolescents in 57 countries. Personal. Individ. Differ. 2023, 217, 112455. [Google Scholar] [CrossRef]
- Nigg, J.T. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 2000, 126, 220–246. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Shah, P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar] [CrossRef]
- Irvan, R.; Tsapali, M. The role of Inhibitory Control in Achievement in Early Childhood Education. Camb. Educ. Res E-J. 2020, 7, 168–190. [Google Scholar]
- Privitera, A.J.; Zhou, Y.; Xie, X. Inhibitory control as a significant predictor of academic performance in Chinese high schoolers. Child Neuropsychol. 2022, 29, 457–473. [Google Scholar] [CrossRef]
- Dvorak, M. Inhibitory control and academic achievement—A study of the relationship between Stroop Effect and university students’ academic performance. BMC Psychol. 2024, 12, 498. [Google Scholar] [CrossRef]
- Jones, D.T.; Graff-Radford, J. Executive Dysfunction and the Prefrontal Cortex. Contin. Lifelong Learn. Neurol. 2021, 27, 1586–1601. [Google Scholar] [CrossRef]
- Dick, A.S.; Garic, D.; Graziano, P.; Tremblay, P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex 2019, 111, 148–163. [Google Scholar] [CrossRef]
- Buss, A.T.; Spencer, J.P. Changes in frontal and posterior cortical activity underlie the early emergence of executive function. Dev. Sci. 2018, 21, e12602. [Google Scholar] [CrossRef]
- Asanowicz, D.; Panek, B.; Kotlewska, I. Selection for Action: The Medial Frontal Cortex Is an Executive Hub for Stimulus and Response Selection. J. Cogn. Neurosci. 2021, 33, 1442–1469. [Google Scholar] [CrossRef]
- Acerbi, A.; McNamara, P.; Nunn, C.L. To sleep or not to sleep: The ecology of sleep in artificial organisms. BMC Ecol. 2008, 8, 10. [Google Scholar] [CrossRef]
- Frank, M.G. The Ontogenesis of Mammalian Sleep: Form and Function. Curr. Sleep Med. Rep. 2020, 6, 267–279. [Google Scholar] [CrossRef]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Xia, Z.; Storm, D. Role of circadian rhythm and REM sleep for memory consolidation. Neurosci. Res. 2017, 118, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Raho, E.M.; Sensi, M.; Di Lorenzo, F.; Fadiga, L.; Koch, G. A new perspective on positive symptoms: Expression of damage or self-defence mechanism of the brain? Neurol. Sci. 2024, 45, 2347–2351. [Google Scholar] [CrossRef] [PubMed]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Weissbluth, M. Naps in Children: 6 Months–7 Years. Sleep 1995, 18, 82–87. [Google Scholar] [CrossRef]
- Staton, S.; Rankin, P.S.; Harding, M.; Smith, S.S.; Westwood, E.; LeBourgeois, M.K.; Thorpe, K.J. Many naps, one nap, none: A systematic review and meta-analysis of napping patterns in children 0–12 years. Sleep Med. Rev. 2020, 50, 101247. [Google Scholar] [CrossRef]
- Diekelmann, S. Sleep for cognitive enhancement. Front. Syst. Neurosci. 2014, 8, 46. [Google Scholar] [CrossRef]
- Mason, G.M.; Lokhandwala, S.; Riggins, T.; Spencer, R.M. Sleep and human cognitive development. Sleep Med. Rev. 2021, 57, 101472. [Google Scholar] [CrossRef]
- Vidueira, V.F.; Booth, J.N.; Saunders, D.H.; Sproule, J.; Turner, A.P. Circadian preference and physical and cognitive performance in adolescence: A scoping review. Chronobiol. Int. 2023, 40, 1296–1331. [Google Scholar] [CrossRef]
- Pisch, M.; Wiesemann, F.; Karmiloff-Smith, A. Infant wake after sleep onset serves as a marker for different trajectories in cognitive development. J. Child Psychol. Psychiatry 2018, 60, 189–198. [Google Scholar] [CrossRef]
- Merín, L.; Nieto, M.; Sánchez-Arias, L.; Ros, L.; Latorre, J.M. Actigraphy-assessed sleep duration and quality and executive function in a sample of typically developing preschoolers. Eur. Child Adolesc. Psychiatry 2025, 34, 1379–1390. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, S.; Zhang, M.; Wu, N. Self-Reported Sleep and Executive Function in Early Primary School Children. Front. Psychol. 2021, 12, 793000. [Google Scholar] [CrossRef]
- Ashworth, A.; Hill, C.M.; Karmiloff-Smith, A.; Dimitriou, D. Sleep enhances memory consolidation in children. J. Sleep Res. 2013, 23, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Bodizs, R.; Gombos, F.; Ujma, P.P.; Kovacs, I. Sleep spindling and fluid intelligence across adolescent development: Sex matters. Front. Hum. Neurosci. 2014, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Turan, O.; Garner, J.; Isaiah, A.; Palatino, M.; Ernst, T.; Wang, Z.; Chang, L. Fitbit-measured sleep duration in young adolescents is associated with functional connectivity in attentional, executive control, memory, and sensory networks. Sleep 2025. [Google Scholar] [CrossRef]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian Typology: A Comprehensive Review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef]
- Ujma, P.P.; Scherrer, V. Circadian preference and intelligence—An updated meta-analysis. Chronobiol. Int. 2021, 38, 1215–1229. [Google Scholar] [CrossRef]
- Ma, N.; Dinges, D.F.; Basner, M.; Rao, H. How Acute Total Sleep Loss Affects the Attending Brain: A Meta-Analysis of Neuroimaging Studies. Sleep 2015, 38, 233–240. [Google Scholar] [CrossRef]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.; Havekes, R.; Saletin, J.M.; Walker, M.P. Sleep, Plasticity and Memory from Molecules to Whole-Brain Networks. Curr. Biol. 2013, 23, R774–R788. [Google Scholar] [CrossRef]
- Wolfson, A.R. Adolescents and Emerging Adults’ Sleep Patterns: New Developments. J. Adolesc. Health 2010, 46, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, C.L.; Simpson, M.R.; Sivertsen, B.; Kallestad, H.; Langsrud, K.; Scott, J.; Vedaa, Ø. Weekday-to-weekend sleep duration patterns among young adults and outcomes related to health and academic performance. Sleep Sci. Pract. 2024, 8, 15. [Google Scholar] [CrossRef]
- Tonetti, L.; Fabbri, M.; Natale, V. Sex Difference in Sleep-Time Preference and Sleep Need: A Cross-Sectional Survey among Italian Pre-Adolescents, Adolescents, and Adults. Chronobiol. Int. 2008, 25, 745–759. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Randler, C. Age and Gender Differences in Morningness–Eveningness During Adolescence. J. Genet. Psychol. 2011, 172, 302–308. [Google Scholar] [CrossRef]
- Silva, E.J.; Wang, W.; Ronda, J.M.; Wyatt, J.K.; Duffy, J.F. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep 2010, 33, 481–490. [Google Scholar] [CrossRef]
- Whiting, W.L.; Murdock, K.K. Emerging adults’ sleep patterns and attentional capture: The pivotal role of consistency. Cogn. Process. 2016, 17, 155–162. [Google Scholar] [CrossRef]
- Jiang, F.; VanDyke, R.D.; Zhang, J.; Li, F.; Gozal, D.; Shen, X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J. Clin. Exp. Neuropsychol. 2011, 33, 892–900. [Google Scholar] [CrossRef]
- Ling, J.; Sun, W.; Chan, N.Y.; Zhang, J.; Lam, S.P.; Li, A.M.; Chan, J.W.Y.; Kyle, S.D.; Li, S.X. Effects of insomnia symptoms and objective short sleep duration on memory performance in youths. J. Sleep Res. 2020, 29, e13049. [Google Scholar] [CrossRef]
- Gilstrap, S.R.; Hobson, J.M.; Dark, H.E.; Gloston, G.F.; Cody, S.L.; Goodin, B.R.; Thomas, S.J. Disordered sleep and its association with academic performance and functioning. Sleep Biol. Rhythm. 2022, 21, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Al Salmani, A.A.; Al Shidhani, A.; Al Qassabi, S.S.; Al Yaaribi, S.A.; Al Musharfi, A.M. Prevalence of sleep disorders among university students and its impact on academic performance. Int. J. Adolesc. Youth 2020, 25, 974–981. [Google Scholar] [CrossRef]
- Okano, K.; Kaczmarzyk, J.R.; Dave, N.; Gabrieli, J.D.E.; Grossman, J.C. Sleep quality, duration, and consistency are associated with better academic performance in college students. npj Sci. Learn. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Ong, J.L.; Leong, R.L.; Gooley, J.J.; Chee, M.W. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep 2016, 39, 687–698. [Google Scholar] [CrossRef]
- Tai, X.Y.; Chen, C.; Manohar, S.; Husain, M. Impact of sleep duration on executive function and brain structure. Commun. Biol. 2022, 5, 201. [Google Scholar] [CrossRef]
- Warren, C.; Riggs, N.; Pentz, M.A. Executive function mediates prospective relationships between sleep duration and sedentary behavior in children. Prev. Med. 2016, 91, 82–88. [Google Scholar] [CrossRef]
- D’Angiulli, A.; Byczynski, G.; Yeh, W.; Garrett, G.; Goldfield, G.; Devenyi, P.; Devenyi, T.; Leisman, G. Cognitive control, bedtime patterns, and testing time in female adolescent students: Behavioral and neuro-electrophysiological correlates. Front. Public Health 2023, 11, 1022731. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, S.; Yang, X.; Peng, Y.; Du, M.; Zhang, R.; Sima, J.; Zou, F.; Wu, X.; Wang, Y.; et al. Neuroelectrophysiological alteration associated with cognitive flexibility after 24 h sleep deprivation in adolescents. Conscious. Cogn. 2024, 124, 103734. [Google Scholar] [CrossRef]
- Cohen-Zion, M.; Shiloh, E. Evening chronotype and sleepiness predict impairment in executive abilities and academic performance of adolescents. Chronobiol. Int. 2017, 35, 137–145. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Hutcherson, C.A.; Bemporad, B.; Morgan, A.; Kumar, A.; Hobson, J.A.; Stickgold, R. Failure to Find Executive Function Deficits Following One Night’s Total Sleep Deprivation in University Students Under Naturalistic Conditions. Behav. Sleep Med. 2009, 7, 136–163. [Google Scholar] [CrossRef]
- Yeung, M.K.; Lee, T.L.; Cheung, W.K.; Chan, A.S. Frontal Underactivation During Working Memory Processing in Adults With Acute Partial Sleep Deprivation: A Near-Infrared Spectroscopy Study. Front. Psychol. 2018, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Dai, C.; Ba, Y.; Zhang, L.; Shao, Y.; Tian, J. Effect of Sleep Deprivation on the Working Memory-Related N2-P3 Components of the Event-Related Potential Waveform. Front. Neurosci. 2020, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Ballesio, A.; Ferlazzo, F.; Lucidi, F.; Lombardo, C. Decreased inhibitory control after partial sleep deprivation in individuals reporting binge eating: Preliminary findings. PeerJ 2020, 8, e9252. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ye, E.; Qi, J.; Wang, L.; Lei, Y.; Chen, P.; Mi, G.; Zou, F.; Shao, Y.; Yang, Z. Recovery Sleep Reverses Impaired Response Inhibition due to Sleep Restriction: Evidence from a Visual Event Related Potentials Study. PLoS ONE 2015, 10, e0142361. [Google Scholar] [CrossRef]
- Del Angel, J.; Cortez, J.; Juárez, D.; Guerrero, M.; García, A.; Ramírez, C.; Valdez, P. Effects of sleep reduction on the phonological and visuospatial components of working memory. Sleep Sci. 2015, 8, 68–74. [Google Scholar] [CrossRef]
- Ballesio, A.; Cerolini, S.; Ferlazzo, F.; Cellini, N.; Lombardo, C. The effects of one night of partial sleep deprivation on executive functions in individuals reporting chronic insomnia and good sleepers. J. Behav. Ther. Exp. Psychiatry 2018, 60, 42–45. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Richter, M.; Gendolla, G.H.E.; van der Linden, M. Young poor sleepers mobilize extra effort in an easy memory task: Evidence from cardiovascular measures. J. Sleep Res. 2010, 19, 487–495. [Google Scholar] [CrossRef]
- Lau, E.Y.; Wong, M.L.; Lau, K.N.; Hui, F.W.; Tseng, C.H. Rapid-Eye-Movement-Sleep (REM) Associated Enhancement of Working Memory Performance after a Daytime Nap. PLoS ONE 2015, 10, e0125752. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L.; Davis, J.E.; Corbett, C.F. Sleep quality: An evolutionary concept analysis. Nurs. Forum 2022, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, F.; Zhang, J.; Zhou, H.; Sun, N.; Li, L.; Liang, L.; Ning, N.; Wu, Q.; Zhao, M. Sleep Quality of Students from Elementary School to University: A Cross-Sectional Study. Nat. Sci. Sleep 2020, 12, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Higgins, H.J. Sleep, Executive Functioning, and Behavioral Problems in School-Age Children. Doctor of Philosophy in the Department of Psychology. Ph.D. Thesis, Idaho State University, Pocatello, ID, USA, 2020. [Google Scholar]
- Anderson, B.; Storfer-Isser, A.; Taylor, H.G.; Rosen, C.L.; Redline, S. Associations of Executive Function With Sleepiness and Sleep Duration in Adolescents. Pediatrics 2009, 123, e701–e707. [Google Scholar] [CrossRef]
- Ouellet, J.; Assaf, R.; Afzali, M.H.; Nourbakhsh, S.; Potvin, S.; Conrod, P. Neurocognitive consequences of adolescent sleep disruptions and their relationship to psychosis vulnerability: A longitudinal cohort study. npj Ment. Health Res. 2024, 3, 18. [Google Scholar] [CrossRef]
- Gong, L.; Wang, M.; Ye, C.; Liu, Q. The impact of sleep quality on visual working memory varied with the duration of maintenance. Front. Psychol. 2024, 15, 1404989. [Google Scholar] [CrossRef]
- Parrilla, M.M.; Kautiainen, R.J.; King, T.Z. Sleep quality and executive function in a diverse sample of healthy young adults. Appl. Neuropsychol. Adult 2024, 1–9. [Google Scholar] [CrossRef]
- Almarzouki, A.F.; Mandili, R.L.; Salloom, J.; Kamal, L.K.; Alharthi, O.; Alharthi, S.; Khayyat, N.; Baglagel, A.M. The Impact of Sleep and Mental Health on Working Memory and Academic Performance: A Longitudinal Study. Brain Sci. 2022, 12, 1525. [Google Scholar] [CrossRef]
- Conner, E.R. The College Experience: Exploring the Relationship between Sleep, Executive Function, and Alcohol Use. Chancellor’s Honor. Program Proj. 2015. [Google Scholar]
- Abbas, N.H.; Bahia, B.; Covacha, A.; Hollett, D.; Kaur, B.; Samson, D.R. The Effects of Sleep Quality on Response Inhibition. Young Anthropol. 2020, 2, 10–16. [Google Scholar]
- Chen, M.; Zhang, X.; Liu, X.; Chen, Y.; Liu, R.; Peng, L.; Li, M. The association between insomnia symptoms and cognitive flexibility among undergraduates: An event-related potential study. Sleep Med. 2024, 121, 343–351. [Google Scholar] [CrossRef]
- Daley, A.J.; Duda, J.L. Self-determination, stage of readiness to change for exercise, and frequency of physical activity in young people. Eur. J. Sport Sci. 2006, 6, 231–243. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs. anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Ambler, PA, USA, 2013. [Google Scholar]
- Wasserman, K. The anaerobic threshold: Definition, physiological significance and identification. Adv. Cardiol. 1986, 35, 1–23. [Google Scholar]
- Temur, H.A.; Vardar, S.A.; Demir, M.; Palabiyik, O.; Karaca, A.; Guksu, Z.; Ortanca, A.; Sut, N. The alteration of NTproCNP plasma levels following anaerobic exercise in physically active young men. Anatol. J. Cardiol. 2015, 15, 97–102. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- Naci, H.; Ioannidis, J.P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 2013, 347, f5577. [Google Scholar] [CrossRef]
- Stensel, D.; Hardman, A.; Gill, J. Physical Activity and Health: The Evidence Explained; Routledge: Milton Park, UK, 2021. [Google Scholar]
- Stubbs, B.; Vancampfort, D.; Hallgren, M.; Firth, J.; Veronese, N.; Solmi, M.; Brand, S.; Cordes, J.; Malchow, B.; Gerber, M.; et al. EPA guidance on physical activity as a treatment for severe mental illness: A meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur. Psychiatry 2018, 54, 124–144. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Chen, Y.; Fang, W.; Li, X.; Wang, R.; Liu, J.; Ma, X. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front. Immunol. 2023, 13, 1080782. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, B.N.; Kim, S.H.; Kim, G.R.; Park, E.C.; Jang, S.I. Association between sedentary time and sleep quality based on the Pittsburgh Sleep Quality Index among South Korean adults. BMC Public Health 2021, 21, 2290. [Google Scholar] [CrossRef]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Armstrong, R.B.; Godfrey, D.A.; Laughlin, M.H.; Ross, C.D.; Wilkerson, M.K. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J. Physiol. 2001, 533, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hart, L. How the Brain Works; Basic Books: New York, NY, USA, 1975. [Google Scholar]
- Walsh, J.J.; Barnes, J.D.; Cameron, J.D.; Goldfield, G.S.; Chaput, J.-P.; Gunnell, K.E.; Ledoux, A.-A.; Zemek, R.L.; Tremblay, M.S. Associations between 24 hour movement behaviours and global cognition in US children: A cross-sectional observational study. Lancet Child Adolesc. Health 2018, 2, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Hinkley, T.; Okely, A.D.; Salmon, J. Tracking physical activity and sedentary behavior in childhood: A systematic review. Am. J. Prev. Med. 2013, 44, 651–658. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef]
- Romeo, R.D.; McEwen, B.S. Stress and the adolescent brain. Ann. N. Y. Acad. Sci. 2006, 1094, 202–214. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Hallal, P.C.; Mielke, G.I.; Menezes, A.M.; Gonçalves, H.; Wehrmeister, F.; Ekelund, U.; Rombaldi, A.J. Physical Activity throughout Adolescence and Cognitive Performance at 18 Years of Age. Med. Sci. Sports Exerc. 2015, 47, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Dumith, S.C.; Gigante, D.P.; Domingues, M.R.; Kohl, H.W. 3° Cambiamento dell’attività fisica durante l’adolescenza: Una revisione sistematica e un’analisi aggregata. Int. J. Epidemiol. 2011, 40, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Pindus, D.M.; Davis, R.D.M.; Hillman, C.H.; Bandelow, S.; Hogervorst, E.; Biddle, S.J.H.; Sherar, L.B. The relationship of moderate-to-vigorous physical activity to cognitive processing in adolescents: Findings from the ALSPAC birth cohort. Psychol. Res. 2014, 79, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.; Yau, P.L.; Convit, A. Obesity, fitness, and brain integrity in adolescence. Appetite 2015, 93, 44–50. [Google Scholar] [CrossRef]
- Herting, M.M.; Nagel, B.J. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 2012, 233, 517–525. [Google Scholar] [CrossRef]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 2008, 40, 1044–1055. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Herting, M.M.; Goddings, A.; Meuwese, R.; Blakemore, S.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.A.; et al. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J. Neurosci. 2017, 37, 3302–3316. [Google Scholar] [CrossRef]
- Whitford, T.J.; Rennie, C.J.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Williams, L.M. Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007, 28, 228–237. [Google Scholar] [CrossRef]
- Haverkamp, B.F.; Wiersma, R.; Vertessen, K.; van Ewijk, H.; Oosterlaan, J.; Hartman, E. Effects of physical activity interventions on cognitive outcomes and academic performance in adolescents and young adults: A meta-analysis. J. Sports Sci. 2020, 38, 2637–2660. [Google Scholar] [CrossRef]
- Wade, N.E.; Kaiver, C.M.; Wallace, A.L.; Hatcher, K.F.; Swartz, A.M.; Lisdahl, K.M. Objective aerobic fitness level and neuropsychological functioning in healthy adolescents and emerging adults: Unique sex effects. Psychol. Sport Exerc. 2020, 51, 101794. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, Z.; Bingquan, L. Research on the effect of different aerobic activity on physical fitness and executive function in primary school students. Sci. Rep. 2024, 14, 7956. [Google Scholar] [CrossRef] [PubMed]
- Erwin, H.; Schreiber, S. Aerobic and Anaerobic Exercise’s Impact on Cognitive Functions in Eighth Grade Students. Int. J. Environ. Res. Public Health 2024, 21, 833. [Google Scholar] [CrossRef]
- Hu, L.; Shen, Q.; Yin, H.; Cui, L. Time-course effects of exercise intervention on executive function in adolescents with obesity. Front. Psychol. 2024, 15, 1346896. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Pühse, U.; Colledge, F. Effects of Aerobic Exercise on Cognitive Performance Among Young Adults in a Higher Education Setting. Res. Q. Exerc. Sport 2018, 89, 164–172. [Google Scholar] [CrossRef]
- Fan, H.; Qi, S.; Huang, G.; Xu, Z. Effect of Acute Aerobic Exercise on Inhibitory Control of College Students with Smartphone Addiction. Evid.-Based Complement. Altern. Med. 2021, 2021, 5530126. [Google Scholar] [CrossRef]
- Li, L.; Men, W.W.; Chang, Y.K.; Fan, M.X.; Ji, L.; Wei, G.X. Acute Aerobic Exercise Increases Cortical Activity during Working Memory: A Functional MRI Study in Female College Students. PLoS ONE 2014, 9, e99222. [Google Scholar] [CrossRef]
- Martínez-Díaz, I.C.; Escobar-Muñoz, M.C.; Carrasco, L. Acute Effects of High-Intensity Interval Training on Brain-Derived Neurotrophic Factor, Cortisol and Working Memory in Physical Education College Students. Int. J. Environ. Res. Public Health 2020, 17, 8216. [Google Scholar] [CrossRef]
- Liu, Y.; Men, W.W.; Chang, Y.K.; Fan, M.X.; Ji, L.; Wei, G.X. Effects of acute rope skipping exercises of different exercise modes on cognitive function in 9–10-year-old children. Sci. Rep. 2024, 14, 29172. [Google Scholar] [CrossRef]
- Peruyero, F.; Zapata, J.; Pastor, D.; Cervelló, E. The Acute Effects of Exercise Intensity on Inhibitory Cognitive Control in Adolescents. Front. Psychol. 2017, 8, 921. [Google Scholar] [CrossRef]
- Budde, H.; Voelcker-Rehage, C.; Pietrassyk-Kendziorra, S.; Machado, S.; Ribeiro, P.; Arafat, A.M. Steroid hormones in the saliva of adolescents after different exercise intensities and their influence on working memory in a school setting. Psychoneuroendocrinology 2010, 35, 382–391. [Google Scholar] [CrossRef]
- Berse, T.; Rolfes, K.; Barenberg, J.; Dutke, S.; Kuhlenbäumer, G.; Völker, K.; Winter, B.; Wittig, M.; Knecht, S. Acute physical exercise improves shifting in adolescents at school: Evidence for a dopaminergic contribution. Front. Behav. Neurosci. 2015, 9, 196. [Google Scholar] [CrossRef]
- Robinson, K.J.; Lubans, D.R.; Mavilidi, M.F.; Hillman, C.H.; Benzing, V.; Valkenborghs, S.R.; Barker, D.; Riley, N. Effects of Classroom-Based Resistance Training With and Without Cognitive Training on Adolescents’ Cognitive Function, On-task Behavior, and Muscular Fitness. Front. Psychol. 2022, 13, 811534. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.M.; Vhavle, S.P.; Manjunath, N. Comparison of yoga versus physical exercise on executive function, attention, and working memory in adolescent schoolchildren: A randomized controlled trial. Int. J. Yoga 2019, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Loaiza, H.; Arias, I.; Bonilla, S.; Ramírez, R.; Ramírez-Herrera, S.; Nanez, J.; Barbosa-Granados, S.; Arenas-Granada, J. Effect of acute physical exercise on inhibitory control in young adults: High-intensity indoor cycling session. Physiol. Behav. 2022, 254, 113902. [Google Scholar] [CrossRef]
- Wen, C.-T.; Chu, C.L.; Chen, H.C.; Chueh, T.Y.; Lin, C.C.; Wu, S.Y.; Hsu, W.C.; Huang, C.J.; Hung, T.M. Effects of acute slackline exercise on executive function in college students. Front. Psychol. 2023, 14, 1092804. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; He, Q.; Li, X.; Zhang, J.; Tang, Y. The relationship between acute aerobic exercise and inhibitory control in college students: The impact of physical and cognitive engagement. Physiol. Behav. 2024, 290, 114779. [Google Scholar] [CrossRef]
- Martínez-Díaz, I.C.; Páez, L.C. Little but Intense: Using a HIIT-Based Strategy to Improve Mood and Cognitive Functioning in College Students. Healthcare 2023, 11, 1880. [Google Scholar] [CrossRef]
- Ji, L.-Y.; Li, X.L.; Liu, Y.; Sun, X.W.; Wang, H.F.; Chen, L.; Gao, L. Time-Dependent Effects of Acute Exercise on University Students’ Cognitive Performance in Temperate and Cold Environments. Front. Psychol. 2017, 8, 1192. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Yang, Q. Tai Chi exercise improves working memory capacity and emotion regulation ability. Front. Psychology. 2023, 14, 1047544. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, H.; Ji, C. Effects of High-Intensity Interval Training on Executive Functions in College Students: Evidence from Different Doses. Brain Sci. 2023, 13, 571. [Google Scholar] [CrossRef]
- Tavakoli, M.H.M.; Masoumeh, S.; Keyvan, M. ffects of physical activity on sleep quality, inhibitory control, working memory and cognitive flexibility among adolescent students. Rev. Iberoam. Psicol. Del Ejerc. Deporte 2024, 19, 398–401. [Google Scholar]
- Sun, F.; Zhang, F.; Ho, K.Y.; Zhang, B.; Wang, Z.; Tse, A.C. Physical Activity and Executive Functions in Adolescents: The Mediating Role of Sleepiness. Int. J. Environ. Res. Public Health 2022, 19, 12972. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, Q.; Zhao, W.; Herold, F.; Cheval, B.; Kong, Z.; Li, J.; Mueller, N.; Kramer, A.F.; Cui, J.; et al. Physical Activity and Inhibitory Control: The Mediating Role of Sleep Quality and Sleep Efficiency. Brain Sci. 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, R. Aerobic Exercise Alleviates the Impairment of Cognitive Control Ability Induced by Sleep Deprivation in College Students: Research Based on Go/NoGo Task. Front. Psychol. 2022, 13, 914568. [Google Scholar] [CrossRef]
- El Hangouche, A.J.; Jniene, A.; Aboudrar, S.; Errguig, L.; Rkain, H.; Cherti, M.; Dakka, T. Relationship between poor quality sleep, excessive daytime sleepiness and low academic performance in medical students. Adv. Med. Educ. Pract. 2018, 9, 631–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafar, M.; Omer, E.O.M.; Hassan, M.E.; Ansari, H.A. Association of sleep disorder with academic performance among medical students in Sudan. ROMJ 2020, 9, 208. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Omidvar, S. The quality of sleep and daytime sleepiness and their association with quality of school life and school achievement among students. J. Educ. Health Promot. 2022, 11, 159. [Google Scholar] [CrossRef]
- Ballesio, A.; Aquino, M.R.J.V.; Kyle, S.D.; Ferlazzo, F.; Lombardo, C. Executive Functions in Insomnia Disorder: A Systematic Review and Exploratory Meta-Analysis. Front. Psychol. 2019, 10, 101. [Google Scholar] [CrossRef]
- Kiss, O.; Arnold, A.; Weiss, H.A.; Baker, F.C. The relationship between sleep and menstrual problems in early adolescent girls. Sleep Sci. Pract. 2024, 8, 20. [Google Scholar] [CrossRef]
- Rugvedh, P.; Gundreddy, P.; Wandile, B. The Menstrual Cycle’s Influence on Sleep Duration and Cardiovascular Health: A Comprehensive Review. Cureus 2023, 15, e47292. [Google Scholar] [CrossRef]
- Jang, D.; Zhang, J.; Elfenbein, H.A. Menstrual cycle effects on cognitive performance: A meta-analysis. PLoS ONE 2025, 20, e0318576. [Google Scholar] [CrossRef]
- Sundström, P.I.; Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014, 8, 380. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluardo, G.; Meneo, D.; Cerolini, S.; Baglioni, C.; De Bartolo, P. Sleep, Physical Activity, and Executive Functions in Students: A Narrative Review. Clocks & Sleep 2025, 7, 47. https://doi.org/10.3390/clockssleep7030047
Belluardo G, Meneo D, Cerolini S, Baglioni C, De Bartolo P. Sleep, Physical Activity, and Executive Functions in Students: A Narrative Review. Clocks & Sleep. 2025; 7(3):47. https://doi.org/10.3390/clockssleep7030047
Chicago/Turabian StyleBelluardo, Giulia, Debora Meneo, Silvia Cerolini, Chiara Baglioni, and Paola De Bartolo. 2025. "Sleep, Physical Activity, and Executive Functions in Students: A Narrative Review" Clocks & Sleep 7, no. 3: 47. https://doi.org/10.3390/clockssleep7030047
APA StyleBelluardo, G., Meneo, D., Cerolini, S., Baglioni, C., & De Bartolo, P. (2025). Sleep, Physical Activity, and Executive Functions in Students: A Narrative Review. Clocks & Sleep, 7(3), 47. https://doi.org/10.3390/clockssleep7030047




