The Role of Sex in the Impact of Sleep Restriction on Appetite- and Weight-Regulating Hormones in Healthy Adults: A Systematic Review of Human Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Data Extraction Process
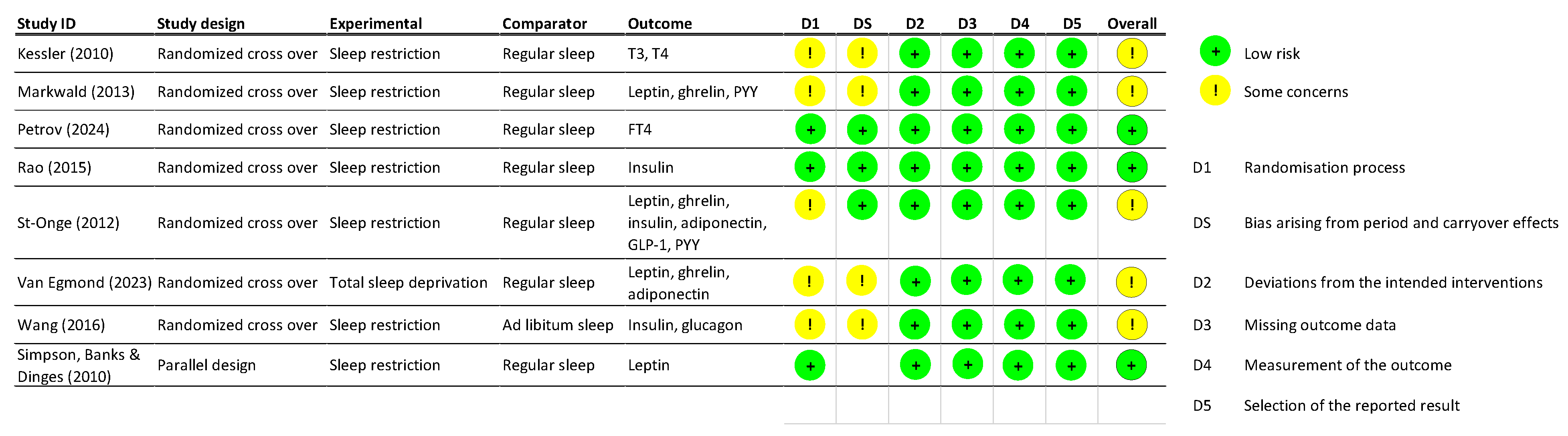
2.3. Risk of Bias Assessment
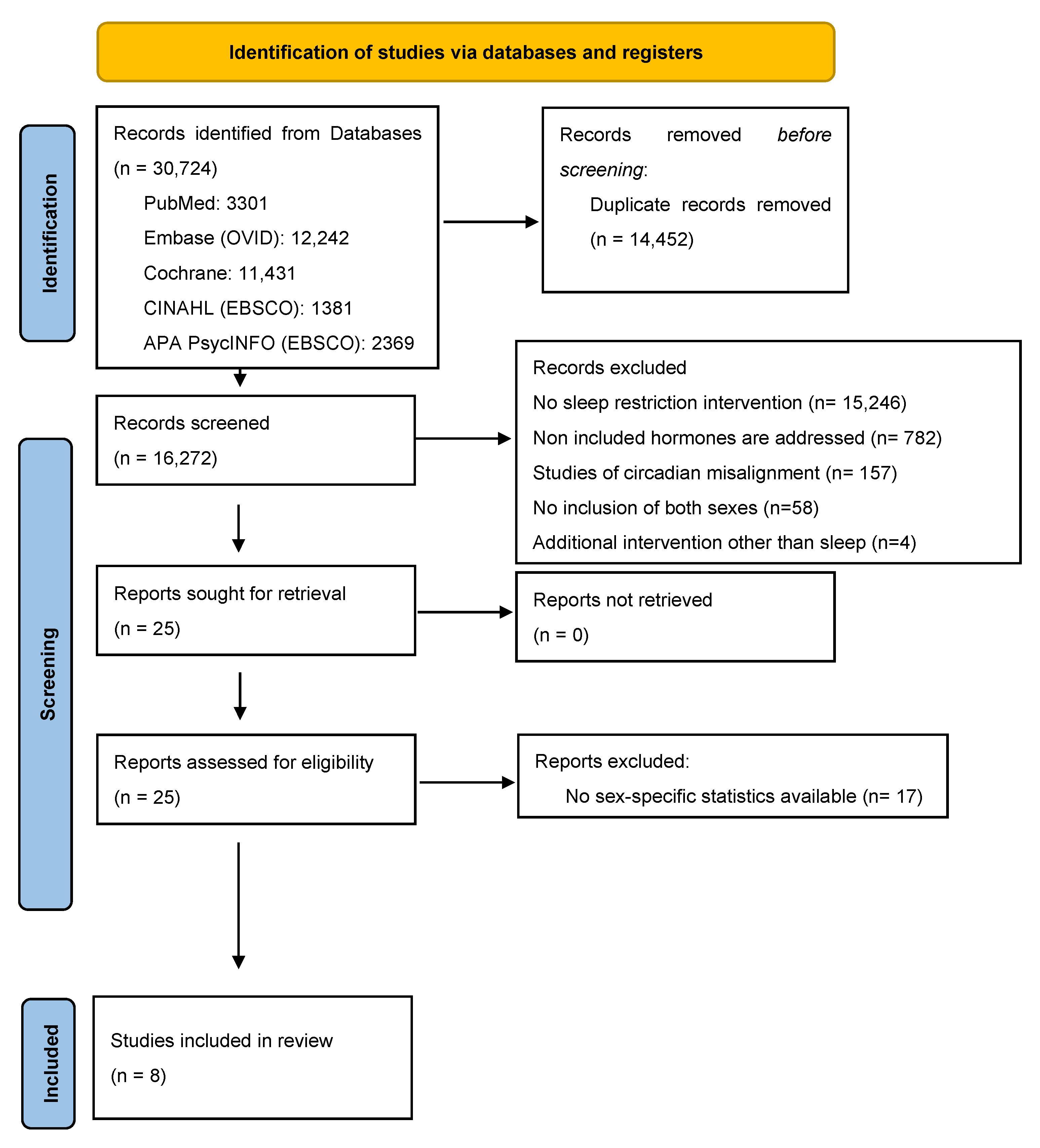
3. Results
4. Sleep Restriction Effect on Appetite Regulating Hormones
4.1. Insulin and Insulin Sensitivity
4.2. Leptin
4.3. Ghrelin
4.4. Peptide YY, GLP-1, Adiponectin, T4 and Glucagon
5. Discussion
Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| GLP-1 | Glucagon-Like Peptide 1 |
| HPA | Hypothalamic–Pituitary–Adrenal |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PYY | Peptide YY |
| RCT | Randomized Controlled Trials |
| RoB | Revised Cochrane Risk-of-Bias Tool for Randomized Trials |
| SEM | Standard Error of the Mean |
| SR | Sleep Restriction |
| TIB | Time In Bed |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TSD | Total Sleep Deprivation |
Appendix A. Search Strategies Used for All Databases
- PubMed
- #1: (sleep interven*[Title/Abstract]) OR (sleep trial[Title/Abstract]) OR (sleep hygiene[Title/Abstract]) OR (sleep chang*[Title/Abstract]) OR (sleep program*[Title/Abstract]) OR (sleep course[Title/Abstract]) OR (sleep length*[Title/Abstract]) OR (sleep decreas*[Title/Abstract]) OR (sleep reduc*[Title/Abstract]) OR (sleep short*[Title/Abstract]) OR (sleep depriv*[Title/Abstract]) OR (sleep disrupt*[Title/Abstract]) OR (sleep disturb*[Title/Abstract]) OR (sleep restrict*[Title/Abstract]) OR (sleep defici*[Title/Abstract]) OR (sleep inadequa*[Title/Abstract]) OR (sleep insufficien*[Title/Abstract]) OR (sleep manipulat*[Title/Abstract]) OR (sleep curtail*[Title/Abstract]) OR (sleep problem*[Title/Abstract]) OR (sleep quality[Title/Abstract]) OR (sleep alter*[Title/Abstract]) OR (sleep debt[Title/Abstract]) OR (sleep duration[Title/Abstract])
- AND
- #2: (hormon*[Title/Abstract]) OR (endocrin*[Title/Abstract]) OR (leptin[Title/Abstract]) OR (ghrelin[Title/Abstract]) OR (adiponectin[Title/Abstract]) OR (insulin[Title/Abstract]) OR (glucagon[Title/Abstract]) OR (PYY[Title/Abstract]) OR (peptide YY[Title/Abstract]) OR (GLP-1[Title/Abstract]) OR (glucagon-like peptide 1[Title/Abstract]) OR (pancreatic polypeptide[Title/Abstract]) OR (T3[Title/Abstract]) OR (T4[Title/Abstract]) OR (food intake[Title/Abstract]) OR (weight[Title/Abstract]) OR (metabolism[Title/Abstract]) OR (appetite[Title/Abstract]) OR (adiposity signal*[Title/Abstract]) OR (gut peptide*[Title/Abstract]) OR (thyroid hormone*[Title/Abstract])
- #3: #1 AND #2
- #3 Filters: English, Humans, Adult: 19+ years, from 1966–2024
- (#4): #3 NOT (review[Publication Type])) NOT (systematic review[Publication Type])) NOT (meta-analysis[Publication Type])
- Embase (OVID)
- #1: (sleep interven* or sleep trial or sleep hygiene or sleep chang* or sleep program* or sleep course or sleep length* or sleep decreas* or sleep reduc* or sleep short* or sleep depriv* or sleep disrupt* or sleep disturb* or sleep restrict* or sleep defici* or sleep inadequa* or sleep insufficien* or sleep manipulat* or sleep curtail* or sleep problem* or sleep quality or sleep alter* or sleep debt or sleep duration).ab.
- #2: Limit #1 to (human and english language and yr = “1934–2024”)
- #3: (hormon* or endocrin* or leptin or ghrelin or adiponectin or insulin or glucagon or PYY or peptide YY or GLP-1 or glucagon-like peptide 1 or pancreatic polypeptide or T3 or T4 or food intake or weight or metabolism or appetite or adiposity signal* or gut peptide* or thyroid hormone*).ab.
- #4: Limit #3 to (human and english language and yr = “1934–2024”)
- #5: #2 and #4
- #1: (sleep interven* or sleep trial or sleep hygiene or sleep chang* or sleep program* or sleep course or sleep length* or sleep decreas* or sleep reduc* or sleep short* or sleep depriv* or sleep disrupt* or sleep disturb* or sleep restrict* or sleep defici* or sleep inadequa* or sleep insufficien* or sleep manipulat* or sleep curtail* or sleep problem* or sleep quality or sleep alter* or sleep debt or sleep duration).ti.
- #2: Limit #1 to (human and english language and yr=“1934–2024”)
- #3: (hormon* or endocrin* or leptin or ghrelin or adiponectin or insulin or glucagon or PYY or peptide YY or GLP-1 or glucagon-like peptide 1 or pancreatic polypeptide or T3 or T4 or food intake or weight or metabolism or appetite or adiposity signal* or gut peptide* or thyroid hormone*).ti.
- #4: Limit #3 to (human and English language and yr=“1934–2024”)
- #5: #2 and #4
- Cochrane
- #1: (sleep interven* OR sleep trial OR sleep hygiene OR sleep chang* OR sleep program* OR sleep course OR sleep length* OR sleep decreas* OR sleep reduc* OR sleep short* OR sleep depriv* OR sleep disrupt* OR sleep disturb* OR sleep restrict* OR sleep defici* OR sleep inadequa* OR sleep insufficien*OR sleep manipulat* OR sleep curtail* OR sleep problem* OR sleep quality OR sleep alter* OR sleep debt OR sleep duration):ti,ab,kw
- with Publication Year from 1950 to 2024, in Trials (Word variations have been searched)
- #2: hormon* OR endocrin* OR leptin OR ghrelin OR adiponectin OR insulin OR glucagon OR PYY OR peptide YY OR GLP-1 OR glucagon-like peptide 1 OR pancreatic polypeptide OR T3 OR T4 OR food intake OR weight OR metabolism OR appetite OR adiposity signal* OR gut peptide* OR thyroid hormone*
- with Publication Year from 1950 to 2024, in Trials (Word variations have been searched)
- #3: #2 AND #3
- CINAHL (EBSCO)
- S1: XB sleep interven* OR XB sleep trial OR XB sleep hygiene OR XB sleep chang* OR XB sleep program* OR XB sleep course OR XB sleep length* OR XB sleep decreas* OR XB sleep reduc* OR XB sleep short* OR XB sleep depriv* OR XB sleep disrupt* OR XB sleep disturb* OR XB sleep restrict* OR XB sleep defici* OR XB sleep inadequa* OR XB sleep insufficien* OR XB sleep manipulat* OR XB sleep curtail* OR XB sleep problem* OR XB sleep quality OR XB sleep alter* OR XB sleep debt OR XB sleep duration
- Limiters—Publication Date: 19760101-20241231; English Language; Human; Age Groups: All Adult; Language: English
- Expanders—Apply equivalent subjects
- S2: XB hormon* OR XB endocrin* OR XB leptin OR XB ghrelin OR XB adiponectin OR XB insulin OR XB glucagon OR XB PYY OR XB peptide YY OR XB GLP-1 OR XB glucagon-like peptide 1 OR XB pancreatic polypeptide OR XB T3 OR XB T4 OR XB food intake OR XB weight OR XB metabolism OR XB appetite OR XB adiposity signal* OR XB gut peptide* OR XB thyroid hormone*
- Limiters—Publication Date: 19760101–20241231; English Language; Human; Age Groups: All Adult; Language: English
- Expanders—Apply equivalent subjects
- S3: S1 AND S2
- APA PsycINFO (EBSCO)
- S1: TI sleep interven* OR TI sleep trial OR TI sleep hygiene OR TI sleep chang* OR TI sleep program* OR TI sleep course OR TI sleep length* OR TI sleep decreas* OR TI sleep reduc* OR TI sleep short* OR TI sleep depriv* OR TI sleep disrupt* OR TI sleep disturb* OR TI sleep restrict* OR TI sleep defici* OR TI sleep inadequa* OR TI sleep insufficien* OR TI sleep manipulat* OR TI sleep curtail* OR TI sleep problem* OR TI sleep quality OR TI sleep alter* OR TI sleep debt OR TI sleep duration
- Limiters—Publication Year: 1976–2024; Publication Date: 19760101–20241231; English language; Language: English; Age Groups: Adulthood (18 yrs & older); Population Group: Human
- Expanders—Apply equivalent subjects
- Search modes—Proximity
- S2: TI hormon* OR TI endocrin* OR TI leptin OR TI ghrelin OR TI adiponectin OR TI insulin OR TI glucagon OR TI PYY OR TI peptide YY OR TI GLP-1 OR TI glucagon-like peptide 1 OR TI pancreatic polypeptide OR TI T3 OR TI T4 OR TI food intake OR TI weight OR TI metabolism OR TI appetite OR TI adiposity signal* OR TI gut peptide* OR TI thyroid hormone*
- Limiters—Publication Year: 1976–2024; Publication Date: 19760101–20241231; English language; Language: English; Age Groups: Adulthood (18 yrs & older); Population Group: Human
- Expanders—Apply equivalent subjects
- Search modes—Proximity
- S3: S1 AND S2
- S4: AB sleep interven* OR AB sleep trial OR AB sleep hygiene OR AB sleep chang* OR AB sleep program* OR AB sleep course OR AB sleep length* OR AB sleep decreas* OR AB sleep reduc* OR AB sleep short* OR AB sleep depriv* OR AB sleep disrupt* OR AB sleep disturb* OR AB sleep restrict* OR AB sleep defici* OR AB sleep inadequa* OR AB sleep insufficien* OR AB sleep manipulat* OR AB sleep curtail* OR AB sleep problem* OR AB sleep quality OR AB sleep alter* OR AB sleep debt OR AB sleep duration
- Limiters—Publication Year: 1976–2024; Publication Date: 19760101–20241231; English language; Language: English; Age Groups: Adulthood (18 yrs & older); Population Group: Human
- Expanders—Apply equivalent subjects
- Search modes—Proximity
- S5: AB hormon* OR AB endocrin* OR AB leptin OR AB ghrelin OR AB adiponectin OR AB insulin OR AB glucagon OR AB PYY OR AB peptide YY OR AB GLP-1 OR AB glucagon-like peptide 1 OR AB pancreatic polypeptide OR AB T3 OR AB T4 OR AB food intake OR AB weight OR AB metabolism OR AB appetite OR AB adiposity signal* OR AB gut peptide* OR AB thyroid hormone*
- Limiters—Publication Year: 1976–2024; Publication Date: 19760101–20241231; English language; Language: English; Age Groups: Adulthood (18 yrs & older); Population Group: Human
- Expanders—Apply equivalent subjects
- Search modes—Proximity
- S6: S4 AND S5
References
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef]
- Khubchandani, J.; Price, J.H. Short Sleep Duration in Working American Adults, 2010–2018. J. Commun. Health 2020, 45, 219–227. [Google Scholar] [CrossRef]
- Kerkhof, G.A. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef]
- How Much Sleep Do We Really Need? Sleep Foundation. 9 March 2021. Available online: https://www.sleepfoundation.org/how-sleep-works/how-much-sleep-do-we-really-need (accessed on 24 July 2023).
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef]
- Sleep Deprivation: Causes, Symptoms, & Treatment. Sleep Foundation. 3 November 2020. Available online: https://www.sleepfoundation.org/sleep-deprivation (accessed on 24 July 2023).
- All-Nighters: Helpful or Harmful? Sleep Foundation. 1 August 2017. Available online: https://www.sleepfoundation.org/sleep-hygiene/why-are-all-nighters-harmful (accessed on 2 August 2023).
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases- a review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [CrossRef]
- Haavisto, M.-L.; Porkka-Heiskanen, T.; Hublin, C.; Härmä, M.; Mutanen, P.; Müller, K.; Virkkala, J.; Sallinen, M. Sleep restriction for the duration of a work week impairs multitasking performance. J. Sleep Res. 2010, 19, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, S.B.; Seaverson, E.L.D.; Anderson, D.R. Association Between Sleep and Productivity Loss Among 598 676 Employees from Multiple Industries. Am. J. Health Promot. 2018, 32, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.; Hébert, M.; Ledoux, E.; Gaudreault, M.; Laberge, L. Relationship of chronotype to sleep, light exposure, and work-related fatigue in student workers. Chronobiol. Int. 2012, 29, 295–304. [Google Scholar] [CrossRef]
- Holliday, E.G.; Magee, C.A.; Kritharides, L.; Banks, E.; Attia, J. Short Sleep Duration Is Associated with Risk of Future Diabetes but Not Cardiovascular Disease: A Prospective Study and Meta-Analysis. PLoS ONE 2013, 8, e82305. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Shankar, A. Sleep Duration and Cardiovascular Disease: Results from the National Health Interview Survey. Sleep 2010, 33, 1037–1042. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex differences in sleep, circadian rhythms, and metabolism: Implications for precision medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef] [PubMed]
- Mallampalli, M.P.; Carter, C.L. Exploring sex and gender differences in sleep health: A Society for Women’s Health Research Report. J. Womens Health 2014, 23, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Haba Rubio, J.; Andries, D.; Tobback, N.; Vollenweider, P.; Waeber, G.; Marques Vidal, P.; Preisig, M.; Heinzer, R.; Tafti, M. Age and gender variations of sleep in subjects without sleep disorders. Ann. Med. 2015, 47, 482–491. [Google Scholar] [CrossRef]
- How a Lack of Sleep May Increase Calorie Consumption. Sleep Foundation. 11 December 2020. Available online: https://www.sleepfoundation.org/sleep-deprivation/lack-sleep-may-increase-calorie-consumption (accessed on 3 September 2023).
- Broussard, J.L.; Klein, S. Insufficient sleep and obesity: Cause or consequence. Obesity 2022, 30, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Abdalla, M.M. Ghrelin–Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, M.-S. Molecular Mechanisms of Appetite Regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. Int. J. Environ. Res. Public Health 2021, 18, 9434. [Google Scholar] [CrossRef]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Makris, C.M.; Alexandrou, A.; Papatsoutsos, G.E.; Malietzis, G.; Tsilimigras, I.D.; Guerron, D.A.; Moris, D. Ghrelin and Obesity: Identifying Gaps and Dispelling Myths. A Reappraisal. in vivo 2017, 31, 1047–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Zhou, Q.; Chen, Y.; Lei, X.; Chen, Y.; Chen, Q. Circulating acyl and des-acyl ghrelin levels in obese adults: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Couillard, C.; Mauriège, P.; Prud’homme, D.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Després, J.-P. Plasma leptin concentrations: Gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia 1997, 40, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.S.; Banks, S.; Arroyo, S.; Dinges, D.F. Effects of sleep restriction on adiponectin levels in healthy men and women. Physiol. Behav. 2010, 101, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; De Amicis, R.; Pellizzari, M.; Bertoli, S.; Ravella, S.; Battezzati, A. Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter? Biol. Sex Differ. 2022, 13, 25. [Google Scholar] [CrossRef]
- Mihalache, L.; Gherasim, A.; Niță, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of ghrelin in energy balance and body weight homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef]
- Gallegos, J.V.; Boege, H.L.; Zuraikat, F.M.; St-Onge, M.-P. Does sex influence the effects of experimental sleep curtailment and circadian misalignment on regulation of appetite? Curr. Opin. Endocr. Metab. Res. 2021, 17, 20–25. [Google Scholar] [CrossRef]
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A Systemic Review and Meta-Analysis of Randomized Controlled Trials of the Impact of Sleep Duration on Adiposity and Components of Energy Balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef]
- Henst, R.H.P.; Pienaar, P.R.; Roden, L.C.; Rae, D.E. The effects of sleep extension on cardiometabolic risk factors: A systematic review. J. Sleep Res. 2019, 28, e12865. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, Y.; Wang, G.; Meng, M.; Zhu, Q.; Mei, H.; Liu, S.; Jiang, F. Associations of short sleep duration with appetite-regulating hormones and adipokines: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13051. [Google Scholar] [CrossRef]
- Soltanieh, S.; Solgi, S.; Ansari, M.; Santos, H.O.; Abbasi, B. Effect of sleep duration on dietary intake, desire to eat, measures of food intake and metabolic hormones: A systematic review of clinical trials. Clin. Nutr. ESPEN 2021, 45, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Shi, C.; Park, C.G.; Zhao, X.; Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2019, 45, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Van Egmond, L.T.; Meth, E.M.S.; Engström, J.; Ilemosoglou, M.; Keller, J.A.; Vogel, H.; Benedict, C. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: A laboratory study. Obesity 2023, 31, 635–641. [Google Scholar] [CrossRef]
- Simpson, N.S.; Banks, S.; Dinges, D.F. Sleep Restriction Is Associated with Increased Morning Plasma Leptin Concentrations, Especially in Women. Biol. Res. Nurs. 2010, 12, 47–53. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; O’Keeffe, M.; Roberts, A.L.; RoyChoudhury, A.; Laferrère, B. Short Sleep Duration, Glucose Dysregulation and Hormonal Regulation of Appetite in Men and Women. Sleep 2012, 35, 1503–1510. [Google Scholar] [CrossRef]
- Wang, X.; Greer, J.; Porter, R.R.; Kaur, K.; Youngstedt, S.D. Short-Term Moderate Sleep Restriction Decreases Insulin Sensitivity in Young Healthy Adults. Sleep Health 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Rao, M.N.; Neylan, T.C.; Grunfeld, C.; Mulligan, K.; Schambelan, M.; Schwarz, J.-M. Subchronic Sleep Restriction Causes Tissue-Specific Insulin Resistance. J. Clin. Endocrinol. Metab. 2015, 100, 1664–1671. [Google Scholar] [CrossRef]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Kenneth, P.; Wright, J. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695. [Google Scholar] [CrossRef]
- Kessler, L.; Nedeltcheva, A.; Imperial, J.; Penev, P.D. Changes in Serum TSH and Free T4 during Human Sleep Restriction. Sleep 2010, 33, 1115–1118. [Google Scholar] [CrossRef]
- Petrov, M.E.; Zuraikat, F.M.; Cheng, B.; Aggarwal, B.; Jelic, S.; Laferrère, B.; St-Onge, M.-P. Impact of sleep restriction on biomarkers of thyroid function: Two pooled randomized trials. Sleep Med. 2024, 124, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Lillioja, S.; Mott, D.M.; Spraul, M.; Ferraro, R.; Foley, J.E.; Ravussin, E.; Knowler, W.C.; Bennett, P.H.; Bogardus, C. Insulin Resistance and Insulin Secretory Dysfunction as Precursors of Non-Insulin-Dependent Diabetes Mellitus: Prospective Studies of Pima Indians. N. Engl. J. Med. 1993, 329, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Viguerie, N.; Massier, L.; Rydén, M.; Astrup, A.; Blaak, E.; Langin, D.; Andersson, D.P. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int. J. Obes. 2024, 48, 934–940. [Google Scholar] [CrossRef]
- Smith, A.; Woodside, B.; Abizaid, A. Ghrelin and the Control of Energy Balance in Females. Front. Endocrinol. 2022, 13, 904754. [Google Scholar] [CrossRef]
- De Souza, G.O.; Wasinski, F.; Donato, J. Characterization of the metabolic differences between male and female C57BL/6 mice. Life Sci. 2022, 301, 120636. [Google Scholar] [CrossRef]
- Börchers, S.; Skibicka, K.P. GLP-1 and Its Analogs: Does Sex Matter? Endocrinology 2025, 166, bqae165. [Google Scholar] [CrossRef]
- Jones, L.A.; Sun, E.W.; Lumsden, A.L.; Thorpe, D.W.; Peterson, R.A.; De Fontgalland, D.; Sposato, L.; Rabbitt, P.; Hollington, P.; Wattchow, D.A.; et al. Alterations in GLP-1 and PYY release with aging and body mass in the human gut. Mol. Cell. Endocrinol. 2023, 578, 112072. [Google Scholar] [CrossRef]
- Villarreal-Molina, M.T.; Antuna-Puente, B. Adiponectin: Anti-inflammatory and cardioprotective effects. Biochimie 2012, 94, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Gradidge, P.J.-L.; Jaff, N.G.; Norris, S.A.; Toman, M.; Crowther, N.J. The negative association of lower body fat mass with cardiometabolic disease risk factors is partially mediated by adiponectin. Endocr. Connect. 2022, 11, e220156. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Beroukhim, G.; Esencan, E.; Seifer, D.B. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: A comprehensive review. Reprod. Biol. Endocrinol. 2022, 20, 16. [Google Scholar] [CrossRef]
- Dunlavey, C.J. Introduction to the Hypothalamic-Pituitary-Adrenal Axis: Healthy and Dysregulated Stress Responses, Developmental Stress and Neurodegeneration. J. Undergrad. Neurosci. Educ. 2018, 16, R59–R60. [Google Scholar] [PubMed]
- Amin, M.R.; Pednekar, D.D.; Azgomi, H.F.; van Wietmarschen, H.; Aschbacher, K.; Faghih, R.T. Sparse System Identification of Leptin Dynamics in Women with Obesity. Front. Endocrinol. 2022, 13, 769951. [Google Scholar] [CrossRef] [PubMed]
- Van Loenen, M.R.; Geenen, B.; Arnoldussen, I.A.C.; Kiliaan, A.J. Ghrelin as a prominent endocrine factor in stress-induced obesity. Nutr. Neurosci. 2022, 25, 1413–1424. [Google Scholar] [CrossRef]
- Van Dalfsen, J.H.; Markus, C.R. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA) axis reactivity: A systematic review. Sleep Med. Rev. 2018, 39, 187–194. [Google Scholar] [CrossRef]
- Goel, N.; Workman, J.L.; Lee, T.T.; Innala, L.; Viau, V. Sex Differences in the HPA Axis. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1121–1155. ISBN 978-0-470-65071-4. [Google Scholar] [CrossRef]
- Liu, P.Y.; Lawrence-Sidebottom, D.; Piotrowska, K.; Zhang, W.; Iranmanesh, A.; Auchus, R.J.; Veldhuis, J.D.; Van Dongen, H.P.A. Clamping Cortisol and Testosterone Mitigates the Development of Insulin Resistance during Sleep Restriction in Men. J. Clin. Endocrinol. Metab. 2021, 106, e3436–e3448. [Google Scholar] [CrossRef]
- Schutte, A.E.; Huisman, H.W.; Schutte, R.; van Rooyen, J.M.; Malan, L.; Malan, N.T. Aging influences the level and functions of fasting plasma ghrelin levels: The POWIRS-Study. Regul. Pept. 2007, 139, 65–71. [Google Scholar] [CrossRef]
- Balaskó, M.; Soós, S.; Székely, M.; Pétervári, E. Leptin and aging: Review and questions with particular emphasis on its role in the central regulation of energy balance. J. Chem. Neuroanat. 2014, 61–62, 248–255. [Google Scholar] [CrossRef]
- Obata, Y.; Yamada, Y.; Takahi, Y.; Baden, M.Y.; Saisho, K.; Tamba, S.; Yamamoto, K.; Umeda, M.; Furubayashi, A.; Matsuzawa, Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin. Endocrinol. 2013, 79, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Horiguchi, K.; Akuzawa, M.; Sakamaki, K.; Yamada, E.; Ozawa, A.; Kobayashi, I.; Shimomura, Y.; Okamoto, Y.; Andou, T.; et al. The Impact of Age- and Sex-Specific Reference Ranges for Serum Thyrotropin and Free Thyroxine on the Diagnosis of Subclinical Thyroid Dysfunction: A Multicenter Study from Japan. Thyroid 2023, 33, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutr. Res. Rev. 2014, 27, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Hinrichs, S.; Jauch-Chara, K.; Hitze, B.; Later, W.; Wilms, B.; Settler, U.; Peters, A.; Kiosz, D.; Müller, M.J. Influence of Partial Sleep Deprivation on Energy Balance and Insulin Sensitivity in Healthy Women. Obes. Facts 2008, 1, 266–273. [Google Scholar] [CrossRef]
- Salem, A.M.; Latif, R.; Rafique, N.; Aldawlan, M.I.; Almulla, L.B.; Alghirash, D.Y.; Fallatah, O.A.; Alotaibi, F.M.; Aljabbari, F.H.; Yar, T. Variations of Ghrelin and Obestatin Hormones During the Menstrual Cycle of Women of Different BMIs. Int. J. Womens Health 2022, 14, 1297–1305. [Google Scholar] [CrossRef]
- Chaput, J.-P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; Costa, B.G.G.d.; Sampasa-Kanyinga, H.; Kenneth, P.; Wright, J. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82. [Google Scholar] [CrossRef]
- Broussard, J.L.; Kilkus, J.M.; Delebecque, F.; Abraham, V.; Day, A.; Whitmore, H.R.; Tasali, E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 2016, 24, 132–138. [Google Scholar] [CrossRef]
- Tajiri, E.; Yoshimura, E.; Tobina, T.; Yamashita, T.; Kume, K.; Hatamoto, Y.; Shimoda, S. Effects of sleep restriction on food intake and appetite under free-living conditions: A randomized crossover trial. Appetite 2023, 189, 106998. [Google Scholar] [CrossRef]
- Lundahl, A.; Nelson, T.D. Sleep and food intake: A multisystem review of mechanisms in children and adults. J. Health Psychol. 2015, 20, 794–805. [Google Scholar] [CrossRef]
| Author (Year) | Country | Participants’ Characteristics [Age (Mean ± SEM), BMI/Weight (Mean ± SEM)] | Sample Size per Sex (Whose Data Are Included for Analysis) | Short Description of the Sleep Restriction | Energy Balance of Participants | Presence/Absence and Duration of Washout Period | Study Setting | Study Design | Sex-Specific Outcome(s) Related to the Studied Hormone(s) (Increase or Decrease)/Significant Result(s) (Mean, SD, Effect Size, Period Effect for Crossover Trials) | Summarized Results (Sex Differences in the Effect of the SR/TSD on the Specified Hormone: None/+/− |
|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | ||||||||||
| Markwald, R (2013) [45] | USA | Age: 22.4 ± 4.8 years, BMI: 22.9 ± 2.4 kg/m2 | 16 (males, n = 8; females, n = 8) | Three baseline days of 9 h/night sleep, then half the participants underwent 5 days of SR (5 h/night), and the other half remained on a 9 h per night sleep. Then participants crossed over to the other condition | Weight maintenance diets during the baseline period. Ad libitum intake during sleep intervention. | ND | In lab | Randomized crossover trial | SR vs normal sleep: Average 24 h leptin levels for both sexes: 6.7 ± 5.1 ng/mL vs. 5.5 ± 5.2 ng/mL, respectively; leptin levels for females were not statistically different across conditions, whereas leptin levels for males were higher in the 5 h condition versus baseline. Not designed to study sex-specific differences. | SR effect on leptin in males: + SR effect on leptin in females: None |
| St-Onge, MP (2012) [42] | USA | Age: 30–45 years, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h/night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting leptin for both sexes: no significant differences Designed to study sex-specific differences. | SR effect on fasting leptin in males: None SR effect on fasting leptin in females: None |
| Van Egmond, L (2023) [40] | Sweden | Age: 24.9 ± 2.9 years, BMI: 27.8 ± 6.7 (males) vs. 27.0 ± 6.1 kg/m2 (females) | 44 (males, n = 24; females, n = 20) | Each participant had 1 night of TSD and 1 night of normal sleep | Standardized dinner followed by an overnight fast | 1 week | In lab | Randomized crossover trial | TSD vs normal sleep: Fasting leptin for females: 25.8 ± 4.3 vs. 28.1 ± 4.7 ng/mL, p = 0.030. Fasting leptin for males: 10.1 ± 2.4 vs. 10.6 ± 2.3 ng/mL, p = 0.458 Designed to study sex-specific differences. | TSD effect on leptin for females: − TSD effect on leptin for males: None |
| Simpson, Banks, and Dinges (2010) [41] | USA | Age: 22–45 years, BMI: 17.7–32.6 kg/m2 | 145 (sleep restriction, n = 136; control, n = 9); among the SR group (n = 136): males = 51%, females = 49% | SR participants: 2 nights (10 h TIB/night), then 5 nights of SR (4 h TIB/night). Of these participants, 27 were randomized to receive 2 additional nights of further SR (0, 2, or 4 h TIB/night), and 37 were randomized to receive 2 nights of increased sleep time (6, 8, or 10 h TIB/night). Control subjects: 10 h TIB on all study nights | Three meals per day plus an optional evening snack; Additional snack food was available ad libitum throughout the study. Participants choose their meals’ timing (EI and EE were not assessed) | NA | In lab | Randomized parallel group study | SR vs. normal sleep: Mean leptin level for both sexes: 10.51 (±8.83) ng/mL vs. 7.88 (±6.59) ng/mL, respectively; Z = −8.43, p < 0.001. Mean leptin level for males: Z = −5.87, p = < 0.001. Mean leptin level for females: Z = −6.07, p = < 0.001. Designed to study sex-specific differences. | SR effect on leptin in females: + SR effect on leptin in males: + A higher effect was observed in females |
| Insulin | ||||||||||
| Rao, M (2015) [44] | USA | Age: 18–45 years, BMI: 24.1 ± 4.1 kg/m2 | 14 (males, n = 8; females, n = 6) | 2 nights of acclimatization, followed by 5 nights of either normal sleep (8 h TIB) or SR (4 h TIB) | An isocaloric metabolic diet consisting of fixed caloric content and proportions of carbohydrate (55%), protein (15%), and fat (30%) Fixed meal times. | 4–10 weeks | In lab | Randomized crossover trial | SR vs. normal sleep: Whole-body insulin sensitivity for both sexes decreased by 25% (p = 0.008). No significant difference between males and females, but males had the most robust decrease in insulin sensitivity (≥30% decrease in whole-body insulin sensitivity). Not designed to study sex-specific differences. | SR effect on whole-body insulin sensitivity in females: − SR effect on whole-body insulin sensitivity in males: − The decrease was more robust in males |
| St-Onge, MP (2012) [42] | USA | Age: 30–45 years, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h/night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting insulin for females: lower level (regression coefficient ± SEM: −1.39 ± 0.62, p = 0.026). Fasting insulin for males: No significant difference. Designed to study sex-specific differences. | SR effect on fasting insulin in females: − SR effect on fasting insulin in males: None |
| Wang, X (2016) [43] | USA | Age: 20.6 ± 1.3 years, BMI: 24.5 ± 3.4 kg/m2 | 15 (males, n = 7; females, n = 8) | Each participant had 3-day SR (usual self-reported TIB reduced by 1–3 h) and 3-day ad libitum sleep | Ad libitum | 2 weeks | Free living | Randomized crossover trial | SR vs. normal sleep: Insulin for both sexes: higher levels (p = 0.034) Not designed to study sex-specific differences. | SR effect on insulin in females: + SR effect on insulin in males: + No sex differences |
| Ghrelin | ||||||||||
| Markwald, R (2013) [45] | USA | Age: 22.4 ± 4.8 years, BMI: 22.9 ± 2.4 kg/m2 | 16 (males, n = 8; females, n = 8) | Three baseline days of 9 h/night sleep then half participants underwent 5 days of SR (5 h/night) and the other half remained on a 9 h per night sleep. Then participants crossed over to the other condition | Weight maintenance diets during baseline period Ad libitum intake during sleep intervention | ND | In lab | Randomized cross over trial | SR vs normal sleep: Average 24 h ghrelin levels for both sexes: 660.2 ± 235.4 pg/mL vs. 794.6 ± 233.8 pg/mL, respectively. Not designed to study sex-specific differences. | SR effect on ghrelin in females: − SR effect on ghrelin in males: − No sex differences |
| St-Onge, MP (2012) [42] | USA | Age: 30–45 years, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h/night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting ghrelin for females: no significant difference. Fasting ghrelin for males: higher ghrelin levels (47.4 ± 24.4 pg/mL, p = 0.054) 24 h total ghrelin for females: no significant difference. 24 h total ghrelin for males: higher morning levels (42.5 ± 20.8 pg/mL, p = 0.042). Fasting active ghrelin for both sexes: no significant differences. Designed to study sex-specific differences. | SR effect on fasting ghrelin in females: None SR effect on fasting ghrelin in males: + SR effect on 24 h ghrelin (morning levels) in males: + SR effect on 24 h ghrelin (morning levels) in females: None SR effect on fasting active ghrelin in both sexes: None with no sex difference |
| Van Egmond, L (2023) [40] | Sweden | Age: 24.9 ± 2.9 years, BMI: 27.8 ± 6.7 (males) vs. 27.0 ± 6.1 kg/m2 (females) | 44 (males, n = 24; females, n = 20) | Each participant had 1 night of TSD and 1 night of normal sleep | Standardized dinner followed by an overnight fast | 1 week | In lab | Randomized crossover trial | TSD vs normal sleep: Fasting ghrelin for males: 703.6 ± 56.6 vs. 616.2 ± 56.1 pg/mL, p = 0.024 Fasting ghrelin for females: 988.8 ± 145.3 vs. 879.1 ± 111.2 pg/mL, p = 0.049 Designed to study sex-specific differences. | TSD effect on ghrelin in females: + TSD effect on ghrelin in males: + No sex differences |
| Adiponectin | ||||||||||
| St-Onge, MP (2012) [42] | USA | Age: 30–45 years, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting adiponectin for both sexes: no significant difference. 24 h adiponectin: lower levels in males (p = 0.0061), not in females. Designed to study sex-specific differences. | SR effect on fasting adiponectin in both sexes: None (no sex differences) SR effect on total adiponectin in males: − SR effect on total adiponectin in females: None |
| Van Egmond, L (2023) [40] | Sweden | Age: 24.9 ± 2.9 years, BMI: 27.8 ± 6.7 (males) vs. 27.0 ± 6.1 kg/m2 (females) | 44 (males, n = 24; females, n = 20) | Each participant had 1 night of TSD and 1 night of normal sleep | Standardized dinner followed by an overnight fast | 1 week | In lab | Randomized crossover trial | TSD vs normal sleep: Fasting adiponectin for males: 5.9 ± 0.5 vs. 5.6 ± 0.6 μg/mL, p = 0.056 Fasting adiponectin for females: 9.4 ± 1.0 vs. 8.4 ± 0.9 μg/mL, p = 0.025 Designed to study sex-specific differences. | TSD effect on adiponectin in females: + TSD effect on adiponectin in males: None |
| GLP−1 | ||||||||||
| St-Onge, MP (2012) [42] | USA | Age: 30–45 years, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h/night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting total GLP-1 for both sexes: no significant differences. 24 h GLP-1 for females: lower afternoon levels (p = 0.016). Designed to study sex-specific differences. | SR effect on fasting total GLP-1 in both sexes: None (no sex differences) SR effect on 24 h GLP-1 in females: − (afternoon levels) SR effect on 24-h GLP-1 in males: None |
| PYY | ||||||||||
| Markwald, R (2013) [45] | USA | Age: 22.4 ± 4.8 years, BMI: 22.9 ± 2.4 kg/m2 | 16 (males, n = 8; females, n = 8) | Three baseline days of 9 h/night sleep, then half the participants underwent 5 days of SR (5 h/night), and the other half remained on a 9 h per night sleep. Then participants crossed over to the other condition | Weight maintenance diets during baseline period Ad libitum intake during sleep intervention | ND | In lab | Randomized crossover trial | SR vs normal sleep: Average 24 h PYY for both sexes: 136.1 ± 44.8 pg/mL vs. 100.5 ± 35.1 pg/mL, respectively. Not designed to study sex-specific differences. | SR effect on PYY in females: + SR effect on PYY in males: + No sex differences |
| St-Onge, MP (2012) [42] | USA | Age: 30–45 yrs, BMI: 22–26 kg/m2 | 27 (males, n = 14; females, n = 13) | 5 nights of SR (4 h/night) versus 5 nights of habitual sleep (9 h/night) | Controlled diet (30% fat, 55% carbohydrates, and 15% protein) with fixed meal times. Energy requirements were estimated using the Harris-Benedict equation. | 3 weeks | In lab | Randomized crossover trial | SR vs normal sleep: Fasting PYY for both sexes: no significant differences. Designed to study sex-specific differences. | SR effect on fasting PYY in females: None SR effect on fasting PYY in males: None No sex differences |
| Glucagon | ||||||||||
| Wang, X (2016) [43] | USA | Age: 20.6 ± 1.3 years, BMI: 24.5 ± 3.4 kg/m2 | 15 (males, n = 7; females, n = 8) | Each participant had 3-day SR (usual self-reported TIB reduced by 1–3 h) and 3-day ad libitum sleep | Ad libitum | 2 weeks | Free living | Randomized crossover trial | SR vs. normal sleep: Fasting glucagon for both sexes: higher levels (p = 0.003) Not designed to study sex-specific differences. | SR effect on glucagon in females: + SR effect on glucagon in males: + No sex differences |
| T3 and T4 | ||||||||||
| Kessler, L (2010) [46] | USA | Age: 39 ± 5 years, BMI: 26.5 ± 1.5 kg/m2 | 11 (males, n = 6; females, n = 5) (6 for rT3) | Each subject completed two 14-day intervention periods with sedentary activity, ad libitum food intake, and scheduled time-in-bed of 5.5 (SR) or 8.5 h/night in random order | Ad libitum intake. EI > EE during both sleep conditions | At least 3 months | In lab | Randomized crossover trial | SR vs. normal sleep: fT4 for males: 0.98 ± 0.06 vs. 1.06 ± 0.05 mcg/dL; p = 0.14. fT4 for females: 1.10 ± 0.03 vs. 1.19 ± 0.05 mcg/dL; p < 0.001 rT3 for both sexes: no significant difference Not designed to study sex-specific differences. | SR effect on fT4 in females: − SR effect on fT4 in males: None SR effect on rT3 in both sexes: None No sex differences |
| Petrov, M (2024) [47] | USA | Age: 36.2 years ± 12.8 yrs, BMI: 26.7 ± 3.1 kg/m2 | 30 (males, n = 10; females, n = 20) | Each subject completed 6 weeks of normal sleep and SR (sleep duration reduced by 1.5 h from the usual sleep duration). | Ad libitum | 2–6 weeks | Free living | Randomized crossover trial | SR vs. normal sleep: FT4 for females: non-significant increases (β = 0.08 ± 0.06 ng/dL, p = 0.177, Cohen’s f2 = 0.05) FT4 for males: non-significant decreases (β = −0.05 ± 0.03 ng/dL, p = 0.127, Cohen’s f2 = 0.08) Designed to study sex-specific differences. | SR effect on FT4 in females: + SR effect on FT4 in males: − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfikany, M.; Sakhr, K.; Kremers, S.; El Khatib, S.; Adam, T.; Meertens, R. The Role of Sex in the Impact of Sleep Restriction on Appetite- and Weight-Regulating Hormones in Healthy Adults: A Systematic Review of Human Studies. Clocks & Sleep 2025, 7, 39. https://doi.org/10.3390/clockssleep7030039
Alfikany M, Sakhr K, Kremers S, El Khatib S, Adam T, Meertens R. The Role of Sex in the Impact of Sleep Restriction on Appetite- and Weight-Regulating Hormones in Healthy Adults: A Systematic Review of Human Studies. Clocks & Sleep. 2025; 7(3):39. https://doi.org/10.3390/clockssleep7030039
Chicago/Turabian StyleAlfikany, Mira, Khaula Sakhr, Stef Kremers, Sami El Khatib, Tanja Adam, and Ree Meertens. 2025. "The Role of Sex in the Impact of Sleep Restriction on Appetite- and Weight-Regulating Hormones in Healthy Adults: A Systematic Review of Human Studies" Clocks & Sleep 7, no. 3: 39. https://doi.org/10.3390/clockssleep7030039
APA StyleAlfikany, M., Sakhr, K., Kremers, S., El Khatib, S., Adam, T., & Meertens, R. (2025). The Role of Sex in the Impact of Sleep Restriction on Appetite- and Weight-Regulating Hormones in Healthy Adults: A Systematic Review of Human Studies. Clocks & Sleep, 7(3), 39. https://doi.org/10.3390/clockssleep7030039








