The Timing of the Melatonin Onset and Phase Angle to Sleep Onset in Older Adults after Uncontrolled vs. Controlled Lighting Conditions
Abstract
1. Introduction
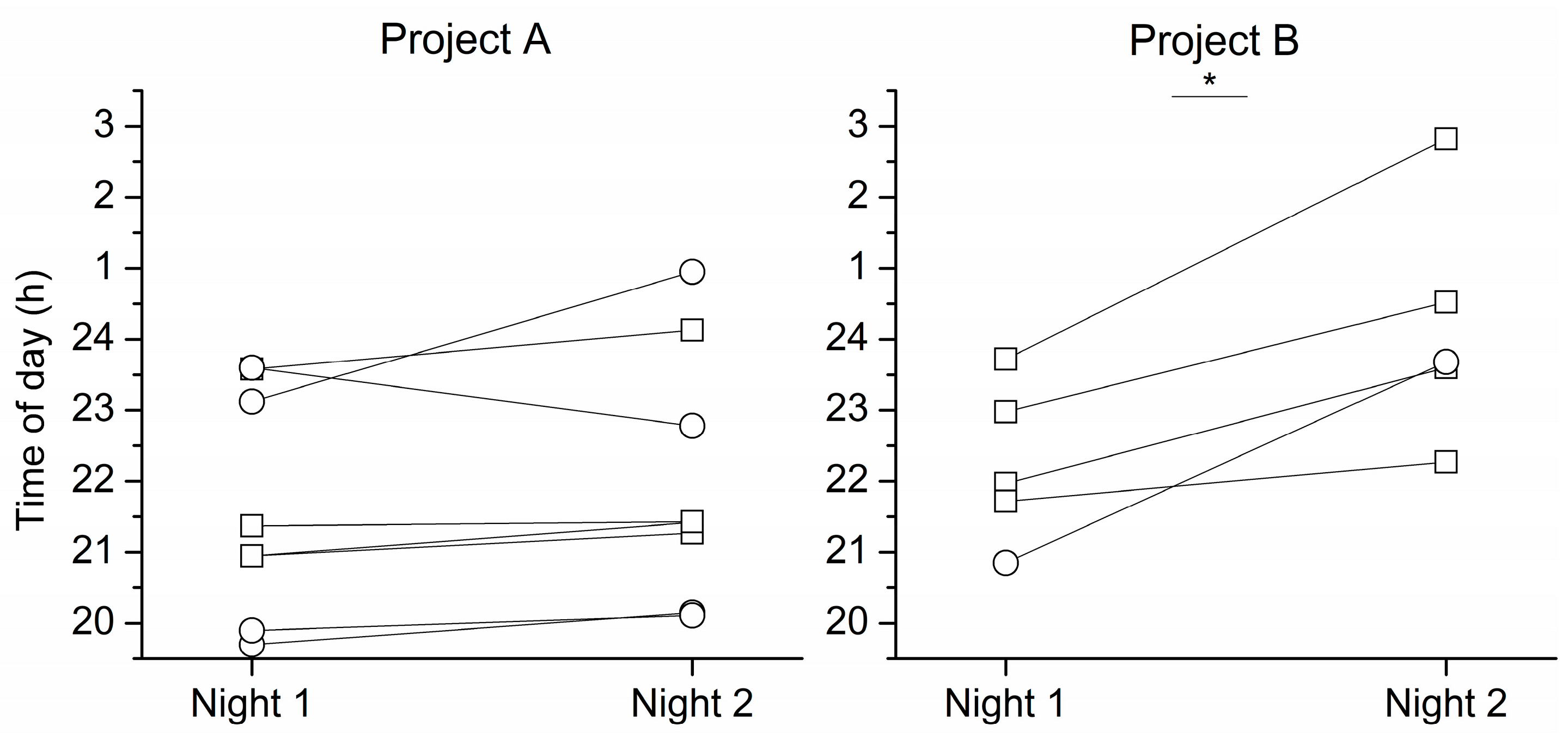
2. Results
3. Discussion
3.1. Limitations
3.2. Future Directions
4. Materials and Methods
4.1. Participants
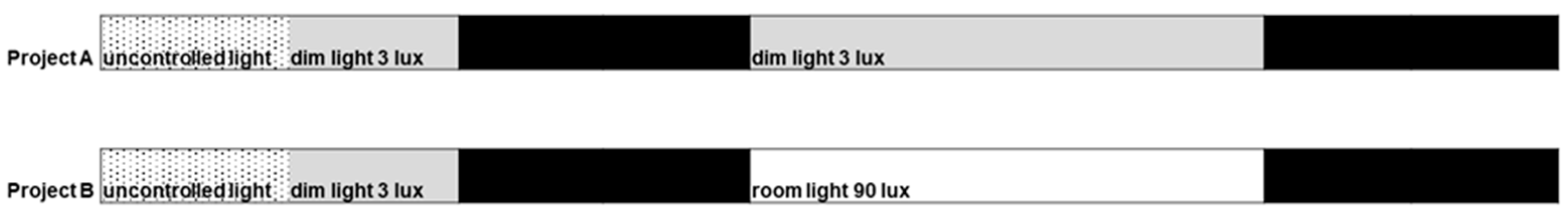
4.2. Study Protocol
4.3. Light Conditions
4.4. DLMO and Melatonin Onset Assessment and Phase Angle to Sleep
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendt, J. Melatonin: Characteristics, concerns, and prospects. J. Biol. Rhythm. 2005, 20, 291–303. [Google Scholar] [CrossRef]
- St Hilaire, M.A.; Ámundadóttir, M.L.; Rahman, S.A.; Rajaratnam, S.M.W.; Rüger, M.; Brainard, G.C.; Czeisler, C.A.; Andersen, M.; Gooley, J.J.; Lockley, S.W. The spectral sensitivity of human circadian phase resetting and melatonin suppression to light changes dynamically with light duration. Proc. Natl. Acad. Sci. USA 2022, 119, e2205301119. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F., 3rd. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Circadian rhythms: Basic neurobiology and clinical applications. Annu. Rev. Med. 1997, 48, 253–266. [Google Scholar] [CrossRef]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [CrossRef]
- Benloucif, S.; Guico, M.J.; Reid, K.J.; Wolfe, L.F.; L’Hermite-Balériaux, M.; Zee, P.C. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. Rhythm. 2005, 20, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Park, M.; Wyatt, J.K.; Rizvydeen, M.; Fogg, L.F. Sleep and circadian variability in people with delayed sleep-wake phase disorder versus healthy controls. Sleep Med. 2017, 34, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Sack, R.L.; Singer, C.M. Immediate and delayed effects of bright light on human melatonin production: Shifting “dawn” and “dusk” shifts the dim light melatonin onset (DLMO). Ann. N. Y. Acad. Sci. 1985, 453, 253–259. [Google Scholar] [CrossRef]
- Conroy, D.A.; Hairston, I.S.; Arnedt, J.T.; Hoffmann, R.F.; Armitage, R.; Brower, K.J. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol. Int. 2012, 29, 35–42. [Google Scholar] [CrossRef]
- Rahman, S.A.; Kayumov, L.; Tchmoutina, E.A.; Shapiro, C.M. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009, 10, 549–555. [Google Scholar] [CrossRef]
- Wyatt, J.K.; Stepanski, E.J.; Kirkby, J. Circadian phase in delayed sleep phase syndrome: Predictors and temporal stability across multiple assessments. Sleep 2006, 29, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Sletten, T.L.; Magee, M.; Gordon, C.; Lovato, N.; Bartlett, D.J.; Kennaway, D.J.; Lack, L.C.; Grunstein, R.R.; Lockley, S.W.; et al. Prevalence of Circadian Misalignment and Its Association With Depressive Symptoms in Delayed Sleep Phase Disorder. Sleep 2017, 40, zsw002. [Google Scholar] [CrossRef]
- Duffy, J.F.; Abbott, S.M.; Burgess, H.J.; Crowley, S.J.; Emens, J.S.; Epstein, L.J.; Gamble, K.L.; Hasler, B.P.; Kristo, D.A.; Malkani, R.G.; et al. Workshop report. Circadian rhythm sleep-wake disorders: Gaps and opportunities. Sleep 2021, 44, zsaa281. [Google Scholar] [CrossRef] [PubMed]
- Pullman, R.E.; Roepke, S.E.; Duffy, J.F. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Med. 2012, 13, 703–706. [Google Scholar] [CrossRef]
- Sletten, T.L.; Vincenzi, S.; Redman, J.R.; Lockley, S.W.; Rajaratnam, S.M. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front. Neurol. 2010, 1, 137. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.; Rajaratnam, S.M.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef]
- Jasser, S.A.; Hanifin, J.P.; Rollag, M.D.; Brainard, G.C. Dim light adaptation attenuates acute melatonin suppression in humans. J. Biol. Rhythm. 2006, 21, 394–404. [Google Scholar] [CrossRef]
- Chang, A.M.; Scheer, F.A.; Czeisler, C.A.; Aeschbach, D. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep 2013, 36, 1239–1246. [Google Scholar] [CrossRef]
- Smith, K.A.; Schoen, M.W.; Czeisler, C.A. Adaptation of human pineal melatonin suppression by recent photic history. J. Clin. Endocrinol. Metab. 2004, 89, 3610–3614. [Google Scholar] [CrossRef]
- Duffy, J.F.; Dijk, D.J.; Hall, E.F.; Czeisler, C.A. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 1999, 47, 141–150. [Google Scholar]
- Duffy, J.F.; Dijk, D.J. Getting through to circadian oscillators: Why use constant routines? J. Biol. Rhythm. 2002, 17, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kawinska, A.; Dumont, M.; Selmaoui, B.; Paquet, J.; Carrier, J. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J. Biol. Rhythm. 2005, 20, 451–460. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; Gronfier, C.; Duffy, J.F.; Czeisler, C.A. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythm. 2005, 20, 168–177. [Google Scholar] [CrossRef]
- Kennaway, D.J. The Dim Light Melatonin Onset (DLMO) across ages, methodologies and sex and its relationship with Morningness/Eveningness. Sleep 2023, 46, zsad033. [Google Scholar] [CrossRef]
- Burns, A.C.; Phillips, A.J.K.; Rutter, M.K.; Saxena, R.; Cain, S.W.; Lane, J.M. Genome-wide gene by environment study of time spent in daylight and chronotype identifies emerging genetic architecture underlying light sensitivity. Sleep 2023, 46, zsac287. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.; Silva, E.J.; Munch, M.; Ronda, J.M.; Wang, W.; Duffy, J.F. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J. Sleep Res. 2009, 18, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Wang, W.; Ronda, J.M.; Czeisler, C.A. High dose melatonin increases sleep duration during nighttime and daytime sleep episodes in older adults. J. Pineal. Res. 2022, 73, e12801. [Google Scholar] [CrossRef]
- Duffy, J.F.; Willson, H.J.; Wang, W.; Czeisler, C.A. Healthy older adults better tolerate sleep deprivation than young adults. J. Am. Geriatr. Soc. 2009, 57, 1245–1251. [Google Scholar] [CrossRef]
- Duffy, J.F.; Zeitzer, J.M.; Czeisler, C.A. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol. Aging 2007, 28, 799–807. [Google Scholar] [CrossRef]
- Amira, S.A.; Bressler, B.L.; Lee, J.H.; Czeisler, C.A.; Duffy, J.F. Psychological Screening for Exceptional Environments: Laboratory Circadian Rhythm and Sleep Research. Clocks Sleep 2020, 2, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrona-Palacios, A.; Lee, J.-H.; Czeisler, C.A.; Duffy, J.F. The Timing of the Melatonin Onset and Phase Angle to Sleep Onset in Older Adults after Uncontrolled vs. Controlled Lighting Conditions. Clocks & Sleep 2023, 5, 350-357. https://doi.org/10.3390/clockssleep5030026
Arrona-Palacios A, Lee J-H, Czeisler CA, Duffy JF. The Timing of the Melatonin Onset and Phase Angle to Sleep Onset in Older Adults after Uncontrolled vs. Controlled Lighting Conditions. Clocks & Sleep. 2023; 5(3):350-357. https://doi.org/10.3390/clockssleep5030026
Chicago/Turabian StyleArrona-Palacios, Arturo, Jung-Hie Lee, Charles A. Czeisler, and Jeanne F. Duffy. 2023. "The Timing of the Melatonin Onset and Phase Angle to Sleep Onset in Older Adults after Uncontrolled vs. Controlled Lighting Conditions" Clocks & Sleep 5, no. 3: 350-357. https://doi.org/10.3390/clockssleep5030026
APA StyleArrona-Palacios, A., Lee, J.-H., Czeisler, C. A., & Duffy, J. F. (2023). The Timing of the Melatonin Onset and Phase Angle to Sleep Onset in Older Adults after Uncontrolled vs. Controlled Lighting Conditions. Clocks & Sleep, 5(3), 350-357. https://doi.org/10.3390/clockssleep5030026






