The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP
Abstract
1. Introduction
2. Results
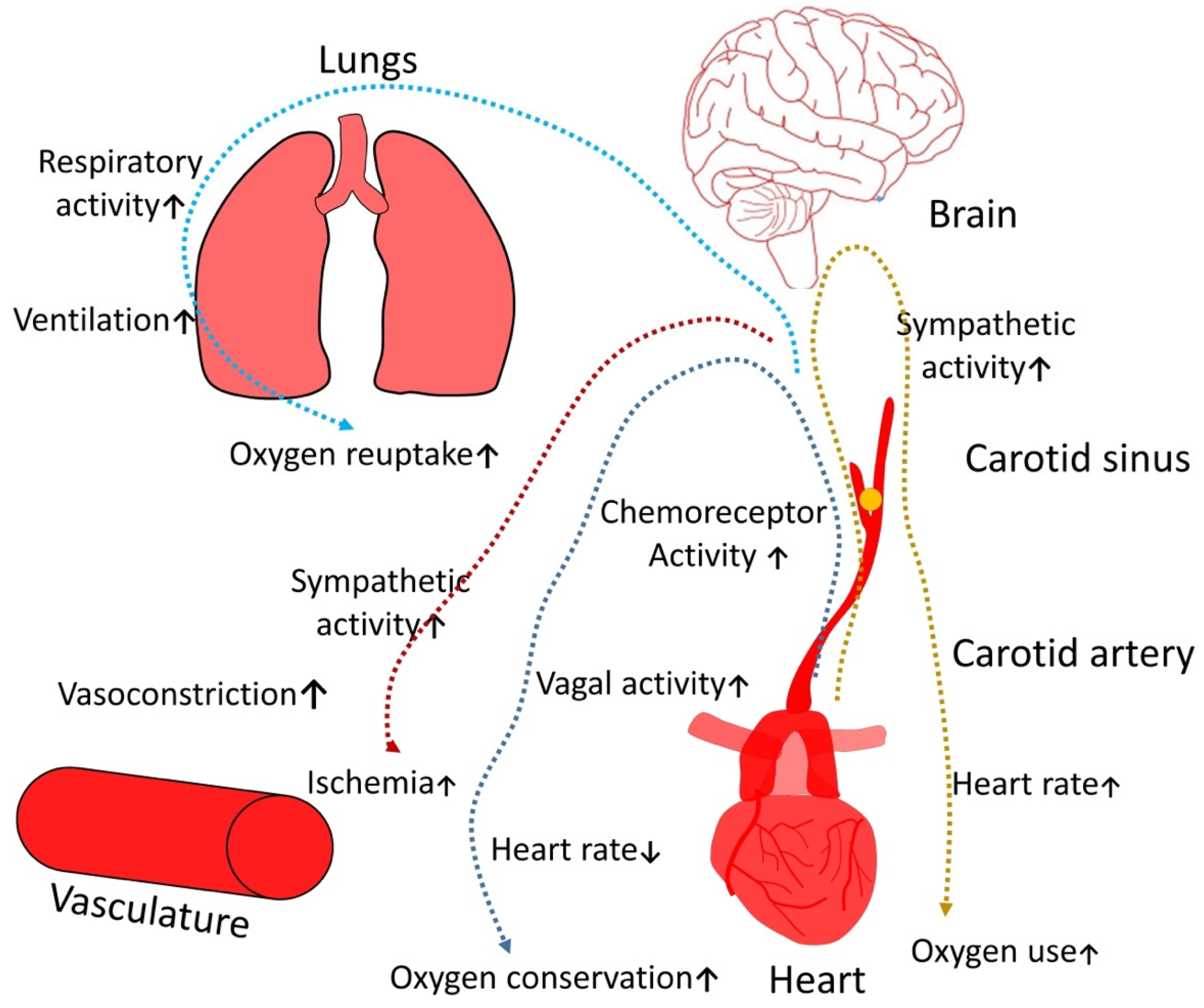
2.1. Cardiovascular Diseases
2.2. Hypertension
2.3. Myocardial Ischemia
2.4. Cardiac Rhythm Disorders
2.5. Neurophyschiatric Deviations
2.6. Cerebrovascular Pathology
2.7. Peripheral Neuropathy
2.8. Depression
2.9. Obesity
2.10. Gastrointestinal Disease
2.11. Nonalcoholic Fatty Liver Disease
2.12. Diabetes Mellitus
2.13. Chronic Kidney Disease
2.14. Malignant Neoplasms
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pack, A.I. Advances in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2006, 173, 7–15. [Google Scholar] [CrossRef]
- Corlateanu, A.; Covantev, S.; Botnaru, V.; Sircu, V.; Nenna, R. To sleep, or not to sleep—That is the question, for polysomnography. Breathe 2017, 13, 137–140. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Lombardi, C.; Hedner, J.; Bonsignore, M.R.; Grote, L.; Tkacova, R.; Lévy, P.; Riha, R.; Bassetti, C.; Narkiewicz, K.; et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur. Respir. J. 2013, 41, 523–538. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Liao, W.-C.; Chung, W.-S.; Muo, C.-H.; Chu, C.-C.; Liu, C.-J.; Kao, C.-H. Association between obstructive sleep apnea and deep vein thrombosis/pulmonary embolism: A population-based retrospective cohort study. Thromb. Res. 2014, 134, 340–345. [Google Scholar] [CrossRef]
- Emiley, P. Sleep Apnea and Risk of Deep Vein Thrombosis: A Non-randomized, Pair-matched Cohort Study: Chou KT, Huang CC, Chen YM, et al. Am J Med 2012;125:374–80. J. Emerg. Med. 2012, 43, 208. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Kukes, V.G.; Corlateanu, A. Coenzyme Q10 in COPD: An Unexplored Opportunity? COPD 2021, 18, 114–122. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.M.; Gersh, B.J.; Somers, V.K. Obstructive Sleep ApneaImplications for Cardiac and Vascular Disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C.; DeBehnke, R.D.; Lovoi, M.S.; Gorin, A.B. Undiagnosed sleep apnea in patients with essential hypertension. Ann. Intern. Med. 1985, 103, 190–195. [Google Scholar] [CrossRef]
- The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA Intern. Med. 1997, 157, 2413–2446. [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.J.T.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2571. [Google Scholar] [CrossRef]
- Whelton, P.K.; Williams, B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: More Similar Than Different Comparison of the 2018 ESC/ESH and 2017 ACC/AHA Hypertension Guidelines Comparison of the 2018 ESC/ESH and 2017 ACC/AHA Hypertension Guidelines. JAMA 2018, 320, 1749–1750. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Kato, M.; Phillips Bradley, G.; Pesek Catherine, A.; Davison Diane, E.; Somers Virend, K. Nocturnal Continuous Positive Airway Pressure Decreases Daytime Sympathetic Traffic in Obstructive Sleep Apnea. Circulation 1999, 100, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Marin-Oto, M.; Vicente, E.E.; Marin, J.M. Long term management of obstructive sleep apnea and its comorbidities. Multidiscip. Respir. Med. 2019, 14, 21. [Google Scholar] [CrossRef]
- Rizza, S.; Longo, S.; Piciucchi, G.; Romanello, D.; Mavilio, M.; Montagna, M.; Coppeta, L.; Martelli, E.; Magrini, A.; Federici, M. Carotid intimal medial thickness in rotating night shift is related to IL1β/IL6 axis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered Breathing and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef]
- Hanly, P.; Sasson, Z.; Zuberi, N.; Lunn, K. ST-segment depression during sleep in obstructive sleep apnea. Am. J. Cardiol. 1993, 71, 1341–1345. [Google Scholar] [CrossRef]
- Franklin, K.A.; Sahlin, C.; Nilsson, J.B.; Näslund, U. Sleep apnoea and nocturnal angina. Lancet 1995, 345, 1085–1087. [Google Scholar] [CrossRef]
- Olafiranye, O.; Reis, S.; Strollo, P.J., Jr. Sleep Apnea and Subclinical Myocardial Injury: Where Do We Stand? Am. J. Respir. Crit. Care Med. 2013, 188, 1394–1395. [Google Scholar] [CrossRef]
- Porto, F.; Sakamoto, Y.S.; Salles, C. Association between Obstructive Sleep Apnea and Myocardial Infarction: A Systematic Review. Arq. Bras. Cardiol. 2017, 108, 361–369. [Google Scholar] [CrossRef]
- Gottlieb Daniel, J.; Yenokyan, G.; Newman Anne, B.; O’Connor George, T.; Punjabi Naresh, M.; Quan Stuart, F.; Redline, S.; Resnick Helaine, E.; Tong Elisa, K.; Diener-West, M.; et al. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Hedner, J.; Kraiczi, H.; Löth, S. Respiratory Disturbance Index. Am. J. Respir. Crit. Care Med. 2000, 162, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hersi, A.S. Obstructive sleep apnea and cardiac arrhythmias. Ann. Thorac. Med. 2010, 5, 10–17. [Google Scholar] [CrossRef]
- Becker, H.F.; Koehler, U.; Stammnitz, A.; Peter, J. Heart block in patients with sleep apnoea. Thorax 1998, 53 (Suppl. S3), S29–S32. [Google Scholar] [CrossRef]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2013, 41, 1439–1451. [Google Scholar] [CrossRef]
- Hoffstein, V.; Mateika, S. Cardiac arrhythmias, snoring, and sleep apnea. Chest 1994, 106, 466–471. [Google Scholar] [CrossRef]
- Djonlagic, I.; Guo, M.; Matteis, P.; Carusona, A.; Stickgold, R.; Malhotra, A. Untreated sleep-disordered breathing: Links to aging-related decline in sleep-dependent memory consolidation. PLoS ONE 2014, 9, e85918. [Google Scholar] [CrossRef] [PubMed]
- Emamian, F.; Khazaie, H.; Tahmasian, M.; Leschziner, G.D.; Morrell, M.J.; Hsiung, G.-Y.R.; Rosenzweig, I.; Sepehry, A.A. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front. Aging Neurosci. 2016, 8, 78. [Google Scholar] [CrossRef]
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, S255–S270. [Google Scholar] [CrossRef]
- Yeh, N.C.; Tien, K.J.; Yang, C.M.; Wang, J.J.; Weng, S.F. Increased Risk of Parkinson’s Disease in Patients with Obstructive Sleep Apnea: A Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine 2016, 95, e2293. [Google Scholar] [CrossRef] [PubMed]
- Good, D.C.; Henkle, J.Q.; Gelber, D.; Welsh, J.; Verhulst, S. Sleep-Disordered Breathing and Poor Functional Outcome After Stroke. Stroke 1996, 27, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.; Werner, P.; Jochums, I.; Lehmann, M.; Strohl, K.P. Blood Flow of the Middle Cerebral Artery with Sleep-Disordered Breathing. Stroke 1998, 29, 87–93. [Google Scholar] [CrossRef]
- Alexiev, F.; Brill, A.-K.; Ott, S.R.; Duss, S.; Schmidt, M.; Bassetti, C.L. Sleep-disordered breathing and stroke: Chicken or egg? J. Thorac. Dis. 2018, 10, S4244–S4252. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Jiang, Z.H.; Zhang, L.G. Therapeutic effects of long-term continuous positive airway pressure treatment on improving leptomeningeal collateral circulation in obstructive sleep apnea syndrome patients. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4261–4267. [Google Scholar] [CrossRef]
- Ifergane, G.; Ovanyan, A.; Toledano, R.; Goldbart, A.; Abu-Salame, I.; Tal, A.; Stavsky, M.; Novack, V. Obstructive Sleep Apnea in Acute Stroke. Stroke 2016, 47, 1207–1212. [Google Scholar] [CrossRef]
- Tosun, A.; Köktürk, O.; Karata, G.K.; Çiftçi, T.U.; Sepici, V. Obstructive sleep apnea in ischemic stroke patients. Clinics 2008, 63, 625–630. [Google Scholar] [CrossRef]
- Sharma, S.; Culebras, A. Sleep apnoea and stroke. Stroke Vasc. Neurol. 2016, 1, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Odajiu, I.; Covantsev, S.; Sivapalan, P.; Mathioudakis, A.G.; Jensen, J.S.; Davidescu, E.I.; Chatzimavridou-Grigoriadou, V.; Corlateanu, A. Peripheral neuropathy: A neglected cause of disability in COPD—A narrative review. Respir. Med. 2022, 201, 106952. [Google Scholar] [CrossRef]
- Mayer, P.; Dematteis, M.; Pépin, J.L.; Wuyam, B.; Veale, D.; Vila, A.; Lévy, P. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am. J. Respir. Crit. Care Med. 1999, 159, 213–219. [Google Scholar] [CrossRef]
- Lüdemann, P.; Dziewas, R.; Sörös, P.; Happe, S.; Frese, A. Axonal polyneuropathy in obstructive sleep apnoea. J. Neurol. Neurosurg. Psychiatry 2001, 70, 685–687. [Google Scholar] [CrossRef]
- Altintas, N.; Tutar, U.; Sariaydin, M.; Tiras, R. A novel connection: Obstructive sleep apnoea and diabetic neuropathy. Eur. Respir. J. 2013, 42, P2539. [Google Scholar]
- Dziewas, R.; Schilling, M.; Engel, P.; Boentert, M.; Hor, H.; Okegwo, A.; Lüdemann, P.; Ringelstein, E.B.; Young, P. Treatment for obstructive sleep apnoea: Effect on peripheral nerve function. J. Neurol. Neurosurg. Psychiatry 2007, 78 (Suppl. S57), 295–297. [Google Scholar] [CrossRef]
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innov. Clin. Neurosci. 2011, 8, 17–25. [Google Scholar]
- Shoib, S.; Malik, J.A.; Masoodi, S. Depression as a Manifestation of Obstructive Sleep Apnea. J. Neurosci. Rural Pr. 2017, 8, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.A.; Tomlinson, G.A.; Leung, R.S. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: A historical cohort study. Eur. Respir. J. 2017, 49, 1601361. [Google Scholar] [CrossRef] [PubMed]
- Aloia, M.S.; Arnedt, J.T.; Smith, L.; Skrekas, J.; Stanchina, M.; Millman, R.P. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005, 6, 115–121. [Google Scholar] [CrossRef]
- Schröder, C.M.; O’Hara, R. Depression and Obstructive Sleep Apnea (OSA). Ann. Gen. Psychiatry 2005, 4, 13. [Google Scholar] [CrossRef]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [PubMed]
- Baron, K.G.; Reid, K.J.; Kim, T.; Van Horn, L.; Attarian, H.; Wolfe, L.; Siddique, J.; Santostasi, G.; Zee, P.C. Circadian timing and alignment in healthy adults: Associations with BMI, body fat, caloric intake and physical activity. Int. J. Obes. 2017, 41, 203–209. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Tan, M.M.C.; Jin, X.; Taylor, C.; Low, A.K.; Le Page, P.; Martin, D.; Li, A.; Joseph, D.; Kormas, N. Long-Term Trajectories in Weight and Health Outcomes Following Multidisciplinary Publicly Funded Bariatric Surgery in Patients with Clinically Severe Obesity (≥3 Associated Comorbidities): A Nine-Year Prospective Cohort Study in Australia. J. Clin. Med. 2022, 11, 4466. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Khanijow, V.; Prakash, P.; Emsellem, H.A.; Borum, M.L.; Doman, D.B. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol. Hepatol. 2015, 11, 817–825. [Google Scholar]
- Li, Q.; Xu, T.; Shao, C.; Gao, W.; Wang, M.; Dong, Y.; Wang, X.; Lu, F.; Li, D.; Tan, H.; et al. Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function. Sci. Rep. 2023, 13, 778. [Google Scholar] [CrossRef]
- Koo, D.L.; Nam, H. The Relationship between Obstructive Sleep Apnea and Functional Dyspepsia. J. Sleep Med. 2020, 17, 73–77. [Google Scholar] [CrossRef]
- Hyun, M.K.; Baek, Y.; Lee, S. Association between digestive symptoms and sleep disturbance: A cross-sectional community-based study. BMC Gastroenterol. 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, F.; Amra, B.; Sebghatollahi, V.; Azimian, F. Association of irritable bowel syndrome and sleep apnea in patients referred to sleep laboratory. J. Res. Med. Sci. 2017, 22, 72. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Petta, S.; Marrone, O.; Torres, D.; Buttacavoli, M.; Cammà, C.; Di Marco, V.; Licata, A.; Lo Bue, A.; Parrinello, G.; Pinto, A.; et al. Obstructive Sleep Apnea Is Associated with Liver Damage and Atherosclerosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0142210. [Google Scholar] [CrossRef]
- Hirono, H.; Watanabe, K.; Hasegawa, K.; Kohno, M.; Terai, S.; Ohkoshi, S. Impact of continuous positive airway pressure therapy for nonalcoholic fatty liver disease in patients with obstructive sleep apnea. World J. Clin. Cases 2021, 9, 5112–5125. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Lin, L.; Zhang, L.J.; Zeng, H.X.; Wu, Q.Y.; Hu, M.F.; Xie, J.J.; Liu, J.N. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin. Respir. J. 2018, 12, 373–381. [Google Scholar] [CrossRef]
- Hobzova, M.; Ludka, O.; Stepanova, R.; Sova, M.; Sovova, E. The positive effect of CPAP therapy on the level of liver enzymes in obstructive sleep apnea patients. Eur. Respir. J. 2015, 46 (Suppl. S59), PA2332. [Google Scholar] [CrossRef]
- Einhorn, D.; Stewart, D.A.; Erman, M.K.; Gordon, N.; Philis-Tsimikas, A.; Casal, E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr. Pract. 2007, 13, 355–362. [Google Scholar] [CrossRef] [PubMed]
- West, S.D.; Nicoll, D.J.; Stradling, J.R. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006, 61, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Luzi, A.; Mavilio, M.; Ballanti, M.; Massimi, A.; Porzio, O.; Magrini, A.; Hannemann, J.; Menghini, R.; Lehrke, M.; et al. Alterations in Rev-ERBα/BMAL1 ratio and glycated hemoglobin in rotating shift workers: The EuRhythDia study. Acta Diabetol. 2021, 58, 1111–1117. [Google Scholar] [CrossRef]
- Koren, D.; Levitt Katz, L.E.; Brar, P.C.; Gallagher, P.R.; Berkowitz, R.I.; Brooks, L.J. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care 2011, 34, 2442–2447. [Google Scholar] [CrossRef]
- Leproult, R.; Copinschi, G.; Buxton, O.; Van Cauter, E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997, 20, 865–870. [Google Scholar]
- Kelly, A.; Dougherty, S.; Cucchiara, A.; Marcus, C.L.; Brooks, L.J. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep 2010, 33, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Steiropoulos, P.; Nena, E.; Tzouvelekis, A.; Maltezos, E.; Trakada, G.; Bouros, D. HbA1c is associated with severity of obstructive sleep apnea hypopnea syndrome in nondiabetic men. Vasc. Health Risk Manag. 2009, 5, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Tatti, P.; Strollo, F.; Passali, D. Sleep apnea, sleep disturbance, and fasting glucose variability: A pilot study. J. Diabetes Sci. Technol. 2013, 7, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Yagihara, F.; Lucchesi, L.M.; D’Almeida, V.; de Mello, M.T.; Tufik, S.; Bittencourt, L.R.A. Oxidative stress and quality of life in elderly patients with obstructive sleep apnea syndrome: Are there differences after six months of Continuous Positive Airway Pressure treatment? Clinics 2012, 67, 565–572. [Google Scholar] [CrossRef]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative stress in obstructive sleep apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.; Lam, B.; Yao, T.J.; Lai, A.Y.; Ooi, C.G.; Tam, S.; Lam, K.S.; Ip, M.S. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 138–145. [Google Scholar] [CrossRef]
- Guest, J.F.; Panca, M.; Sladkevicius, E.; Taheri, S.; Stradling, J. Clinical Outcomes and Cost-effectiveness of Continuous Positive Airway Pressure to Manage Obstructive Sleep Apnea in Patients with Type 2 Diabetes in the U.K. Diabetes Care 2014, 37, 1263–1271. [Google Scholar] [CrossRef]
- Xu, P.H.; Hui, C.K.M.; Lui, M.M.S.; Lam, D.C.L.; Fong, D.Y.T.; Ip, M.S.M. Incident Type 2 Diabetes in OSA and Effect of CPAP Treatment: A Retrospective Clinic Cohort Study. Chest 2019, 156, 743–753. [Google Scholar] [CrossRef]
- West, S.D.; Nicoll, D.J.; Wallace, T.M.; Matthews, D.R.; Stradling, J.R. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007, 62, 969–974. [Google Scholar] [CrossRef]
- Nicholl, D.D.M.; Ahmed, S.B.; Loewen, A.H.S.; Hemmelgarn, B.R.; Sola, D.Y.; Beecroft, J.M.; Turin, T.C.; Hanly, P.J. Declining Kidney Function Increases the Prevalence of Sleep Apnea and Nocturnal Hypoxia. Chest 2012, 141, 1422–1430. [Google Scholar] [CrossRef]
- Hanly, P.J. Consider the Kidney when Managing Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 845–846. [Google Scholar] [CrossRef]
- Lin, C.H.; Perger, E.; Lyons, O.D. Obstructive sleep apnea and chronic kidney disease. Curr. Opin. Pulm. Med. 2018, 24, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Marrone, O.; Bonsignore, M.R. Sleep Apnea and the Kidney. Curr. Sleep Med. Rep. 2020, 6, 85–93. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lurie, R.C.; Lyons, O.D. Sleep Apnea and Chronic Kidney Disease: A State-of-the-Art Review. Chest 2020, 157, 673–685. [Google Scholar] [CrossRef]
- Zamarrón, E.; Jaureguizar, A.; García-Sánchez, A.; Díaz-Cambriles, T.; Alonso-Fernández, A.; Lores, V.; Mediano, O.; Rodríguez-Rodríguez, P.; Cabello-Pelegrín, S.; Morales-Ruíz, E.; et al. Obstructive sleep apnea is associated with impaired renal function in patients with diabetic kidney disease. Sci. Rep. 2021, 11, 5675. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Murata, M.; Honma, S.; Iwazu, Y.; Sasaki, N.; Ogura, M.; Onishi, A.; Ando, Y.; Muto, S.; Shimada, K.; et al. Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2289–2295. [Google Scholar] [CrossRef]
- Ozkok, A.; Kanbay, A.; Odabas, A.R.; Covic, A.; Kanbay, M. Obstructive sleep apnea syndrome and chronic kidney disease: A new cardiorenal risk factor. Clin. Exp. Hypertens. 2014, 36, 211–216. [Google Scholar] [CrossRef]
- Wingfield Digby, J.; Mathioudakis, A.; Heartshorne, R.; Mohammad, M.; Tewkesbury, D.; Needham, M. CPAP appears to protect kidney function of patients with OSA. Eur. Respir. J. 2017, 50 (Suppl. S61), OA3210. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Raneri, J.K.; Ahmed, S.B.; Hirsch Allen, A.J.M.; Nocon, A.; Gomes, T.; Gakwaya, S.; Series, F.; Kimoff, J.; Skomro, R.P.; et al. Risk of chronic kidney disease in patients with obstructive sleep apnea. Sleep 2021, 45, zsab267. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Song, P.; Hang, K.; Chen, Z.; Zhu, Z.; Zhang, Y.; Xu, J.; Qin, J.; Wang, B.; Qu, W.; et al. Sleep Deprivation Disturbs Immune Surveillance and Promotes the Progression of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 727959. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.; Wang, Y.; Zhang, S.X.L.; Zheng, J.; Yolcu, E.S.; Carreras, A.; Khalyfa, A.; Shirwan, H.; Almendros, I.; Gozal, D. Fragmented Sleep Accelerates Tumor Growth and Progression through Recruitment of Tumor-Associated Macrophages and TLR4 Signaling. Cancer Res. 2014, 74, 1329–1337. [Google Scholar] [CrossRef]
- Lee, D.-B.; An, S.-Y.; Pyo, S.-S.; Kim, J.; Kim, S.-W.; Yoon, D.-W. Sleep Fragmentation Accelerates Carcinogenesis in a Chemical-Induced Colon Cancer Model. Int. J. Mol. Sci. 2023, 24, 4547. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Gaustad, J.-V.; Egeland, T.A.M.; Mathiesen, B.; Galappathi, K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int. J. Cancer 2010, 127, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Almendros, I.; Hakim, F. Sleep apnea awakens cancer. OncoImmunology 2014, 3, e28326. [Google Scholar] [CrossRef]
- Kontogianni, K.; Messini-Nikolaki, N.; Christou, K.; Gourgoulianis, K.; Tsilimigaki, S.; Piperakis, S.M. DNA damage and repair capacity in lymphocytes from obstructive sleep apnea patients. Environ. Mol. Mutagen. 2007, 48, 722–727. [Google Scholar] [CrossRef]
- McElroy, J.A.; Newcomb, P.A.; Titus-Ernstoff, L.; Trentham-Dietz, A.; Hampton, J.M.; Egan, K.M. Duration of sleep and breast cancer risk in a large population-based case–control study. J. Sleep Res. 2006, 15, 241–249. [Google Scholar] [CrossRef]
- Luo, J.; Sands, M.; Wactawski-Wende, J.; Song, Y.; Margolis, K.L. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am. J. Epidemiol. 2013, 177, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farré, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Martinez, M.; Duran-Cantolla, J.; Peña Mde, L.; Masdeu, M.J.; Gonzalez, M.; Campo, F.; Gallego, I.; Marin, J.M.; et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef]
- Chen, J.C.; Hwang, J.H. Sleep apnea increased incidence of primary central nervous system cancers: A nationwide cohort study. Sleep Med. 2014, 15, 749–754. [Google Scholar] [CrossRef]
- Kendzerska, T.; Povitz, M.; Leung, R.S.; Boulos, M.I.; McIsaac, D.I.; Murray, B.J.; Bryson, G.L.; Talarico, R.; Hilton, J.F.; Malhotra, A.; et al. Obstructive Sleep Apnea and Incident Cancer: A Large Retrospective Multicenter Clinical Cohort Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P.; Liu, M.E.; Chang, W.C.; Yang, A.C.; Ku, Y.C.; Pai, J.T.; Lin, Y.W.; Tsai, S.J. Sleep apnea and the subsequent risk of breast cancer in women: A nationwide population-based cohort study. Sleep Med. 2014, 15, 1016–1020. [Google Scholar] [CrossRef]
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018, 29, 987–994. [Google Scholar] [CrossRef]
- Gozal, D.; Ham, S.A.; Mokhlesi, B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep 2016, 39, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hu, J.M.; Shen, C.J.; Chou, Y.C.; Tian, Y.F.; Chen, Y.C.; You, S.L.; Hung, C.F.; Lin, T.C.; Hsiao, C.W.; et al. Increased incidence of colorectal cancer with obstructive sleep apnea: A nationwide population-based cohort study. Sleep Med. 2020, 66, 15–20. [Google Scholar] [CrossRef] [PubMed]


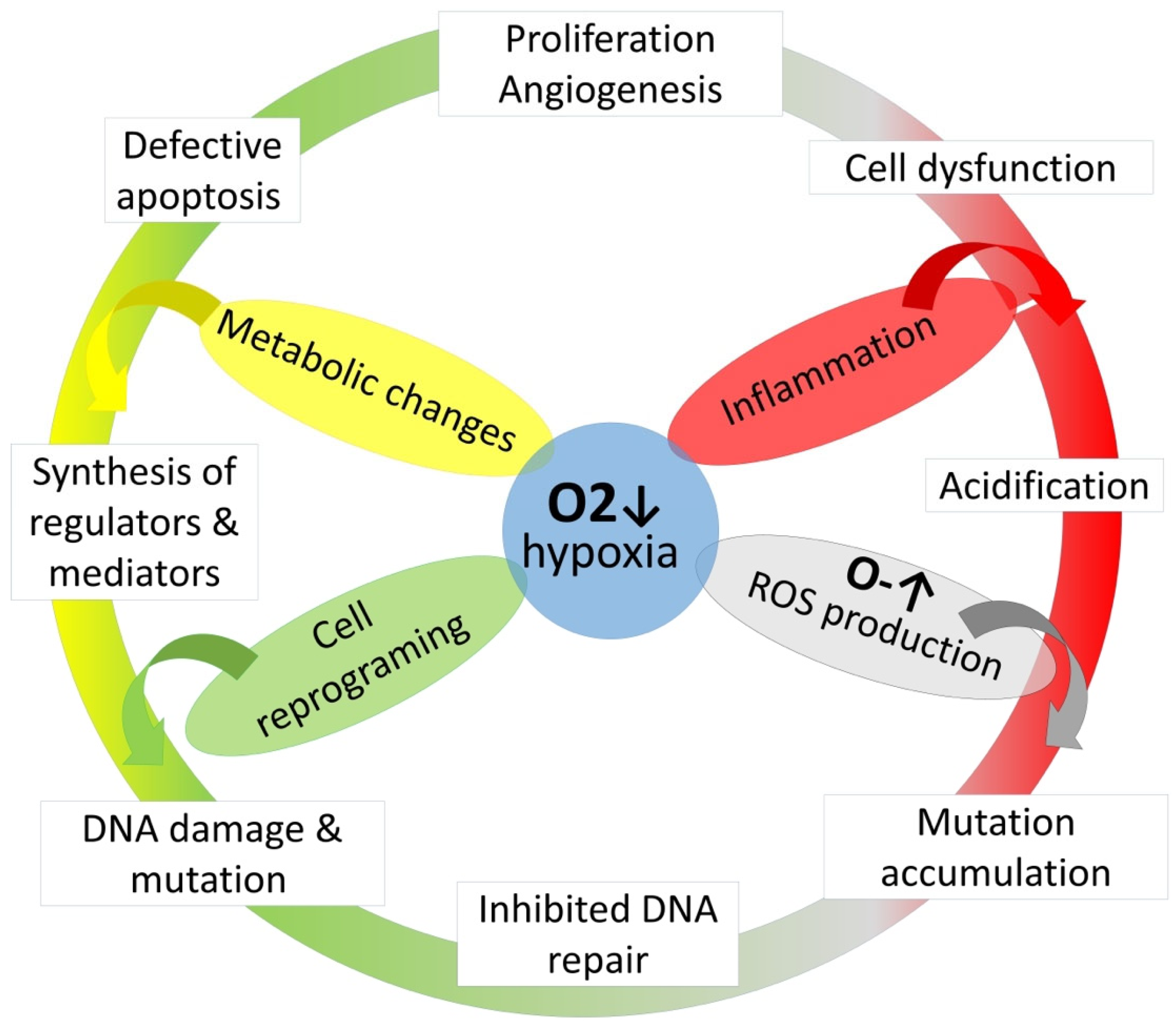
| Malignant Neoplasia | Possible Risk |
|---|---|
| CNS neoplasms | The overall risk for developing primary CNS cancers was found to be significantly higher in the OSAS group (aHR, 1.54; p = 0.046) after adjusting for age, gender, and obesity among other variables. Subgroup analysis revealed a significantly higher risk for primary brain cancers but not for primary spinal cord cancers [101]. |
| Lung cancer | The data on lung cancer differs from study to study. Kendzerska and coworkers have reported a higher risk of developing lung cancer in a subgroup of OSAS patients with AHI Q4 vs. Q1 (1.78 [1.03–3.10] [102]. Sillah and coworkers have reported a protective effect of OSAS on the lungs (SIR 0.66, 95% CI 0.54–0.79) [104]. |
| Melanoma | The risk of melanoma tends to increase with more severe AHI 2.49 (1.03–6.05) AHI: Q4 vs. Q1 [102]. Other studies have also demonstrated an increased risk of melanoma (HR = 1.13, CI = 1.09–1.18 and SIR 1.71, 95% CI 1.42–2.03) [105]. |
| Breast cancer | The aHR of breast cancer in patients with OSAS was found to be higher [HR, 2.09; 95% confidence interval (CI), 1.06–4.12; p < 0.05] than that of the controls during a 5-year follow-up. Despite not meeting statistical significance, the authors reported an increase in the risk of breast cancer in women aged 30–59 years (HR, 2.06; 95% CI, 0.90–4.70) and ≥60 years (HR, 3.05; 95% CI, 0.90–10.32) compared with those aged 0–29 years [103]. |
| Colorectal cancer | Patients with OSAS tend to have a higher risk of colorectal cancer (1.63 [1.12–2.38]) [102]. Another study has demonstrated similar results: after adjusting for potential confounders, patients with OSAS were associated with a significantly higher risk than those without OSAS (aHR, 1.80; 95% CI, 1.28–2.52). Moreover, the cumulative incidence of colorectal cancer was significantly higher in the OSAS cohort than in the comparison cohort [106].Nevertheless, several other studies have demonstrated a decreased risk of colorectal cancer [104]. |
| Pancreatic cancer | Patients with OSAS tend to have an increased risk of pancreatic cancer (HR = 1.14, CI = 1.06–1.23) [106]. |
| Kidney cancer | The risk of kidney cancer is debatable. Kendzerska and coworkers found no association between kidney cancer and OSAS [102]. Other studies have demonstrated an increased risk (HR = 1.30, CI = 1.23–1.37; SIR 2.24, 95% CI 1.82–2.72) [105]. |
| Prostate cancer | One of the studies has demonstrated an increased risk of prostate cancer 1.63 (1.06–2.51) [102] while another demonstrated a protective effect (HR = 0.93, CI = 0.90–0.96 in both) [105]. |
| Urinary cancer | Severe OSAS tends to increase urinary cancer 1.72 (1.08–2.75) [102]. |
| Uterus | Uterus cancer is more frequent in OSAS patients (SIR 2.80, 95% CI 2.24–2.47) [104,105]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sircu, V.; Colesnic, S.-I.; Covantsev, S.; Corlateanu, O.; Sukhotko, A.; Popovici, C.; Corlateanu, A. The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP. Clocks & Sleep 2023, 5, 333-349. https://doi.org/10.3390/clockssleep5020025
Sircu V, Colesnic S-I, Covantsev S, Corlateanu O, Sukhotko A, Popovici C, Corlateanu A. The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP. Clocks & Sleep. 2023; 5(2):333-349. https://doi.org/10.3390/clockssleep5020025
Chicago/Turabian StyleSircu, Victoria, Silvia-Iaroslava Colesnic, Serghei Covantsev, Olga Corlateanu, Anna Sukhotko, Cristian Popovici, and Alexandru Corlateanu. 2023. "The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP" Clocks & Sleep 5, no. 2: 333-349. https://doi.org/10.3390/clockssleep5020025
APA StyleSircu, V., Colesnic, S.-I., Covantsev, S., Corlateanu, O., Sukhotko, A., Popovici, C., & Corlateanu, A. (2023). The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP. Clocks & Sleep, 5(2), 333-349. https://doi.org/10.3390/clockssleep5020025







