The Acute Effects of Morning Bright Light on the Human White Adipose Tissue Transcriptome: Exploratory Post Hoc Analysis
Abstract
1. Introduction
2. Results
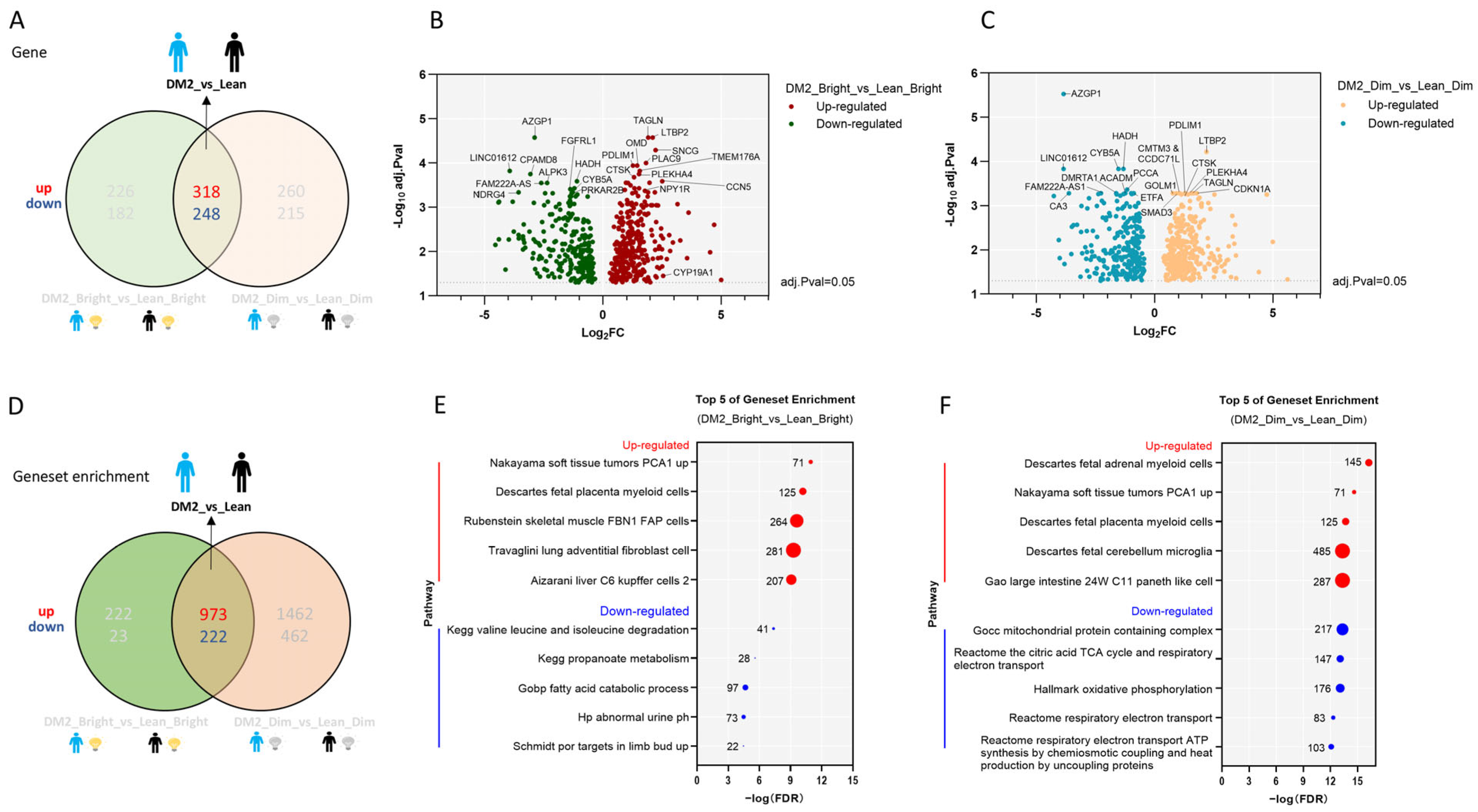
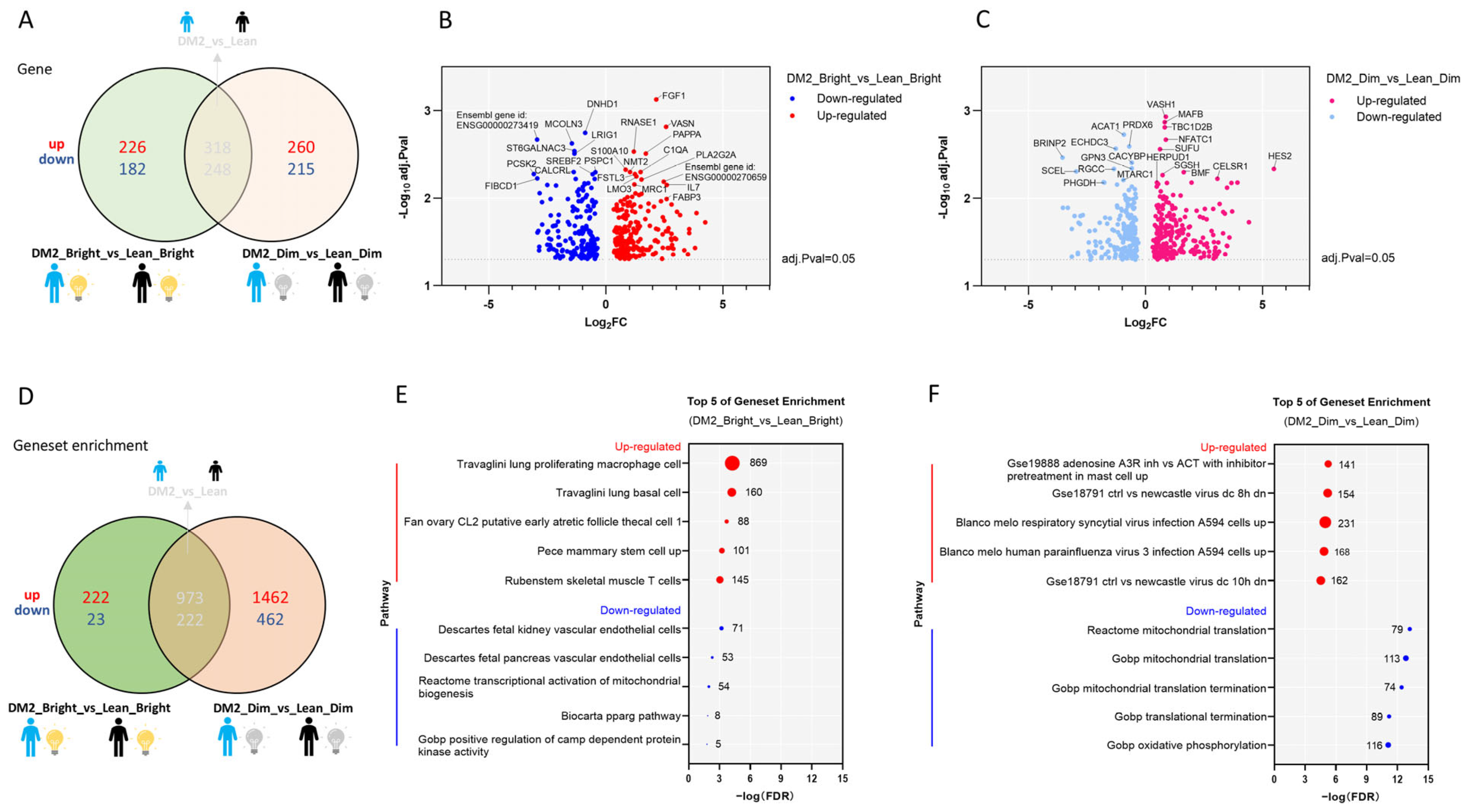
2.1. Consistent Differences in the WAT Transcriptome Between Men with Obesity and DM2 and Lean, Healthy Men
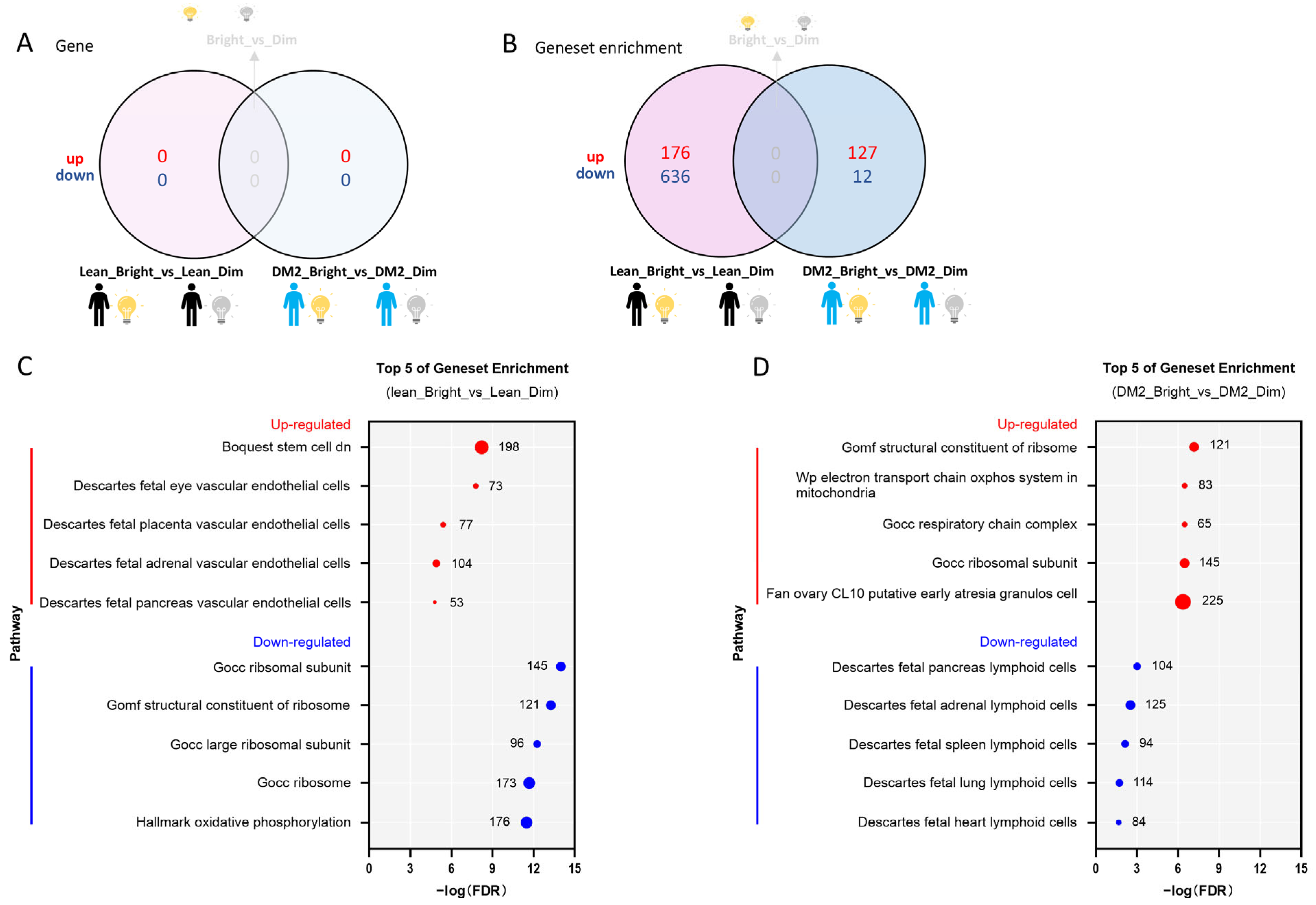
2.2. Effects of Bright-Light Exposure on the WAT Transcriptome
2.2.1. There Is No Consistent Effect of Bright Light on the WAT Transcriptome Among Men with Obesity and DM2 and Lean, Healthy Men
2.2.2. The Effect of Bright Light on the WAT Transcriptome in Men with Both Obesity and DM2 and Lean, Healthy Men
2.2.3. Effects of Bright or Dim Light on the WAT Transcriptome Differ Between Men with Obesity and DM2 and Lean, Healthy Men
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Participants and Screening Procedures
4.3. Study Design
4.4. Lighting Conditions
4.5. Adipose Tissue Biopsy
4.6. RNA Sequencing
4.7. Statistical Analysis
4.8. Power Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Do, M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- van der Spek, R.; Kreier, F.; Fliers, E.; Kalsbeek, A. Circadian rhythms in white adipose tissue. Prog. Brain Res. 2012, 199, 183–201. [Google Scholar] [PubMed]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Garaulet, M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Heyde, I.; Begemann, K.; Oster, H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021, 162, bqab009. [Google Scholar] [CrossRef]
- Vázquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Mayoral, L.P.C.; Andrade, G.M.; Mayoral, E.P.C.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar]
- Passaro, A.; Miselli, M.A.; Sanz, J.M.; Nora, E.D.; Morieri, M.L.; Colonna, R.; Pišot, R.; Zuliani, G. Gene expression regional differences in human subcutaneous adipose tissue. BMC Genom. 2017, 18, 202. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef]
- Coomans, C.P.; Berg, S.A.A.; Houben, T.; Klinken, J.; Berg, R.; Pronk, A.C.M.; Havekes, L.M.; Romijn, J.A.; Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, J.-F.; Wefers, J.; Doligkeit, D.; Schlangen, L.; Dautzenberg, B.; Rense, P.; van Moorsel, D.; Hoeks, J.; Moonen-Kornips, E.; Gordijn, M.C.M.; et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day. Diabetologia 2022, 65, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Benedito-Silva, A.A.; Evans, S.; Mendes, J.V.; Castro, J.; Gonçalves, B.d.S.B.; Ruiz, F.S.; Beijamini, F.; Evangelista, F.S.; Vallada, H.; Krieger, J.E.; et al. Association between light exposure and metabolic syndrome in a rural Brazilian town. PLoS ONE 2020, 15, e0238772. [Google Scholar] [CrossRef]
- Zheng, R.; Xin, Z.; Li, M.; Wang, T.; Xu, M.; Lu, J.; Dai, M.; Zhang, D.; Chen, Y.; Wang, S.; et al. Outdoor light at night in relation to glucose homoeostasis and diabetes in Chinese adults: A national and cross-sectional study of 98,658 participants from 162 study sites. Diabetologia 2023, 66, 336–345. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Stenvers, D.J.; Visintainer, D.; Linnenbank, A.; Tanck, M.W.; Zwanenburg, G.; Smilde, A.K.; Fliers, E.; Kalsbeek, A.; Serlie, M.J.; et al. Acute Effects of Morning Light on Plasma Glucose and Triglycerides in Healthy Men and Men with Type 2 Diabetes. J. Biol. Rhythm. 2017, 32, 130–142. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef]
- Maury, E.; Navez, B.; Brichard, S.M. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 2021, 12, 2388. [Google Scholar] [CrossRef]
- Dixon, J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Diez-Caballero, A.; Martínez-Cruz, L.A.; Gil, M.J.; García-Foncillas, J.; Cienfuegos, J.A.; Salvador, J.; Mato, J.M.; Frühbeck, G. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 2004, 18, 215–217. [Google Scholar] [CrossRef]
- Hinte, L.C.; Castellano-Castillo, D.; Ghosh, A.; Melrose, K.; Gasser, E.; Noé, F.; Massier, L.; Dong, H.; Sun, W.; Hoffmann, A.; et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 2024, 636, 457–465. [Google Scholar] [CrossRef]
- Barros, R.P.D.A.; Morani, A.; Moriscot, A.; Machado, U.F. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol. Cell. Endocrinol. 2008, 295, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, Y.; Liu, Q.; Cai, J.; Tang, X.; Liu, S.; Zhang, J.; Xu, M.; Wei, C.; Mo, X.; et al. Association of CYP19A1 Gene, Plasma Zinc, and Urinary Zinc with the Risk of Type 2 Diabetes Mellitus in a Chinese Population. Biol. Trace Elem. Res. 2023, 201, 4205–4215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, H.; Chen, S.; Zhu, H.; Lai, S. Interaction of sex steroid hormones and obesity on insulin resistance and type 2 diabetes in men: The Third National Health and Nutrition Examination Survey. J. Diabetes Its Complicat. 2017, 31, 318–327. [Google Scholar] [CrossRef]
- Ahmed, F.; Hetty, S.; Laterveer, R.; Surucu, E.B.; Mathioudaki, A.; Hornbrinck, E.; Patsoukaki, V.; Olausson, J.; Sundbom, M.; Svensson, M.K.; et al. Altered Expression of Aromatase and Estrogen Receptors in Adipose Tissue From Men With Obesity or Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2025, dgaf038. [Google Scholar] [CrossRef]
- Livingstone, C.; Collison, M. Sex steroids and insulin resistance. Clin. Sci. 2002, 102, 151–166. [Google Scholar] [CrossRef]
- Sidon, E.; Shemesh, S.S.; Mor-Yossef Moldovan, L.; Wiesenfeld, Y.; Ohana, N.; Benayahu, D. Molecular profile of ultrastructure changes of the ligamentum flavum related to lumbar spinal canal stenosis. J. Cell. Biochem. 2019, 120, 11716–11725. [Google Scholar] [CrossRef]
- Ribot, J.; Caliaperoumal, G.; Paquet, J.; Boisson-Vidal, C.; Petite, H.; Anagnostou, F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J. Cell. Mol. Med. 2017, 21, 349–363. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Chen, J.; Yao, H.; Mao, R.; Li, C.; Zhang, G.; Chen, Z.; Xu, X.; Wang, C. Identification of Immune Infiltration and the Potential Biomarkers in Diabetic Peripheral Neuropathy through Bioinformatics and Machine Learning Methods. Biomolecules 2022, 13, 39. [Google Scholar] [CrossRef]
- Varberg, K.M.; Garretson, R.O.; Blue, E.K.; Chu, C.; Gohn, C.R.; Tu, W.; Haneline, L.S. Transgelin induces dysfunction of fetal endothelial colony-forming cells from gestational diabetic pregnancies. Am. J. Physiol. Cell Physiol. 2018, 315, C502–C515. [Google Scholar] [CrossRef]
- Bui, L.; Edwards, S.; Hall, E.; Alderfer, L.; Round, K.; Owen, M.; Sainaghi, P.; Zhang, S.; Nallathamby, P.D.; Haneline, L.S.; et al. Engineering bioactive nanoparticles to rejuvenate vascular progenitor cells. Commun. Biol. 2022, 5, 635. [Google Scholar] [CrossRef]
- Dunn, T.N.; Akiyama, T.; Lee, H.W.; Kim, J.B.; Knotts, T.A.; Smith, S.R.; Sears, D.D.; Carstens, E.; Adams, S.H. Evaluation of the synuclein-γ (SNCG) gene as a PPARγ target in murine adipocytes, dorsal root ganglia somatosensory neurons, and human adipose tissue. PLoS ONE 2015, 10, e0115830. [Google Scholar] [CrossRef]
- Afrisham, R.; Farrokhi, V.; Ayyoubzadeh, S.M.; Vatannejad, A.; Fadaei, R.; Moradi, N.; Jadidi, Y.; Alizadeh, S. CCN5/WISP2 serum levels in patients with coronary artery disease and type 2 diabetes and its correlation with inflammation and insulin resistance; a machine learning approach. Biochem. Biophys. Rep. 2024, 40, 101857. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.T.; Schröder, T.; Kosacka, J.; Nowicki, M.; Klöting, N.; Spanel-Borowski, K. Neuropeptide Y impairs insulin-stimulated translocation of glucose transporter 4 in 3T3-L1 adipocytes through the Y1 receptor. Mol. Cell. Endocrinol. 2012, 348, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Ann-Onda, D.; Lin, X.; Fynch, S.; Nadarajah, S.; Pappas, E.G.; Liu, X.; Scott, J.W.; Oakhill, J.S.; Galic, S.; et al. Neuropeptide Y1 receptor antagonism protects β-cells and improves glycemic control in type 2 diabetes. Mol. Metab. 2022, 55, 101413. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, L.; Gallo-Ferraz, A.L.; Bombassaro, B.; Simões, M.R.; Abe, I.; Chen, J.; Sarker, G.; Ciccarelli, A.; Zhou, L.; et al. Sympathetic neuropeptide Y protects from obesity by sustaining thermogenic fat. Nature 2024, 634, 243–250. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Stenvers, D.J.; Kalsbeek, A.; Bisschop, P.H.; Serlie, M.J.; la Fleur, S.E. Nutrition in the spotlight: Metabolic effects of environmental light. Proc. Nutr. Soc. 2016, 75, 451–463. [Google Scholar] [CrossRef]
- Ma, M.; Liu, H.; Yu, J.; He, S.; Li, P.; Ma, C.; Zhang, H.; Xu, L.; Ping, F.; Li, W.; et al. Triglyceride is independently correlated with insulin resistance and islet beta cell function: A study in population with different glucose and lipid metabolism states. Lipids Health Dis. 2020, 19, 121. [Google Scholar] [CrossRef]
- Nies, V.J.M.; Struik, D.; Liu, S.; Liu, W.; Kruit, J.K.; Downes, M.; van Zutphen, T.; Verkade, H.J.; Evans, R.M.; Jonker, J.W. Autocrine FGF1 signaling promotes glucose uptake in adipocytes. Proc. Natl. Acad. Sci. USA 2022, 119, e2122382119. [Google Scholar] [CrossRef]
- Samulin, J.; Berget, I.; Lien, S.; Sundvold, H. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Brown, K.K.; Chen, L.; Carrick, K.M.; Clifton, L.G.; McNulty, J.A.; Winegar, D.A.; Strum, J.C.; Stimpson, S.A.; Pahel, G.L. Serum adiponectin as a biomarker for in vivo PPARgamma activation and PPARgamma agonist-induced efficacy on insulin sensitization/lipid lowering in rats. BMC Pharmacol. 2004, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Imai, Y.; Kumagai, H.; Nosaka, T.; Morikawa, Y.; Hisaoka, T.; Manabe, I.; Maemura, K.; Nakaoka, T.; Imamura, T.; et al. Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 10732–10737. [Google Scholar] [CrossRef]
- Ahn, J.M.; Kim, B.G.; Yu, M.H.; Lee, I.K.; Cho, J.Y. Identification of diabetic nephropathy-selective proteins in human plasma by multi-lectin affinity chromatography and LC-MS/MS. Proteom. Clin. Appl. 2010, 4, 644–653. [Google Scholar] [CrossRef]
- Xue, D.; Narisu, N.; Taylor, D.L.; Zhang, M.; Grenko, C.; Taylor, H.J.; Yan, T.; Tang, X.; Sinha, N.; Zhu, J.; et al. Functional interrogation of twenty type 2 diabetes-associated genes using isogenic human embryonic stem cell-derived β-like cells. Cell Metab. 2023, 35, 1897–1914.e11. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Lamiral, Z.; Xhaard, C.; Duarte, K.; Bresso, E.; Devignes, M.-D.; Le Floch, E.; Roulland, C.D.; Deleuze, J.-F.; Wagner, S.; et al. Circulating plasma proteins and new-onset diabetes in a population-based study: Proteomic and genomic insights from the STANISLAS cohort. Eur. J. Endocrinol. 2020, 183, 285–295. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Guan, S.; Liu, L.; Wang, H.; Chen, Y.; Zhou, Q.; Xu, F.; Zhang, Y. Common ground on immune infiltration landscape and diagnostic biomarkers in diabetes-complicated atherosclerosis: An integrated bioinformatics analysis. Front. Endocrinol. 2024, 15, 1381229. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chae, S.; Kim, H.; Mun, D.-G.; Back, S.; Choi, H.Y.; Park, K.S.; Hwang, D.; Choi, S.H.; Lee, S.-W. A Protein Profile of Visceral Adipose Tissues Linked to Early Pathogenesis of Type 2 Diabetes Mellitus*. Mol. Cell. Proteom. 2014, 13, 811–822. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. Follistatin and follistatin-like 3 in metabolic disorders. Prostaglandins Other Lipid Mediat. 2023, 169, 106785. [Google Scholar] [CrossRef]
- Brandt, C.; Pedersen, M.; Rinnov, A.R.; Andreasen, A.S.; Møller, K.; Hojman, P.; Pedersen, B.K.; Plomgaard, P. Obesity and Low-Grade Inflammation Increase Plasma Follistatin-Like 3 in Humans. Mediat. Inflamm. 2014, 2014, 364209. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Kokkinos, A.; Peradze, N.; Tentolouris, N.; Ghaly, W.; Tsilingiris, D.; Alexandrou, A.; Mantzoros, C.S. Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes. Metab. 2019, 21, 683–690. [Google Scholar] [CrossRef]
- Iyer, A.; Lim, J.; Poudyal, H.; Reid, R.C.; Suen, J.Y.; Webster, J.; Prins, J.B.; Whitehead, J.P.; Fairlie, D.P.; Brown, L. An Inhibitor of Phospholipase A2 Group IIA Modulates Adipocyte Signaling and Protects Against Diet-Induced Metabolic Syndrome in Rats. Diabetes 2012, 61, 2320–2329. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.X.; Deng, H.; Yang, X.P.; Tang, Z.H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Bikker, A.; Hack, C.E.; Lafeber, F.P.; van Roon, J.A. Interleukin-7: A key mediator in T cell-driven autoimmunity, inflammation, and tissue destruction. Curr. Pharm. Des. 2012, 18, 2347–2356. [Google Scholar] [CrossRef]
- Sumaya, I.C.; Rienzi, B.M.; Deegan, J.F., II; Moss, D.E. Bright Light Treatment Decreases Depression in Institutionalized Older Adults: A Placebo-Controlled Crossover Study. J. Gerontol. Ser. A 2001, 56, M356–M360. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, M.; Tanaka, M.; Takanao, T.; Nishinoue, N.; Kaku, A.; Kato, N.; Tagaya, H.; Miyaoka, H. Brief morning exposure to bright light improves subjective symptoms and performance in nurses with rapidly rotating shifts. J. Occup. Health 2011, 53, 258–266. [Google Scholar] [CrossRef]
- Sayols, S.; Scherzinger, D.; Klein, H. dupRadar: A Bioconductor package for the assessment of PCR artifacts in RNA-Seq data. BMC Bioinform. 2016, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Vijay, J.; Gauthier, M.-F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot- and disease-specific cell types. Nat. Metab. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- Messa, L.; Rey, F.; Pandini, C.; Barzaghini, B.; Micheletto, G.; Raimondi, M.T.; Bertoli, S.; Cereda, C.; Zuccotti, G.; Cancello, R.; et al. RNA-seq dataset of subcutaneous adipose tissue: Transcriptional differences between obesity and healthy women. Data Brief 2021, 39, 107647. [Google Scholar] [CrossRef]

| Characteristic | |
|---|---|
| Age, years | 23 (21–24) |
| BMI, kg/m2 | 22 (22–23) |
| HbA1c, % | 5.2 (5.1–5.5) |
| HbA1c, mmol/mol | 34 (33–37) |
| Fasting glucose, mmol/L | 5.0 (4.8–5.0) |
| Fasting insulin, pmol/L | 40 (12–62) |
| Sleep parameters from diary | |
| Onset of sleep, time | 23:48 h ± 13 min |
| End of sleep, time | 08:02 h ± 6 min |
| Characteristic | |
|---|---|
| Age, years | 60 (54–63) |
| BMI, kg/m2 | 30 (28–35) |
| HbA1c, % | 6.8 (6.7–8.0) |
| HbA1c, mmol/mol | 51 (50–65) |
| Fasting glucose, mmol/L | 8.4 (6.2–10) |
| Fasting insulin, pmol/L | 119 (111–184) |
| Medication, n (%) | |
| Metformin a | 8 (100) |
| Lipid-lowering drugs | 4 (50) |
| Antihypertensives | 3 (37.5) |
| Proton pump inhibitor | 1 (12.5) |
| Sleep parameters from diary | |
| Onset of sleep, time | 00:05 h ± 7 min |
| End of sleep, time | 07:40 h ± 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Vreijling, J.; Jongejan, A.; Rumanova, V.S.; Versteeg, R.I.; Kalsbeek, A.; Serlie, M.J.; la Fleur, S.E.; Bisschop, P.H.; Baas, F.; et al. The Acute Effects of Morning Bright Light on the Human White Adipose Tissue Transcriptome: Exploratory Post Hoc Analysis. Clocks & Sleep 2025, 7, 45. https://doi.org/10.3390/clockssleep7030045
Wang A, Vreijling J, Jongejan A, Rumanova VS, Versteeg RI, Kalsbeek A, Serlie MJ, la Fleur SE, Bisschop PH, Baas F, et al. The Acute Effects of Morning Bright Light on the Human White Adipose Tissue Transcriptome: Exploratory Post Hoc Analysis. Clocks & Sleep. 2025; 7(3):45. https://doi.org/10.3390/clockssleep7030045
Chicago/Turabian StyleWang, Anhui, Jeroen Vreijling, Aldo Jongejan, Valentina S. Rumanova, Ruth I. Versteeg, Andries Kalsbeek, Mireille J. Serlie, Susanne E. la Fleur, Peter H. Bisschop, Frank Baas, and et al. 2025. "The Acute Effects of Morning Bright Light on the Human White Adipose Tissue Transcriptome: Exploratory Post Hoc Analysis" Clocks & Sleep 7, no. 3: 45. https://doi.org/10.3390/clockssleep7030045
APA StyleWang, A., Vreijling, J., Jongejan, A., Rumanova, V. S., Versteeg, R. I., Kalsbeek, A., Serlie, M. J., la Fleur, S. E., Bisschop, P. H., Baas, F., & Stenvers, D. J. (2025). The Acute Effects of Morning Bright Light on the Human White Adipose Tissue Transcriptome: Exploratory Post Hoc Analysis. Clocks & Sleep, 7(3), 45. https://doi.org/10.3390/clockssleep7030045







