Efficacy of Morning Shorter Wavelength Lighting in the Visible (Blue) Range and Broad-Spectrum or Blue-Enriched Bright White Light in Regulating Sleep, Mood, and Fatigue in Traumatic Brain Injury: A Systematic Review
Abstract
1. Introduction
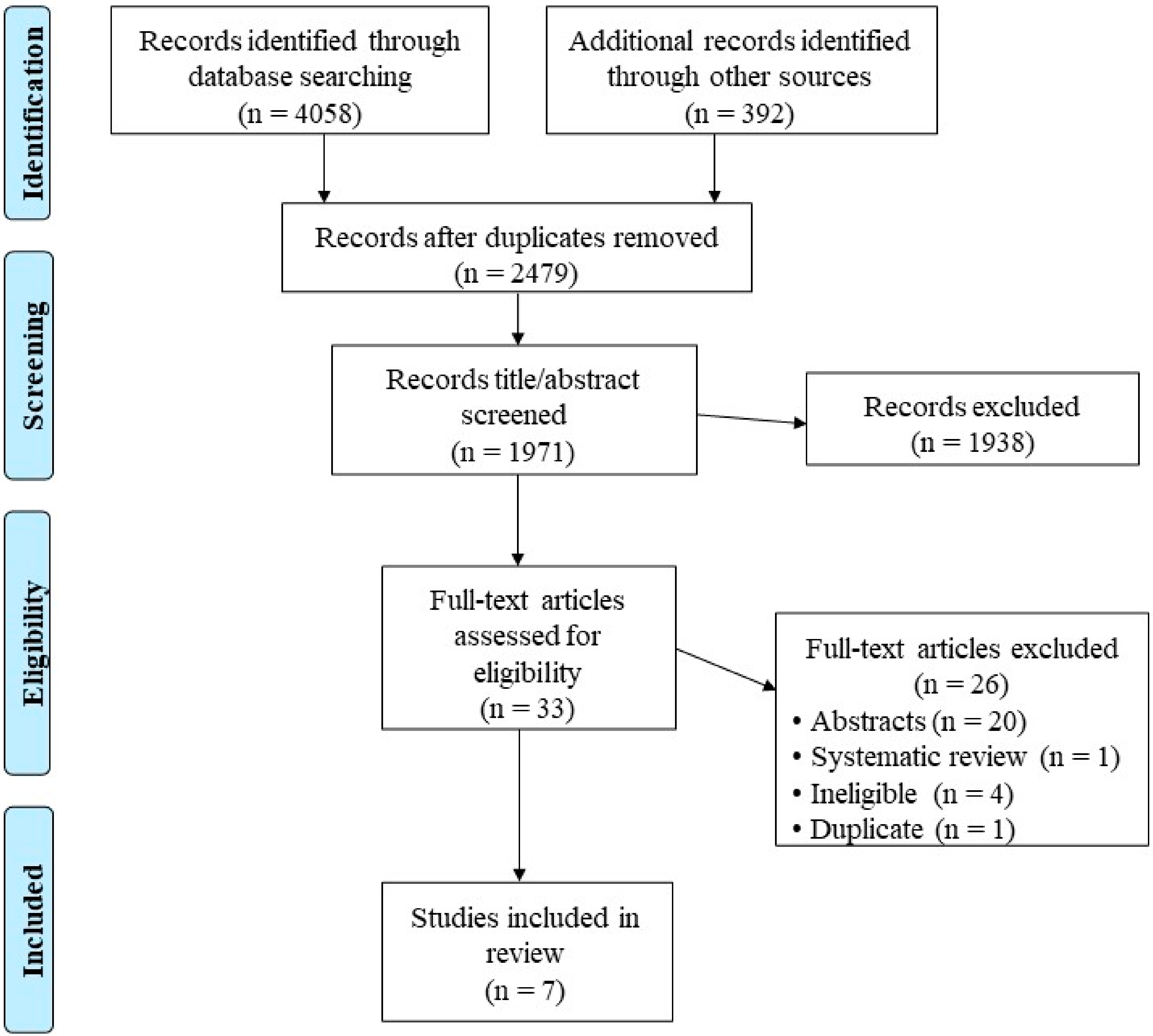
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Outcomes and Data Extraction
2.4. Risk of Bias
3. Results
3.1. Study and Participant Characteristics
3.2. Sleep–Wake Timing and Sleep Variables
3.3. Mild TBI (mTBI)
3.4. Moderate–Severe and Severe TBI (m-sTBI and sTBI)
3.5. Sleepiness, Mood, and Fatigue
3.6. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, M.; Ponsford, J.; Lannin, N.A.; O’Brien, T.J.; Cameron, P.; Cooper, D.J.; Rushworth, N.; Gabbe, B. AUS-TBI: The Australian Health Informatics Approach to Predict Outcomes and Monitor Intervention Efficacy after Moderate-to-Severe Traumatic Brain Injury. Neurotrauma Rep. 2022, 3, 217–223. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Jourdan, C.; Azouvi, P.; Genêt, F.; Selly, N.; Josseran, L.; Schnitzler, A. Disability and Health Consequences of Traumatic Brain Injury: National Prevalence. Am. J. Phys. Med. Rehabil. 2018, 97, 323–331. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef]
- Saksvik, S.B.; Karaliute, M.; Kallestad, H.; Follestad, T.; Asarnow, R.; Vik, A.; Håberg, A.K.; Skandsen, T.; Olsen, A. The Prevalence and Stability of Sleep-Wake Disturbance and Fatigue throughout the First Year after Mild Traumatic Brain Injury. J Neurotrauma 2020, 37, 2528–2541. [Google Scholar] [CrossRef]
- Duclos, C.; Dumont, M.; Wiseman-Hakes, C.; Arbour, C.; Mongrain, V.; Gaudreault, P.O.; Khoury, S.; Lavigne, G.; Desautels, A.; Gosselin, N. Sleep and wake disturbances following traumatic brain injury. Pathol. Biol. 2014, 62, 252–261. [Google Scholar] [CrossRef]
- Ayalon, L.; Borodkin, K.; Dishon, L.; Kanety, H.; Dagan, Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007, 68, 1136–1140. [Google Scholar] [CrossRef]
- Balba, N.M.; Elliott, J.E.; Weymann, K.B.; Opel, R.A.; Duke, J.W.; Oken, B.S.; Morasco, B.J.; Heinricher, M.M.; Lim, M.M. Increased Sleep Disturbances and Pain in Veterans With Comorbid Traumatic Brain Injury and Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2018, 14, 1865–1878. [Google Scholar] [CrossRef]
- Elliott, J.E.; Opel, R.A.; Weymann, K.B.; Chau, A.Q.; Papesh, M.A.; Callahan, M.L.; Storzbach, D.; Lim, M.M. Sleep Disturbances in Traumatic Brain Injury: Associations with Sensory Sensitivity. J. Clin. Sleep Med. 2018, 14, 1177–1186. [Google Scholar] [CrossRef]
- Castriotta, R.J.; Murthy, J.N. Sleep disorders in patients with traumatic brain injury: A review. CNS Drugs 2011, 25, 175–185. [Google Scholar] [CrossRef]
- Coogan, A.N.; Wyse, C.A. Neuroimmunology of the circadian clock. Brain Res. 2008, 1232, 104–112. [Google Scholar] [CrossRef]
- Quera Salva, M.A.; Azabou, E.; Hartley, S.; Sauvagnac, R.; Leotard, A.; Vaugier, I.; Pradat Diehl, P.; Vallat-Azouvi, C.; Barbot, F.; Azouvi, P. Blue-Enriched White Light Therapy Reduces Fatigue in Survivors of Severe Traumatic Brain Injury: A Randomized Controlled Trial. J. Head Trauma Rehabil. 2020, 35, E78–E85. [Google Scholar] [CrossRef]
- Raikes, A.C.; Dailey, N.S.; Shane, B.R.; Forbeck, B.; Alkozei, A.; Killgore, W.D.S. Daily Morning Blue Light Therapy Improves Daytime Sleepiness, Sleep Quality, and Quality of Life Following a Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, E405–E421. [Google Scholar] [CrossRef]
- Sinclair, K.L.; Ponsford, J.L.; Taffe, J.; Lockley, S.W.; Rajaratnam, S.M. Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabilit. Neural Repair 2014, 28, 303–313. [Google Scholar] [CrossRef]
- Srisurapanont, K.; Samakarn, Y.; Kamklong, B.; Siratrairat, P.; Bumiputra, A.; Jaikwang, M.; Srisurapanont, M. Blue-wavelength light therapy for post-traumatic brain injury sleepiness, sleep disturbance, depression, and fatigue: A systematic review and network meta-analysis. PLoS ONE 2021, 16, e0246172. [Google Scholar] [CrossRef]
- Elliott, J.E.; McBride, A.A.; Balba, N.M.; Thomas, S.V.; Pattinson, C.L.; Morasco, B.J.; Wilkerson, A.; Gill, J.M.; Lim, M.M. Feasibility and preliminary efficacy for morning bright light therapy to improve sleep and plasma biomarkers in US Veterans with TBI. A prospective, open-label, single-arm trial. PLoS ONE 2022, 17, e0262955. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Vanuk, J.R.; Shane, B.R.; Weber, M.; Bajaj, S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol. Dis. 2020, 134, 104679. [Google Scholar] [CrossRef]
- Bailes, H.J.; Lucas, R.J. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122987. [Google Scholar]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Duda, M.; Domagalik, A.; Orlowska-Feuer, P.; Krzysztynska-Kuleta, O.; Beldzik, E.; Smyk, M.K.; Stachurska, A.; Oginska, H.; Jeczmien-Lazur, J.S.; Fafrowicz, M.; et al. Melanopsin: From a small molecule to brain functions. Neurosci. Biobehav. Rev. 2020, 113, 190–203. [Google Scholar] [CrossRef]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef]
- Figueiro, M.G. Disruption of Circadian Rhythms by Light During Day and Night. Curr. Sleep Med. Rep. 2017, 3, 76–84. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology 2019, 8, 13. [Google Scholar] [CrossRef]
- Foster, R.G. Fundamentals of circadian entrainment by light. Lighting Res. Technol. 2021, 53, 377–393. [Google Scholar] [CrossRef]
- Gooley, J.J.; Rajaratnam, S.M.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra3. [Google Scholar] [CrossRef]
- Mardi, A.; Siwi, S.H.; Fatimah, T. A Study of Natural Lighting and Quality of Lighting in Production House for Cinematography (Case Study: Residential House at Cinere). In 3rd Tarumanagara International Conference on the Applications of Social Sciences and Humanities (TICASH 2021); Atlantis Press: Amsterdam, The Netherlands, 2022; pp. 241–246. [Google Scholar]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef]
- Cajochen, C. Alerting effects of light. Sleep Med. Rev. 2007, 11, 453–464. [Google Scholar] [CrossRef]
- Stephenson, K.M.; Schroder, C.M.; Bertschy, G.; Bourgin, P. Complex interaction of circadian and non-circadian effects of light on mood: Shedding new light on an old story. Sleep Med. Rev. 2012, 16, 445–454. [Google Scholar] [CrossRef]
- Duffy, J.F.; Wright, K.P., Jr. Entrainment of the human circadian system by light. J. Biol. Rhythm. 2005, 20, 326–338. [Google Scholar] [CrossRef]
- St Hilaire, M.A.; Gooley, J.J.; Khalsa, S.B.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Human phase response curve to a 1 h pulse of bright white light. J. Physiol. 2012, 590, 3035–3045. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Fröberg, J.E. Twenty-four-hour patterns in human performance, subjective and physiological variables and differences between morning and evening active subjects. Biol. Psychol. 1977, 5, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Systematic reviews of effectiveness. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: North Adelaide, Australia, 2017; Volume 3. [Google Scholar]
- Bajaj, S.; Vanuk, J.R.; Smith, R.; Dailey, N.S.; Killgore, W.D.S. Blue-Light Therapy following Mild Traumatic Brain Injury: Effects on White Matter Water Diffusion in the Brain. Front. Neurol. 2017, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.R.; Fogelberg, D.; Barber, J.; Nakase-Richardson, R.; Zumsteg, J.M.; Dubiel, R.; Dams-O’Connor, K.; Hoffman, J.M. The effect of phototherapy on sleep during acute rehabilitation after traumatic brain injury: A randomized controlled trial. Brain Inj. 2021, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Statements, Q. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J. Rehabil. Res. Dev. 2009, 46, 1–60. [Google Scholar]
- Mutch, C.A.; Talbott, J.F.; Gean, A. Imaging Evaluation of Acute Traumatic Brain Injury. Neurosurg. Clin. N. Am 2016, 27, 409–439. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H. Diagnostic Problems in Diffuse Axonal Injury. Diagnostics 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Beersma, D.G.; Daan, S.; Lewy, A.J. Bright morning light advances the human circadian system without affecting NREM sleep homeostasis. Am. J. Physiol. 1989, 256, R106–R111. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Fogg, L.F.; Young, M.A.; Eastman, C.I. Bright light therapy for winter depression—Is phase advancing beneficial? Chronobiol. Int. 2004, 21, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Lack, L.C.; Micic, G.; Lovato, N. Circadian aspects in the aetiology and pathophysiology of insomnia. J. Sleep Res. 2023, 32, e13976. [Google Scholar] [CrossRef] [PubMed]
- Oginska, H.; Pokorski, J. Fatigue and mood correlates of sleep length in three age-social groups: School children, students, and employees. Chronobiol. Int. 2006, 23, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 2006, 29, 161–168. [Google Scholar] [PubMed]
- StatistaResearch. (2023, 13.12.2023). Gender Distribution of Active Duty Officers in the United States Department of Defense in 2022. Available online: https://www.statista.com/statistics/232608/gender-of-us-dod-officers-in-2010-by-service-branch/ (accessed on 11 March 2024).
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock-Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Design/ Participants | History of TBI and Severity | Type | Wavelength and Intensity (Intervention) | Wavelength and Intensity (Control) | Timing/Duration/Length of Exposure |
|---|---|---|---|---|---|---|
| Bajaj et al. [38] | RCT (13 m, 15 f) Blue light (n = 14): 21.8 ± 4.4 y, 8 females); comparator placebo group (n = 14), mean age = 21.2 ± 3.1 y | Injury due to sports or vehicular/household accidents (≤12 mth). Mild TBI defined by VA/DoD practice guidelines. | GoLITE Blu®, Philips Electronics | Blue light 469 nm, 214 lux | Amber light, 578 nm, 188 lux. | Within 2 h of awakening, but before 11:00 a.m., homebased. 30 min, 6 weeks. |
| Bell et al. [39] | RCT (89 m, 42 f) BWL (n = 65); control red light (n = 66) | Injury caused by an external mechanical force (≤90 days). Moderate-to-severe TBI defined by Glasgow Coma Scale of 3–12. | Litebook® device | BWL 440–480 nm, 1260 lux | Red light: no light emitted between 440 and 480 nm, <450 lux. | Between 7:30–9:30 a.m. during acute rehabilitation hospitalization. 30 min daily, 10 days. |
| Elliot et al. [16] | Single-arm, open-label pre–post-intervention (30 m, 3 f); 53 ± 18 y (n = 33) | 3–55 y post-injury (military combat blast, blunt force, fall, sports, unknown). Mild TBI defined by VA/DoD practice guidelines. | Light box (LightPad Mini, Aurora Light Solutions Inc) | BWL (555 nm), 10,000 lux | No control group. | Morning for 60 min, 4 weeks. |
| Killgore et al. [17] | RCT (15 m, 17 f) Blue light (n = 16): 23.2 ± 7.1 y; amber light control (n = 16): 23.3 ± 7.4 y | Injury ≤ 18 mth (external force (e.g., head impact, blast wave)). Mild TBI defined by VA/DoD practice guidelines. | GoLITE Blu®, Philips Electronics | Blue light 469 nm, 214 lux | Amber light 578 nm, 188 lux. | Within two hours of awakening, but no later than 11:00 a.m. 30 min, 6 weeks. |
| Quera Salva et al. [12] | RCT (11 m, 9 f) BWL (10): 34.2 ± 10.7 y; control (n = 10): 39.0 ± 9.8 y | Cause of injury not reported (≤6 mth). Severe TBI, initial Glasgow Coma Scale score 8 or less. | Light device (Luminette Lucimed Belgium) | BWL at 468 nm, 392.2 μW/cm2 of one intensity of 1703 lux | No light exposure. | At awakening. 30 min, 4 weeks |
| Raikes et al. [13] | RCT (13 m, 22 f) Blue light (n = 17): 25.5 ± 8.7 y, n = 17; amber light (n = 18): 26.2 ± 7.6 y | Non-blast mTBI (≤18 mth). Mild TBI defined by VA/DoD practice guidelines. | GoLITE Blu®, Philips Electronic | Blue light ∼480 nm | Amber light ~530 nm. | Within 2 h of waking each morning between 8:00–10:00 a.m. 30 min, 6 weeks at home. |
| Sinclair et al. [14] | RCT (24 m, 6 f) Blue light (n = 10): 47.2 ± 13.7 y, yellow light (n = 10): 36.2 ± 13 y; control (n = 10): 42.5 ± 12.9 y, (no treatment) | TBI at least 3 mth earlier (motor vehicle injury, falls) Severe TBI based on medical records. | GoLITE Blu®, Philips Apollo Health | Blue light: 465 nm, 84.8 μW/cm2, 39.5 lux | Yellow GoLite: 574 nm, 18.5 μW/cm2, 68 lux. | Morning 2 h after waking, homebased. 45 min each morning, 4 weeks. |
| Study | Glasgow Coma Scale | TBI Classification | Circadian Phase Shift | Sleep Variables | Insomnia Severity Index | Sleepiness | Alertness/ Mood | Fatigue |
|---|---|---|---|---|---|---|---|---|
| Bajaj et al. [38] | Other 1 | Mild | NM | NR | NM | Reduced sleepiness, (↑ MSLT) | NM | NM |
| Bell et al. [39] | 3–12 | Moderate–severe | NM | ↔ variables | NM | ↔ KSS | ↔ mood | ↔ fatigue |
| Elliot et al. [16] | Other 1 | Mild | Phase advance of bedtime and mid-sleep time | ↑ TIB, ↑ TST, ↔ in other variables | Improved (from moderate to mild) | NM | ↑ mood | NM |
| Killgore et al. [17] | Other 1 | Mild | Phase advance (sleep onset and offset, mid-sleep time) | ↔ TST | NM | Reduced sleepiness (↓ ESS, ↑ MSLT) | NM | NM |
| Quera Salva et al. [12] | ≤8 | Severe | NM | ↔ PSQI | NM | ↔ ESS | NM | ↓ fatigue |
| Raikes et al. [13] | Other 1 | Mild | NM | ↔ WASO ↔ SL | NM | ↓ ESS | NM | NM |
| Sinclair et al. [14] | Other 2 | Severe | NM | ↔ PSQI | NM | ↓ ESS | NM | ↓ fatigue |
| RCT | q1 | q2 | q3 | q4 | q5 | q6 | q7 | q8 | q9 | q10 | q11 | q12 | q13 | q14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bajaj et al. [38] | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Bell et al. [39] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Killgore et al. [17] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Quera Salva et al. [12] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Raikes et al. [13] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Sinclair et al. [14] | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| Quasi | q1 | q2 | q3 | q4 | q5 | q6 | q7 | q8 | q9 | q10 | ||||
| Elliot et al. [16] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, C.M.; Ekanayake, K.; Hackett, D. Efficacy of Morning Shorter Wavelength Lighting in the Visible (Blue) Range and Broad-Spectrum or Blue-Enriched Bright White Light in Regulating Sleep, Mood, and Fatigue in Traumatic Brain Injury: A Systematic Review. Clocks & Sleep 2024, 6, 255-266. https://doi.org/10.3390/clockssleep6020018
Chow CM, Ekanayake K, Hackett D. Efficacy of Morning Shorter Wavelength Lighting in the Visible (Blue) Range and Broad-Spectrum or Blue-Enriched Bright White Light in Regulating Sleep, Mood, and Fatigue in Traumatic Brain Injury: A Systematic Review. Clocks & Sleep. 2024; 6(2):255-266. https://doi.org/10.3390/clockssleep6020018
Chicago/Turabian StyleChow, Chin Moi, Kanchana Ekanayake, and Daniel Hackett. 2024. "Efficacy of Morning Shorter Wavelength Lighting in the Visible (Blue) Range and Broad-Spectrum or Blue-Enriched Bright White Light in Regulating Sleep, Mood, and Fatigue in Traumatic Brain Injury: A Systematic Review" Clocks & Sleep 6, no. 2: 255-266. https://doi.org/10.3390/clockssleep6020018
APA StyleChow, C. M., Ekanayake, K., & Hackett, D. (2024). Efficacy of Morning Shorter Wavelength Lighting in the Visible (Blue) Range and Broad-Spectrum or Blue-Enriched Bright White Light in Regulating Sleep, Mood, and Fatigue in Traumatic Brain Injury: A Systematic Review. Clocks & Sleep, 6(2), 255-266. https://doi.org/10.3390/clockssleep6020018







