Bright Light Therapy for Major Depressive Disorder in Adolescent Outpatients: A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Acceptability and Feasibility
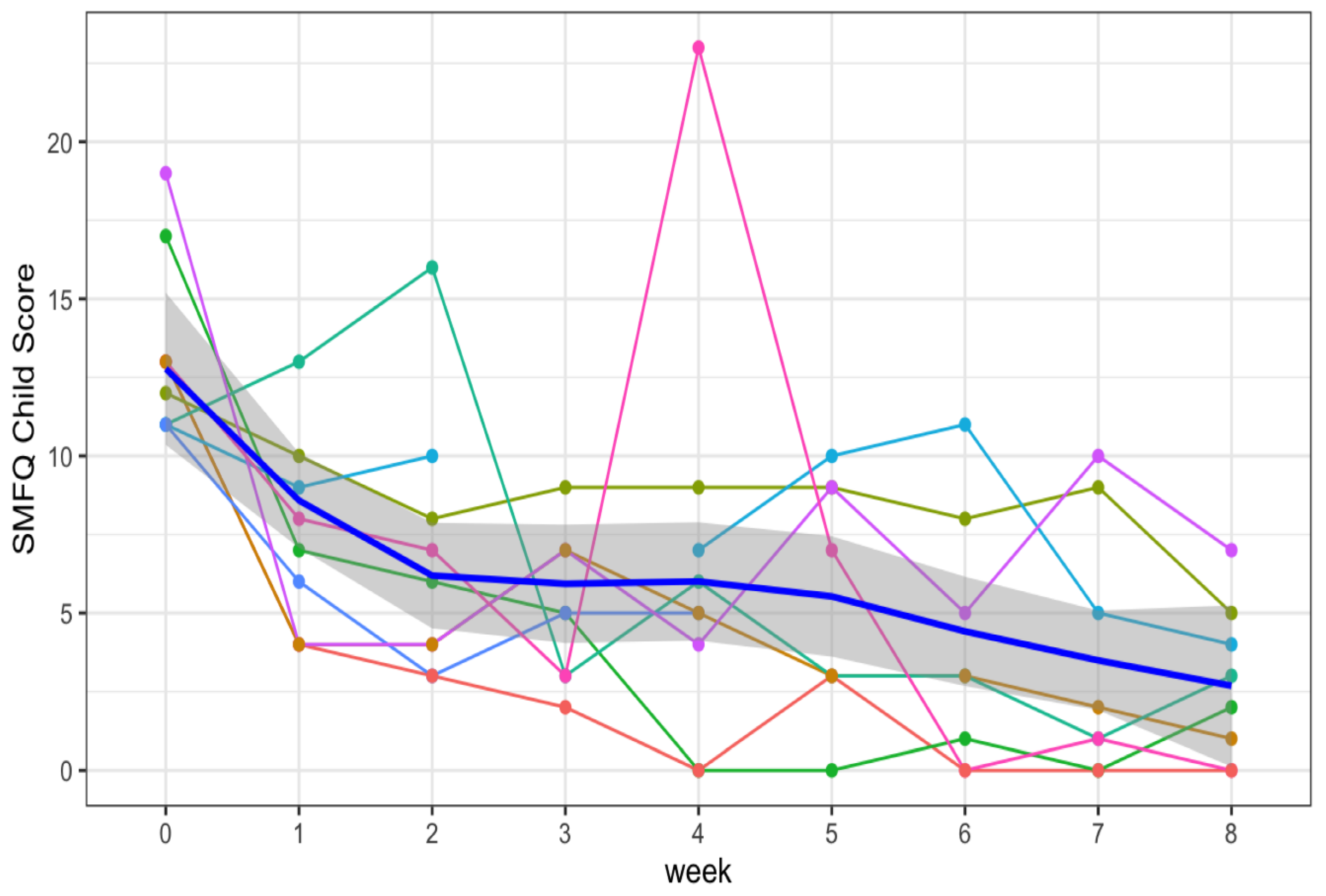
2.3. Mood Ratings
2.4. Adverse Events
2.5. Expectations
2.6. Actigraphy Measures
3. Discussion
3.1. Recruitment
3.2. Feasibility and Acceptability in Adolescent Outpatients
3.3. Parent–Adolescent Correlation in Assessment of Adolescent Depression
3.4. Anxiety
3.5. Placebo Response and Expectations
3.6. Time of Day and Optimal Response to BLT
3.7. Adverse Events
3.8. Actigraphy
3.9. Strengths/Limitations
4. Methods
4.1. Stakeholder Engagement and Focus Group Interviews
4.2. Inclusion Criteria
4.3. Diagnostic Interview and Clinical Ratings
4.3.1. Short Mood and Feelings Questionnaire (SMFQ)
4.3.2. Clinical Global Impression—Severity and Improvement Scales (CGI-S and -I)
4.3.3. Screen for Child Anxiety Related Emotional Disorders (SCARED)
4.3.4. Seasonal Pattern Assessment Questionnaire (SPAQ)
4.4. Assessments
4.5. Light Therapy Protocol with Placebo Lead-In
4.6. Adherence
4.7. Safety Checks
4.8. Actigraphic Assessments
4.9. Data Analysis
4.10. Statistical Analyses of Actigraphy Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLT | bright light therapy |
| CGI-S and -I | Clinical Global Impression Scale Severity and Improvement Scales |
| CMRS | brief Child Mania Rating Scale |
| DRL | dim red light |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| IQR | interquartile range |
| MEQ | Morningness–Eveningness Questionnaire |
| MDD | major depressive disorder |
| MINI-KID | Mini-International Neuropsychiatric Interview for Children and Adolescents |
| PHQ-9 | Patient Health Questionnaire-9 |
| SAD | seasonal affective disorder |
| SCARED | Screen for Child Anxiety Related Disorders |
| SAFTEE | Systematic Assessment for Treatment Emergent Effects |
| ShARP | stakeholder academic resource panel (ShARP) |
| SMFQ | Short Mood and Feelings Questionnaire |
| SPAQ | Seasonal Pattern Assessment Questionnaire |
References
- Avenevoli, S.; Swendsen, J.; He, J.-P.; Burstein, M.; Merikangas, K. Major Depression in the National Comorbidity Survey-Adolescent Supplement: Prevalence, Correlates, and Treatment. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 37–44.e2. [Google Scholar] [CrossRef]
- Bodden, D.; Stikkelbroek, Y.; Dirksen, C. Societal burden of adolescent depression, an overview and cost-of-illness study. J. Affect. Disord. 2018, 241, 256–262. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; A Furukawa, T.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Emslie, G.J.; Mayes, T.; Porta, G.; Vitiello, B.; Clarke, G.; Wagner, K.D.; Asarnow, J.R.; Spirito, A.; Birmaher, B.; Ryan, N.; et al. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. Am. J. Psychiatry 2010, 167, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.I.; Young, M.A.; Fogg, L.F.; Liu, L.; Meaden, P.M. Bright light treatment of winter depression: A placebo-controlled trial. Arch. Gen. Psychiatry 1998, 55, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Levitt, A.J.; Levitan, R.D.; Enns, M.W.; Morehouse, R.; Michalak, E.E.; Tam, E.M. The Can-SAD study: A randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am. J. Psychiatry 2006, 163, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, N.E.; Sack, D.A.; Gillin, J.C.; Lewy, A.J.; Goodwin, F.K.; Davenport, Y.; Mueller, P.S.; Newsome, D.A.; Wehr, T.A. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 1984, 41, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Terman, J.S. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectr. 2005, 10, 647–663. [Google Scholar] [CrossRef]
- Goel, N.; Terman, M.; Terman, J.S.; Macchi, M.M.; Stewart, J.W. Controlled trial of bright light and negative air ions for chronic depression. Psychol. Med. 2005, 35, 945–955. [Google Scholar] [CrossRef]
- Golden, R.N.; Gaynes, B.N.; Ekstrom, R.D.; Hamer, R.M.; Jacobsen, F.M.; Suppes, T.; Wisner, K.L.; Nemeroff, C.B. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am. J. Psychiatry 2005, 162, 656–662. [Google Scholar] [CrossRef]
- Lam, R.W.; Levitt, A.J.; Levitan, R.D.; Michalak, E.E.; Cheung, A.H.; Morehouse, R.; Ramasubbu, R.; Yatham, L.N.; Tam, E.M. Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 56–63. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Bader, A.; Frisch, U.; Stieglitz, R.-D.; Alder, J.; Bitzer, J.; Hösli, I.; Jazbec, S.; Benedetti, F.; Terman, M.; et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J. Clin. Psychiatry 2011, 72, 986–993. [Google Scholar] [CrossRef]
- Gottlieb, J.F.; Benedetti, F.; Geoffroy, P.A.; Henriksen, T.E.G.; Lam, R.W.; Murray, G.; Phelps, J.; Sit, D.; Swartz, H.A.; Crowe, M.; et al. The chronotherapeutic treatment of bipolar disorders: A systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019, 21, 741–773. [Google Scholar] [CrossRef]
- Sit, D.K.; McGowan, J.; Wiltrout, C.; Diler, R.S.; Dills, J.; Luther, J.; Yang, A.; Ciolino, J.D.; Seltman, H.; Wisniewski, S.R.; et al. Adjunctive Bright Light Therapy for Bipolar Depression: A Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2018, 175, 131–139. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Schroder, C.M.; Reynaud, E.; Bourgin, P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 48, 101213. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Freeman, M.P.; Markowitz, J.C.; Rosenbaum, J.F.; Thase, M.E.; Trivedi, M.H.; Van Rhoads, R.S. Work Group on Major Depressive Disorder: Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 2010; p. 62. [Google Scholar]
- Ballard, R.; Parkhurst, J.; Julian, K.; Pasetes, L.N.; Fawcett, A.; Li, A.; Goel, N.; Sit, D.K. Light Therapy for Adolescent Depression: A Scoping Review. Curr. Psychiatry Rep. 2023, 25, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Monti, J.M.; Burman, D.; Karthikeyan, R.; BaHammam, A.S.; Spence, D.W.; Brown, G.M.; Narashimhan, M. Clarifying the role of sleep in depression: A narrative review. Psychiatry Res. 2020, 291, 113239. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, N.E.; Carpenter, C.J.; James, S.P.; Parry, B.L.; Rogers, S.L.; A Wehr, T. Seasonal affective disorder in children and adolescents. Am. J. Psychiatry 1986, 143, 356–358. [Google Scholar]
- Sharkey, K.M.; Carskadon, M.A.; Figueiro, M.G.; Zhu, Y.; Rea, M.S. Effects of an Advanced Sleep Schedule and Morning Short Wavelength Light Exposure on Circadian Phase in Young Adults with Late Sleep Schedules. Sleep Med. 2011, 12, 685–692. [Google Scholar] [CrossRef]
- Williamson, D.E.; Birmaher, B.; Brent, D.A.; Balach, L.; Dahl, R.E.; Ryan, N.D. Atypical Symptoms of Depression in a Sample of Depressed Child and Adolescent Outpatients. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Amira, L.; Terman, J.S.; Ross, D.C. Predictors of response and nonresponse to light treatment for winter depression. Am. J. Psychiatry 1996, 153, 1423–1429. [Google Scholar]
- Janas-Kozik, M.; Krzystanek, M.; Stachowicz, M.; Krupka-Matuszczyk, I.; Janas, A.; Rybakowski, J.K. Bright light treatment of depressive symptoms in patients with restrictive type of anorexia nervosa. J. Affect. Disord. 2011, 130, 462–465. [Google Scholar] [CrossRef]
- LaRosa, K.N.; MacArthur, E.; Wang, F.; Zhang, H.; Pan, H.; Brigden, J.; Pappo, A.; Wilson, M.W.; Crabtree, V.M. Light Therapy for QoL/Depression in AYA With Cancer: A Randomized Trial. J. Pediatr. Psychol. 2022, 47, 306–317. [Google Scholar] [CrossRef]
- Sit, D.; Wisner, K.L.; Hanusa, B.H.; Stull, S.; Terman, M. Light therapy for bipolar disorder: A case series in women. Bipolar Disord. 2007, 9, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A.; Benedetti, F.; Terman, T. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light and Wake Therapy, Revised Edition, 2nd ed.; S. Karger: Basel, Switzerland, 2013; p. 220. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rhew, I.C.; Simpson, K.; Tracy, M.; Lymp, J.; McCauley, E.; Tsuang, D.; Stoep, A.V. Criterion validity of the Short Mood and Feelings Questionnaire and one- and two-item depression screens in young adolescents. Child Adolesc. Psychiatry Ment. Health 2010, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Joinson, C.; Peters, T.J.; Wiles, N.; Lewis, G. Validity of the Short Mood and Feelings Questionnaire in late adolescence. Psychol. Assess. 2014, 26, 752–762. [Google Scholar] [CrossRef]
- Bogen, S.; Legenbauer, T.; Gest, S.; Holtmann, M. Lighting the mood of depressed youth: Feasibility and efficacy of a 2 week-placebo controlled bright light treatment for juvenile inpatients. J. Affect. Disord. 2016, 190, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Allen, A.J.; Glod, C.A.; Clark, C.H.; Teicher, M.H.; Richter, D.; Hoffman, C.; Hamburger, S.D.; Dow, S.; Brown, C.; et al. A controlled trial of light therapy for the treatment of pediatric seasonal affective disorder. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Sonis, W.A.; Yellin, A.M.; Garfinkel, B.D.; Hoberman, H.H. The antidepressant effect of light in seasonal affective disorder of childhood and adolescence. Psychopharmacol. Bull. 1987, 23, 360–363. [Google Scholar] [PubMed]
- Niederhofer, H.; von Klitzing, K. Bright light treatment as add-on therapy for depression in 28 adolescents: A randomized trial. Prim. Care Companion CNS Disord. 2011, 13, 26720. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Gradisar, M. Depressed mood and repetitive negative thinking in Delayed Sleep-Wake Phase Disorder: Treatment effects and a comparison with good sleepers. J. Sleep Res. 2022, 31, e13452. [Google Scholar] [CrossRef] [PubMed]
- De Los Reyes, A.; Augenstein, T.M.; Wang, M.; Thomas, S.A.; Drabick, D.A.; Burgers, D.E.; Rabinowitz, J. The Validity of the Multi-Informant Approach to Assessing Child and Adolescent Mental Health. Psychol. Bull. 2015, 141, 858–900. [Google Scholar] [CrossRef] [PubMed]
- Beesdo, K.; Pine, D.S.; Lieb, R.; Wittchen, H.U. Incidence and Risk Patterns of Anxiety and Depressive Disorders and Categorization of Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2010, 67, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Niederhofer, H. Bright light treatment of depression for adolescents. Eur. Psychiatry 2011, 26, 664. [Google Scholar] [CrossRef]
- Piaggio, G.; Elbourne, D.R.; Pocock, S.J.; Evans, S.J.; Altman, D.G.; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA 2012, 308, 2594–2604. [Google Scholar] [CrossRef]
- Locher, C.; Gaab, J.; Blease, C. When a Placebo Is Not a Placebo: Problems and Solutions to the Gold Standard in Psychotherapy Research. Front. Psychol. 2018, 9, 2317. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Graw, P.; Kräuchi, K.; Gisin, B.; Jochum, A.; Arendt, J.; Fisch, H.-U.; Buddeberg, C.; Pöldinger, W. Light therapy in seasonal affective disorder is independent of time of day or circadian phase. Arch. Gen. Psychiatry 1993, 50, 929–937. [Google Scholar] [CrossRef]
- He, C.; Xiao, L.; Xu, J.; Cui, Y.; Huang, Y.; Li, Y.; Tang, Y.; Xu, S.; Wang, H.; Cai, Y.; et al. Effect of sleep deprivation plus existing therapies on depression: A systematic review and meta-analysis of randomized controlled trials. Int. J. Psychophysiol. 2023, 184, 1–11. [Google Scholar] [CrossRef]
- Grandner, M.A.; Kripke, D.F.; Elliott, J.; Cole, R. Short wavelength light administered just prior to waking: A pilot study. Biol. Rhythm. Res. 2013, 44, 13–32. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Guy, W. Clinical Global Impressions, ECDEU Assessment Manual for Psychopharmacology, Revised; National Institute of Mental Health: Rockville, MD, USA, 1976; pp. 218–222. [Google Scholar]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Boyle, M.H.; Duncan, L.; Georgiades, K.; Bennett, K.; Gonzalez, A.; Van Lieshout, R.J.; Szatmari, P.; MacMillan, H.L.; Kata, A.; Ferro, M.A.; et al. Classifying child and adolescent psychiatric disorder by problem checklists and standardized interviews. Int. J. Methods Psychiatr. Res. 2017, 26, e1544. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.; Georgiades, K.; Wang, L.; Van Lieshout, R.J.; MacMillan, H.L.; Ferro, M.A.; Lipman, E.L.; Szatmari, P.; Bennett, K.; Kata, A.; et al. Psychometric evaluation of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). Psychol. Assess. 2018, 30, 916–928. [Google Scholar] [CrossRef]
- Angold, A.; Erkanli, A.; Silberg, J.; Eaves, L.; Costello, E.J. Depression Scale Scores in 8–17-year-olds: Effects of Age and Gender. J. Child Psychol. Psychiatry 2002, 43, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Eyre, O.; Jones, R.B.; Agha, S.S.; E Wootton, R.; Thapar, A.K.; Stergiakouli, E.; Langley, K.; Collishaw, S.; Thapar, A.; Riglin, L. Validation of the short Mood and Feelings Questionnaire in young adulthood. J. Affect. Disord. 2021, 294, 883–888. [Google Scholar] [CrossRef]
- Birmaher, B.; Khetarpal, S.; Brent, D.; Cully, M.; Balach, L.; Kaufman, J.; Neer, S.M. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Behrens, B.; Swetlitz, C.; Pine, D.S.; Pagliaccio, D. The screen for child anxiety related emotional disorders (SCARED): Informant discrepancy, measurement invariance, and test–retest reliability. Child Psychiatry Hum. Dev. 2019, 50, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, B.; Pagliaccio, D.; Pine, D.; Klein, D.; Jarcho, J. Discriminant validity, diagnostic utility, and parent-child agreement on the Screen for Child Anxiety Related Emotional Disorders (SCARED) in treatment- and non-treatment-seeking youth. J. Anxiety Disord. 2017, 51, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Brent, D.A.; Chiappetta, L.; Bridge, J.; Monga, S.; Baugher, M. Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A Replication Study. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 1230–1236. [Google Scholar] [CrossRef]
- Magnusson, A. Validation of the Seasonal Pattern Assessment Questionnaire (SPAQ). J. Affect. Disord. 1996, 40, 121–129. [Google Scholar] [CrossRef]
- Levine, J.; Schooler, N.R. SAFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacol. Bull. 1986, 22, 343–381. [Google Scholar] [PubMed]
- Rand Corporation. Sleep Scale Survey. Available online: https://www.rand.org/health-care/surveys_tools/mos/sleep-scale.html (accessed on 15 December 2021).
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Henry, D.B.; Pavuluri, M.N.; Youngstrom, E.; Birmaher, B. Accuracy of brief and full forms of the child mania rating scale. J. Clin. Psychol. 2008, 64, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Garbazza, C.; Cirignotta, F.; D’Agostino, A.; Cicolin, A.; Hackethal, S.; Wirz-Justice, A.; Cajochen, C.; Manconi, M.; the “Life-ON” study group. Sustained remission from perinatal depression after bright light therapy: A pilot randomised, placebo-controlled trial. Acta Psychiatr. Scand. 2022, 146, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Oren, D.A.; Brainard, G.C.; Johnston, S.H.; Joseph-Vanderpool, J.R.; Sorek, E.; Rosenthal, N.E. Treatment of seasonal affective disorder with green light and red light. Am. J. Psychiatry 1991, 148, 509–511. [Google Scholar] [PubMed]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Terman, J.S.; Ross, D.C. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch. Gen. Psychiatry 1998, 55, 875–882. [Google Scholar] [CrossRef]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef]
- Brieva, T.E.; Casale, C.E.; Yamazaki, E.M.; Antler, C.A.; Goel, N. Cognitive throughput and working memory raw scores consistently differentiate resilient and vulnerable groups to sleep loss. Sleep 2021, 44, zsab197. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; von Scheven, G.; Feiveson, A.; Bürkle, A.; Wu, H.; Goel, N. The degree of radiation-induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep 2018, 41, zsy067. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.M.; A Antler, C.; Lasek, C.R.; Goel, N. Residual, differential neurobehavioral deficits linger after multiple recovery nights following chronic sleep restriction or acute total sleep deprivation. Sleep 2021, 44, zsaa224. [Google Scholar] [CrossRef]
- Yamazaki, E.M.; Rosendahl-Garcia, K.M.; Casale, C.E.; MacMullen, L.E.; Ecker, A.J.; Kirkpatrick, J.N.; Goel, N. Left Ventricular Ejection Time Measured by Echocardiography Differentiates Neurobehavioral Resilience and Vulnerability to Sleep Loss and Stress. Front. Physiol. 2022, 12, 795321. [Google Scholar] [CrossRef]
- Yamazaki, E.M.; Goel, N. Robust stability of trait-like vulnerability or resilience to common types of sleep deprivation in a large sample of adults. Sleep 2020, 43, zsz292. [Google Scholar] [CrossRef]
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947, 12, 153–157. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
| Measure | T1 | T2 | Median Change | V stat; p_adj | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|
| SMFQ_child_score | week_0 (N = 9) | week_2 (N = 9) | 7 | 42; 0.042 * | 1.10 |
| SMFQ_child_score | week_0 (N = 9) | week_8 (N = 8) | 10 | 36; 0.042 * | 3.52 |
| SMFQ_child_score | week_2 (N = 9) | week_8 (N = 8) | 4 | 32; 0.058 | 0.99 |
| SMFQ_parent_score | week_0 (N = 9) | week_2 (N = 9) | 5 | 39.5; 0.1 | 0.99 |
| SMFQ_parent_score | week_0 (N = 9) | week_8 (N = 8) | 7 | 28; 0.066 | 1.35 |
| SMFQ_parent_score | week_2 (N = 9) | week_8 (N = 8) | 1 | 13.5; 0.598 | 0.22 |
| CGI-S_clinician | week_0 (N = 9) | week_2 (N = 9) | 2 | 21; 0.067 | 1.12 |
| CGI-S_clinician | week_0 (N = 9) | week_8 (N = 7) | 2 | 28; 0.064 | 2.00 |
| CGI-S_clinician | week_2 (N = 9) | week_8 (N = 7) | 1 | 15; 0.067 | 1.73 |
| DRL | BLT | ||
|---|---|---|---|
| N = 6 | N = 6 | ||
| Sleep Variable | Total Sleep Time (min) | 381.59 ± 43.66 | 388.06 ± 55.22 |
| Sleep Duration (min) | 431.05 ± 42.55 | 437.77 ± 54.40 | |
| Sleep Onset Latency (min) | 14.23 ± 8.31 | 9.39 ± 5.60 | |
| Sleep Onset (clock hour) | 24.13 ± 1.33 | 24.51 ± 0.83 | |
| Sleep Offset (clock hour) | 7.32 ± 1.17 | 7.80 ± 1.15 | |
| Sleep Midpoint (clock hour) | 3.73 ± 1.20 | 4.16 ± 0.89 | |
| Wake Time (%) | 11.17 ± 1.85 | 11.10 ± 1.70 | |
| Sleep Time (%) | 88.83 ± 1.85 | 88.90 ± 1.70 | |
| Wake After Sleep Onset (min) | 47.60 ± 5.03 | 48.30 ± 5.87 | |
| Sleep Efficiency (%) | 83.84 ± 2.07 | 85.00 ± 2.48 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballard, R.; Parkhurst, J.T.; Gadek, L.K.; Julian, K.M.; Yang, A.; Pasetes, L.N.; Goel, N.; Sit, D.K. Bright Light Therapy for Major Depressive Disorder in Adolescent Outpatients: A Preliminary Study. Clocks & Sleep 2024, 6, 56-71. https://doi.org/10.3390/clockssleep6010005
Ballard R, Parkhurst JT, Gadek LK, Julian KM, Yang A, Pasetes LN, Goel N, Sit DK. Bright Light Therapy for Major Depressive Disorder in Adolescent Outpatients: A Preliminary Study. Clocks & Sleep. 2024; 6(1):56-71. https://doi.org/10.3390/clockssleep6010005
Chicago/Turabian StyleBallard, Rachel, John T. Parkhurst, Lisa K. Gadek, Kelsey M. Julian, Amy Yang, Lauren N. Pasetes, Namni Goel, and Dorothy K. Sit. 2024. "Bright Light Therapy for Major Depressive Disorder in Adolescent Outpatients: A Preliminary Study" Clocks & Sleep 6, no. 1: 56-71. https://doi.org/10.3390/clockssleep6010005
APA StyleBallard, R., Parkhurst, J. T., Gadek, L. K., Julian, K. M., Yang, A., Pasetes, L. N., Goel, N., & Sit, D. K. (2024). Bright Light Therapy for Major Depressive Disorder in Adolescent Outpatients: A Preliminary Study. Clocks & Sleep, 6(1), 56-71. https://doi.org/10.3390/clockssleep6010005






