The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts
Abstract
1. Introduction
2. Results
2.1. Sleep Timing between Shifts and Type of Day
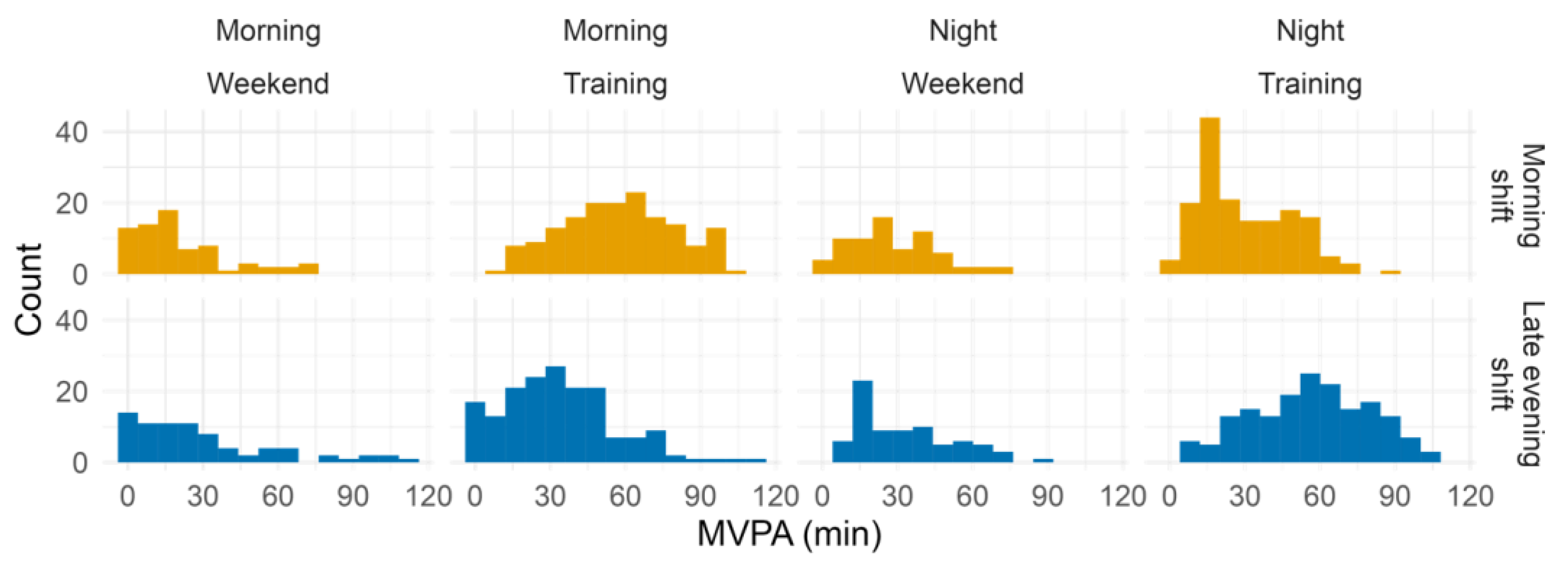
2.2. Training Shifts Involve Differences in the Timing of Light Exposure and Physical Activity
2.3. Sleep Pattern Is Influenced by Several Factors Related with Training in Different Shifts
3. Discussion
3.1. What Do Dance-Training Shifts Entail?
3.2. What Factors Shape the Sleep Pattern?
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Sleep Onset | Sleep End | Sleep Duration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b ± SE | t | df | p | b ± SE | t | df | p | b ± SE | t | df | p | |
| Intercept | 0.7 ± 0.4 | 2.0 | 199.8 | 0.052 | 9.6 ± 0.4 | 25.3 | 113.4 | <0.001 | 8.8 ± 0.4 | 24.6 | 242.5 | <0.001 |
| Training shift (1 = Late evening) | 1.5 ± 0.3 | 5.2 | 31.4 | <0.001 | 1.3 ± 0.4 | 3.6 | 31.1 | <0.001 | −0.1 ± 0.2 | −0.6 | 30.6 | 0.575 |
| Type of day (1 = Weekend day) | 2.0 ± 0.2 | 12.6 | 461.1 | <0.001 | 1.2 ± 0.1 | 8.5 | 456.4 | <0.001 | −0.8 ± 0.2 | −4.7 | 464.4 | <0.001 |
| Alarm (1 = Yes) | 0.0 ± 0.2 | 0.3 | 476.8 | 0.788 | −1.7 ± 0.1 | −12.3 | 465.7 | <0.001 | −1.7 ± 0.2 | −10.3 | 482.3 | <0.001 |
| Daily illuminance (log10 lux) | −0.3 ± 0.1 | −2.4 | 482.4 | 0.015 | −0.3 ± 0.1 | −3.3 | 471.8 | 0.001 | −0.1 ± 0.1 | −0.5 | 480.0 | 0.652 |
| Daily MVPA (minutes) | 0.0 ± 0.1 | 0.2 | 472.5 | 0.838 | 0.1 ± 0.1 | 1.0 | 481.1 | 0.328 | 0.0 ± 0.1 | 0.2 | 430.0 | 0.854 |
| Pseudo-R2 marginal/conditional | 0.38/0.52 | 0.46/0.69 | 0.18/0.30 | |||||||||
| Sleep Onset | Sleep End | Sleep Duration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b ± SE | t | df | p | b ± SE | t | df | p | b ± SE | t | df | p | |
| Intercept | 0.3 ± 0.2 | 1.5 | 73.9 | 0.144 | 8.9 ± 0.3 | 31.3 | 49.2 | <0.001 | 8.6 ± 0.2 | 36.1 | 90.1 | <0.001 |
| Training shift (1 = Late evening) | 1.0 ± 0.3 | 3.9 | 33.1 | <0.001 | 1.2 ± 0.4 | 3.4 | 32.5 | 0.002 | 0.0 ± 0.3 | 0.2 | 37.4 | 0.866 |
| Type of day (1 = Weekend day) | 1.8 ± 0.2 | 11.5 | 400.7 | <0.001 | 1.1 ± 0.1 | 7.3 | 395.4 | <0.001 | −0.8 ± 0.2 | −4.6 | 404.4 | <0.001 |
| Alarm (1 = Yes) | 0.1 ± 0.2 | 0.7 | 414.9 | 0.489 | −1.7 ± 0.1 | −11.4 | 402.6 | <0.001 | −1.8 ± 0.2 | −10.0 | 418.0 | <0.001 |
| Morning illuminance (log10 lux) | −0.3 ± 0.1 | −4.4 | 420.9 | <0.001 | −0.1 ± 0.1 | −1.2 | 410.8 | 0.218 | 0.2 ± 0.1 | 2.9 | 419.0 | 0.004 |
| Late evening illuminance (log10 lux) | 0.1 ± 0.1 | 1.7 | 421.0 | 0.087 | 0.1 ± 0.1 | 2.0 | 409.4 | 0.051 | 0.0 ± 0.1 | 0.4 | 419.5 | 0.658 |
| Morning MVPA (minutes) | 0.1 ± 0.1 | 0.7 | 410.1 | 0.477 | −0.0 ± 0.1 | −0.2 | 417.2 | 0.816 | −0.0 ± 0.1 | −0.5 | 399.7 | 0.640 |
| Late evening MVPA (minutes) | 0.4 ± 0.1 | 4.7 | 420.7 | <0.001 | 0.1 ± 0.1 | 1.2 | 410.8 | 0.217 | −0.3 ± 0.1 | −3.1 | 418.1 | 0.002 |
| Previous sleep end | 0.3 ± 0.1 | 2.6 | 409.9 | 0.011 | 0.1 ± 0.1 | 1.4 | 417.0 | 0.158 | 0.0 ± 0.1 | 0.3 | 398.8 | 0.745 |
| Pseudo-R2 marginal/conditional | 0.47/0.57 | 0.48/0.69 | 0.21/0.32 | |||||||||
References
- Czeisler, C.A. Duration, Timing and Quality of Sleep Are Each Vital for Health, Performance and Safety. Sleep Health J. Natl. Sleep Found. 2015, 1, 5–8. [Google Scholar] [CrossRef]
- Kocevska, D.; Lysen, T.S.; Dotinga, A.; Koopman-Verhoeff, M.E.; Luijk, M.P.C.M.; Antypa, N.; Biermasz, N.R.; Blokstra, A.; Brug, J.; Burk, W.J.; et al. Sleep Characteristics across the Lifespan in 1.1 Million People from the Netherlands, United Kingdom and United States: A Systematic Review and Meta-Analysis. Nat. Hum. Behav. 2021, 5, 113–122. [Google Scholar] [CrossRef]
- Bowers, J.M.; Moyer, A. Effects of School Start Time on Students’ Sleep Duration, Daytime Sleepiness, and Attendance: A Meta-Analysis. Sleep Health 2017, 3, 423–431. [Google Scholar] [CrossRef]
- Peltzer, K.; Pengpid, S. Sleep Duration and Health Correlates among University Students in 26 Countries. Psychol. Health Med. 2016, 21, 208–220. [Google Scholar] [CrossRef]
- Shochat, T.; Santhi, N.; Herer, P.; Dijk, D.-J.; Skeldon, A.C. Sleepiness Is a Signal to Go to Bed: Data and Model Simulations. Sleep 2021, 44, zsab123. [Google Scholar] [CrossRef]
- Czeisler, C.A. Casting Light on Sleep Deficiency. Nature 2013, 497, S13. [Google Scholar] [CrossRef]
- Prayag, A.S.; Münch, M.; Aeschbach, D.; Chellappa, S.L.; Gronfier, C. Light Modulation of Human Clocks, Wake, and Sleep. Clocks Sleep 2019, 1, 193–208. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kantermann, T.; Juda, M.; Vetter, C.; Allebrandt, K.V. Light and the human circadian clock. In Handbook of Experimental Pharmacology; Kramer, A., Merrow, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 217, pp. 311–331. ISBN 978-3-642-25949-4. [Google Scholar]
- Wright Jr, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef]
- Dunster, G.P.; Hua, I.J.; Grahe, A.; Fleischer, J.G.; Panda, S.; Wright, K.P., Jr.; Vetter, C.; Doherty, J.H.; de la Iglesia, H.O. Daytime Light Exposure Is a Strong Predictor of Seasonal Variation in Sleep and Circadian Timing of University Students. J. Pineal Res. 2022, e12843. [Google Scholar] [CrossRef]
- Boubekri, M.; Cheung, I.N.; Reid, K.J.; Wang, C.-H.; Zee, P.C. Impact of Windows and Daylight Exposure on Overall Health and Sleep Quality of Office Workers: A Case-Control Pilot Study. J. Clin. Sleep Med. 2014, 10, 603–611. [Google Scholar] [CrossRef]
- Estevan, I.; Tassino, B.; Vetter, C.; Silva, A. Bidirectional Association between Light Exposure and Sleep in Adolescents. J. Sleep Res. 2022, 31, e13501. [Google Scholar] [CrossRef]
- Figueiro, M.; Rea, M. Office Lighting and Personal Light Exposures in Two Seasons: Impact on Sleep and Mood. Light. Res. Technol. 2016, 48, 352–364. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and Exercise: A Reciprocal Issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef]
- Kline, C.E.; Hillman, C.H.; Bloodgood Sheppard, B.; Tennant, B.; Conroy, D.E.; Macko, R.F.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; Erickson, K.I. Physical Activity and Sleep: An Updated Umbrella Review of the 2018 Physical Activity Guidelines Advisory Committee Report. Sleep Med. Rev. 2021, 58, 101489. [Google Scholar] [CrossRef]
- Lewis, P.; Korf, H.W.; Kuffer, L.; Groß, J.V.; Erren, T.C. Exercise Time Cues (Zeitgebers) for Human Circadian Systems Can Foster Health and Improve Performance: A Systematic Review. BMJ Open Sport Exerc. Med. 2018, 4, e000443. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kline, C.E.; Elliott, J.A.; Zielinski, M.R.; Devlin, T.M.; Moore, T.A. Circadian Phase-Shifting Effects of Bright Light, Exercise, and Bright Light + Exercise. J. Circadian Rhythm. 2016, 14, 2. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human Circadian Phase–Response Curves for Exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef]
- American Sleep Association Sleep Hygiene Tips, Research & Treatments. Available online: https://www.sleepassociation.org/about-sleep/sleep-hygiene-tips/ (accessed on 16 August 2021).
- Frimpong, E.; Mograss, M.; Zvionow, T.; Dang-Vu, T.T. The Effects of Evening High-Intensity Exercise on Sleep in Healthy Adults: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2021, 60, 101535. [Google Scholar] [CrossRef]
- Stutz, J.; Eiholzer, R.; Spengler, C.M. Effects of Evening Exercise on Sleep in Healthy Participants: A Systematic Review and Meta-Analysis. Sport. Med. 2019, 49, 269–287. [Google Scholar] [CrossRef]
- Estevan, I.; Silva, A.; Vetter, C.; Tassino, B. Short Sleep Duration and Extremely Delayed Chronotypes in Uruguayan Youth: The Role of School Start Times and Social Constraints. J. Biol. Rhythm. 2020, 35, 391–404. [Google Scholar] [CrossRef]
- Malheiros, L.E.A.; da Costa, B.G.G.; Lopes, M.V.V.; Silva, K.S. School Schedule Affect Sleep, but Not Physical Activity, Screen Time and Diet Behaviors. Sleep Med. 2021, 85, 54–59. [Google Scholar] [CrossRef]
- Martin, J.S.; Gaudreault, M.M.; Perron, M.; Laberge, L. Chronotype, Light Exposure, Sleep, and Daytime Functioning in High School Students Attending Morning or Afternoon School Shifts: An Actigraphic Study. J. Biol. Rhythm. 2016, 31, 205–217. [Google Scholar] [CrossRef]
- Grundy, A.; Sanchez, M.; Richardson, H.; Tranmer, J.; Borugian, M.; Graham, C.H.; Aronson, K.J. Light Intensity Exposure, Sleep Duration, Physical Activity, and Biomarkers of Melatonin among Rotating Shift Nurses. Chronobiol. Int. 2009, 26, 1443–1461. [Google Scholar] [CrossRef]
- Papantoniou, K.; Pozo, O.J.; Espinosa, A.; Marcos, J.; Castaño-Vinyals, G.; Basagaña, X.; Ribas, F.C.; Mirabent, J.; Martín, J.; Carenys, G.; et al. Circadian Variation of Melatonin, Light Exposure, and Diurnal Preference in Day and Night Shift Workers of Both Sexes. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1176–1186. [Google Scholar] [CrossRef]
- Coirolo, N.; Silva, A.; Tassino, B. The Impact of Training Shifts in Dancers’ Chronotype and Sleep Patterns. Sleep Sci. 2020, 13, 31–35. [Google Scholar] [CrossRef]
- Tassino, B.; Horta, S.; Santana, N.; Levandovski, R.; Silva, A. Extreme Late Chronotypes and Social Jetlag Challenged by Antarctic Conditions in a Population of University Students from Uruguay. Sleep Sci. 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Arrona-Palacios, A.; García, A.; Valdez, P. Sleep–Wake Habits and Circadian Preference in Mexican Secondary School. Sleep Med. 2015, 16, 1259–1264. [Google Scholar] [CrossRef]
- Crowley, S.J.; Molina, T.A.; Burgess, H.J. A Week in the Life of Full-Time Office Workers: Work Day and Weekend Light Exposure in Summer and Winter. Appl. Ergon. 2015, 46, 193–200. [Google Scholar] [CrossRef]
- Zerbini, G.; Winnebeck, E.C.; Merrow, M. Weekly, Seasonal, and Chronotype-Dependent Variation of Dim-Light Melatonin Onset. J. Pineal Res. 2021, 70, e12723. [Google Scholar] [CrossRef]
- Anacleto, T.S.; Adamowicz, T.; Simões da Costa Pinto, L.; Louzada, F.M. School Schedules Affect Sleep Timing in Children and Contribute to Partial Sleep Deprivation. Mind Brain Educ. 2014, 8, 169–174. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s Updated Sleep Duration Recommendations: Final Report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Kripke, D.; Elliott, J.A.; Youngstedt, S.D.; Rex, K. Circadian Phase Response Curves to Light in Older and Young Women and Men. J. Circadian Rhythm. 2007, 5, 4. [Google Scholar] [CrossRef]
- Coirolo, N.; Casaravilla, C.; Tassino, B.; Silva, A. Evaluation of Environmental, Social, and Behavioral Modulations of the Circadian Phase of Dancers Trained in Shifts. eScience 2022, 25, 104676. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kline, C.E. Epidemiology of Exercise and Sleep. Sleep Biol. Rhythm. 2006, 4, 215–221. [Google Scholar] [CrossRef]
- Espie, C.A. Insomnia: Conceptual Issues in the Development, Persistence, and Treatment of Sleep Disorder in Adults. Annu. Rev. Psychol. 2002, 53, 215–243. [Google Scholar] [CrossRef]
- Kahn, M.; Korhonen, T.; Leinonen, L.; Martinmäki, K.; Kuula, L.; Pesonen, A.-K.; Gradisar, M. Is It Time We Stop Discouraging Evening Physical Activity? New Real-World Evidence From 150,000 Nights. Front. Public Health 2021, 9, 772376. [Google Scholar] [CrossRef]
- Oda, S.; Shirakawa, K. Sleep Onset Is Disrupted Following Pre-Sleep Exercise That Causes Large Physiological Excitement at Bedtime. Eur. J. Appl. Physiol. 2014, 114, 1789–1799. [Google Scholar] [CrossRef]
- Stone, J.E.; McGlashan, E.M.; Facer-Childs, E.R.; Cain, S.W.; Phillips, A.J.K. Accuracy of the GENEActiv Device for Measuring Light Exposure in Sleep and Circadian Research. Clocks Sleep 2020, 2, 143–152. [Google Scholar] [CrossRef]
- Joyce, D.S.; Zele, A.J.; Feigl, B.; Adhikari, P. The Accuracy of Artificial and Natural Light Measurements by Actigraphs. J. Sleep Res. 2019, 29, e12963. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for Daytime, Evening, and Nighttime Indoor Light Exposure to Best Support Physiology, Sleep, and Wakefulness in Healthy Adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef]
- van Hees, V.T.; Gorzelniak, L.; León, E.C.D.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef]
- Sabia, S.; van Hees, V.T.; Shipley, M.J.; Trenell, M.I.; Hagger-Johnson, G.; Elbaz, A.; Kivimaki, M.; Singh-Manoux, A. Association Between Questionnaire- and Accelerometer-Assessed Physical Activity: The Role of Sociodemographic Factors. Am. J. Epidemiol. 2014, 179, 781–790. [Google Scholar] [CrossRef]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Med. Sci. Sport. Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R, 2019, Version 1.47.1. Available online: https://CRAN.R-project.org/package=MuMIn.
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]

| Sleep | Morning Shift | Late Evening Shift | ||
|---|---|---|---|---|
| Training Day | Weekend Day | Training Day | Weekend Day | |
| Onset (h) | 00:04 ± 00:43 | 02:10 ± 01:06 | 01:39 ± 00:51 | 03:31 ± 01:43 |
| End (h) | 07:04 ± 00:33 | 09:49 ± 01:00 | 08:59 ± 01:27 | 10:23 ± 01:25 |
| Duration | 7.0 ± 0.6 | 7.7 ± 0.8 | 7.3 ± 0.9 | 6.9 ± 1.2 |
| Time Window | Morning Shift (n = 15) | Late Evening Shift (n = 16) | Training Shift | Type of Day | Training Shift * Type of Day | |||
|---|---|---|---|---|---|---|---|---|
| Training Day | Weekend Day | Training Day | Weekend Day | |||||
| Moderate-to-vigorous physical activity (MVPA, minutes) 1 | Daily | 184.5 ± 40.0 | 143.7 ± 36.9 | 182.6 ± 37.2 | 190.7 ± 57.6 | F(1,30.1) = 2.6, p = 0.114 | F(1,450.3) = 11.3, p < 0.001 | F(1,450.3) = 26.9, p < 0.001 |
| Morning | 57.1 ± 13.2 | 20.7 ± 10.9 | 33.5 ± 15.5 | 29.3 ± 16.1 | F(1,30.8) = 2.6, p = 0.118 | F(1,450.3) = 99.5, p < 0.001 | F(1,450.3) = 67.4, p < 0.001 | |
| Late evening | 30.0 ± 14.1 | 28.0 ± 9.5 | 56.3 ± 7.5 | 32.4 ± 13.6 | F(1,31.8) = 18.8, p < 0.001 | F(1,450.7) = 47.6, p < 0.001 | F(1,450.7) = 35.3, p < 0.001 | |
| Mean illuminance (log10 lux) 1 | Daily | 2.3 ± 0.3 | 2.2 ± 0.4 | 2.2 ± 0.4 | 2.2 ± 0.3 | F(1,31.1) = 0.2, p = 0.68 | F(1,450.3) = 3.7, p = 0.054 | F(1,450.3) = 1.3, p = 0.248 |
| Morning | 2.4 ± 0.4 | 1.9 ± 0.8 | 2.3 ± 0.5 | 1.9 ± 0.8 | F(1,31.6) = 0.0, p = 0.949 | F(1,429.3) = 24.9, p < 0.001 | F(1,429.3) = 0.2, p = 0.659 | |
| Late evening | 0.8 ± 0.4 | 0.7 ± 0.3 | 1.3 ± 0.3 | 0.8 ± 0.6 | F(1,31.5) = 5.9, p = 0.021 | F(1,443.5) = 22.9, p < 0.001 | F(1,443.5) = 14.1, p < 0.001 | |
| Frequency of alarm usage (%) 2 | 92.7 | 26.8 | 72.7 | 41.3 | X2(1,481) = 8.5, p = 0.004 | X2(1,480) = 94.6, p < 0.001 | X2(1,479) = 19.5, p < 0.001 | |
| Sleep Onset | Sleep End | Sleep Duration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b ± SE | t | df | p | b ± SE | t | df | p | b ± SE | t | df | p | |
| Intercept | 0.3 ± 0.2 | 1.2 | 70.1 | 0.241 | 8.8 ± 0.3 | 30.4 | 47.9 | <0.001 | 8.5 ± 0.2 | 36.7 | 86.7 | <0.001 |
| Training shift (1 = Late evening) | 1.1 ± 0.3 | 4.2 | 33.9 | <0.001 | 1.3 ± 0.4 | 3.4 | 32.8 | 0.002 | 0.1 ± 0.3 | 0.4 | 35.4 | 0.709 |
| Type of day (1 = Weekend day) | 1.9 ± 0.2 | 12 | 434.5 | <0.001 | 1.2 ± 0.1 | 8.1 | 429.2 | <0.001 | −0.7 ± 0.2 | −4.2 | 437.9 | <0.001 |
| Alarm (1 = Yes) | 0.1 ± 0.2 | 0.5 | 448.0 | 0.592 | −1.6 ± 0.1 | −11.3 | 436.9 | <0.001 | −1.6 ± 0.2 | −9.8 | 452.3 | < 0.001 |
| Morning illuminance (log10 lux) | −0.3 ± 0.1 | −4.8 | 453.9 | <0.001 | −0.1 ± 0.1 | −2.1 | 445.2 | 0.038 | 0.2 ± 0.1 | 2.3 | 447.8 | 0.024 |
| Late evening illuminance (log10 lux) | 0.1 ± 0.1 | 2.1 | 452.8 | 0.036 | 0.2 ± 0.1 | 2.7 | 440.6 | 0.007 | 0.0 ± 0.1 | 0.5 | 453.5 | 0.589 |
| Morning MVPA (minutes) | −0.0 ± 0.1 | −0.5 | 454.0 | 0.631 | −0.0 ± 0.1 | −0.3 | 443.5 | 0.759 | −0.0 ± 0.1 | −0.1 | 450.7 | 0.890 |
| Late evening MVPA (minutes) | 0.4 ± 0.1 | 4.8 | 453.4 | <0.001 | 0.1 ± 0.1 | 1.4 | 441.7 | 0.172 | −0.3 ± 0.1 | −3.2 | 452.9 | 0.001 |
| Pseudo-R2 marginal/conditional | 0.45/0.57 | 0.47/0.69 | 0.19/0.31 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevan, I.; Coirolo, N.; Tassino, B.; Silva, A. The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts. Clocks & Sleep 2023, 5, 47-61. https://doi.org/10.3390/clockssleep5010006
Estevan I, Coirolo N, Tassino B, Silva A. The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts. Clocks & Sleep. 2023; 5(1):47-61. https://doi.org/10.3390/clockssleep5010006
Chicago/Turabian StyleEstevan, Ignacio, Natalia Coirolo, Bettina Tassino, and Ana Silva. 2023. "The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts" Clocks & Sleep 5, no. 1: 47-61. https://doi.org/10.3390/clockssleep5010006
APA StyleEstevan, I., Coirolo, N., Tassino, B., & Silva, A. (2023). The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts. Clocks & Sleep, 5(1), 47-61. https://doi.org/10.3390/clockssleep5010006






