STOP-Bang Score and Prediction of Severity of Obstructive Sleep Apnea in a First Nation Community in Saskatchewan, Canada
Abstract
1. Introduction
2. Results
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Sample
4.2. Data Collection
4.2.1. Measurements
4.2.2. Definitions
| Condition-OSA | |||
| Present | Absent | ||
| Test Results | Positive | TP | FP |
| Negative | FN | TN | |
- TP = “True Positives”;
- FP = “False Positives”;
- FN = “False Negatives”;
- TN = “True Negatives”;
- Sensitivity = TP/(TP + FN);
- Specificity = TN/(FP + TN);
- Positive predictive value (PPV) = TP/(TP + FP);
- Negative predictive value (NPV) = TN/(FN + TN).
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| PSG | Polysomnography |
| AHI | Apnea–hypopnea index |
| BMI | Body mass index |
| NIHB | Non-insured health benefits |
| CPAP | Continuous positive airway pressure therapy |
| FNSHP | First Nations Sleep Health Project |
| CIHR | Canadian Institutes of Health Research |
| AASM | American Academy of Sleep Medicine |
| ESS | Epworth Sleepiness Scale |
| SPSS | Statistical package for the social sciences |
| SD | Standard deviation |
| OR | Odds ratio |
| CI | Confidence interval |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| ROC | Receiver operating characteristic curve |
| AUC | Area under the ROC curve |
References
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk factors for obstructive sleep apnea in adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Dudley, K.A.; Patel, S.R. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016, 18, 96–102. [Google Scholar] [CrossRef]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Zhang, K.; Nagappa, M.; Saripella, A.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnoea in patients with cardiovascular risk factors: A systematic review and meta-analysis. BMJ Open Respir. Res. 2021, 8, e000848. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pivetta, B.; Nagappa, M.; Saripella, A.; Islam, S.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire for screening of obstructive sleep apnea in the general population and commercial drivers: A systematic review and meta-analysis. Sleep Breath. 2021, 25, 1741–1751. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Chapter 5; John Wiley and Sons: New York, NY, USA, 2000; pp. 160–164. [Google Scholar]
- Šimundić, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar] [PubMed]
- Chung, F.; Subramanyam, R.; Liao, P.; Sasaki, E.; Shapiro, C.; Sun, Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br. J. Anaesth. 2012, 108, 768–775. [Google Scholar] [CrossRef]
- Pivetta, B.; Chen, L.; Nagappa, M.; Saripella, A.; Waseem, R.; Englesakis, M.; Chung, F. Use and Performance of the STOP-Bang Questionnaire for Obstructive Sleep Apnea Screening Across Geographic Regions: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2021, 4, e211009. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J. Clin. Diagn. Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef] [PubMed]
- Erman, M.K.; Stewart, D.; Einhorn, D.; Gordon, N.; Casal, E. Validation of the ApneaLink™ for the screening of sleep apnea: A novel and simple single channel recording device. J. Clin. Sleep Med. 2007, 3, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Dosman, J.A.; Karunanayake, C.P.; Fenton, M.; Ramsden, V.R.; Seeseequasis, J.; Skomro, R.; Kirychuk, S.; Rennie, D.C.; McMullin, K.; Russell, B.P.; et al. Obesity, sex, snoring and severity of OSA in a First Nation community in Saskatchewan, Canada. Clocks Sleep 2022, 4, 100–113. [Google Scholar] [CrossRef]
- Dosman, J.A.; Karunanayake, C.P.; Fenton, M.; Ramsden, V.R.; Skomro, R.; Kirychuk, S.; Rennie, D.C.; Seeseequasis, J.; Bird, C.; McMullin, K.; et al. Prevalence of Insomnia in Two Saskatchewan First Nation Communities. Clocks Sleep 2021, 3, 98–114. [Google Scholar] [CrossRef]
- Karunanayake, C.P.; Fenton, M.; Skomro, R.; Ramsden, V.R.; Kirychuk, S.; Rennie, D.C.; Seeseequasis, J.; Bird, C.; McMullin, K.; Russell, B.P.; et al. Sleep Deprivation in two Saskatchewan First Nation Communities: A Public Health Consideration. Sleep Med. X 2021, 3, 100037. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada, Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans—TCPS 2 (2018), December 2018. Available online: https://ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf (accessed on 31 August 2020).
- Tolonen, H.; Kuulasmaa, K.; Laatikainen, T.; Wolf, H.; European Health Risk Monitoring Project. Recommendation for Indicators, International Collaboration, Protocol and Manual of Operations for Chronic Disease Risk Factor Surveys; Finnish National Public Health Institute: Helsinki, Finland, 2002; Available online: https://www.thl.fi/publications/ehrm/product2/product2.pdf (accessed on 5 August 2018).
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Kirsch, D.B.; Kristo, D.A.; Olson, E.J.; Aurora, R.N.; Carden, K.A.; Chervin, R.D.; Martin, J.L.; Ramar, K.; Rosen, C.L.; et al. Polysomnography for Obstructive Sleep Apnea Should Include Arousal-Based Scoring: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2018, 14, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B. The AASM Manual for The scoring of Sleep and Associated Events, 2nd ed.; American Academy of Sleep Medicine: Chicago, IL, USA, 2017. [Google Scholar]
- Bruno, P. The importance of diagnostic test parameters in the interpretation of clinical test findings: The Prone Hip Extension Test as an example. J. Can. Chiropr. Assoc. 2011, 55, 69–75. [Google Scholar] [PubMed]
- Altman, D.G. Diagnostic Tests. In Statistics with Confidence, 2nd ed.; Altman, D.G., Machin, D., Bryant, T.N., Gardner, M.J., Eds.; BMJ Books: Bristol, UK, 2000; pp. 105–119. [Google Scholar]
- Davidson, M. The interpretation of diagnostic test: A primer for physiotherapists. Aust. J. Physiother. 2002, 48, 227–232. [Google Scholar] [CrossRef]
- Chien, P.F.; Khan, K.S. Evaluation of a clinical test. II: Assessment of validity. BJOG 2001, 108, 568–572. [Google Scholar] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 1991. [Google Scholar]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thoracic. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2012; 752p. [Google Scholar]
- Agresti, A. An Introduction to Categorical Data Analysis, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2018; 400p. [Google Scholar]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]

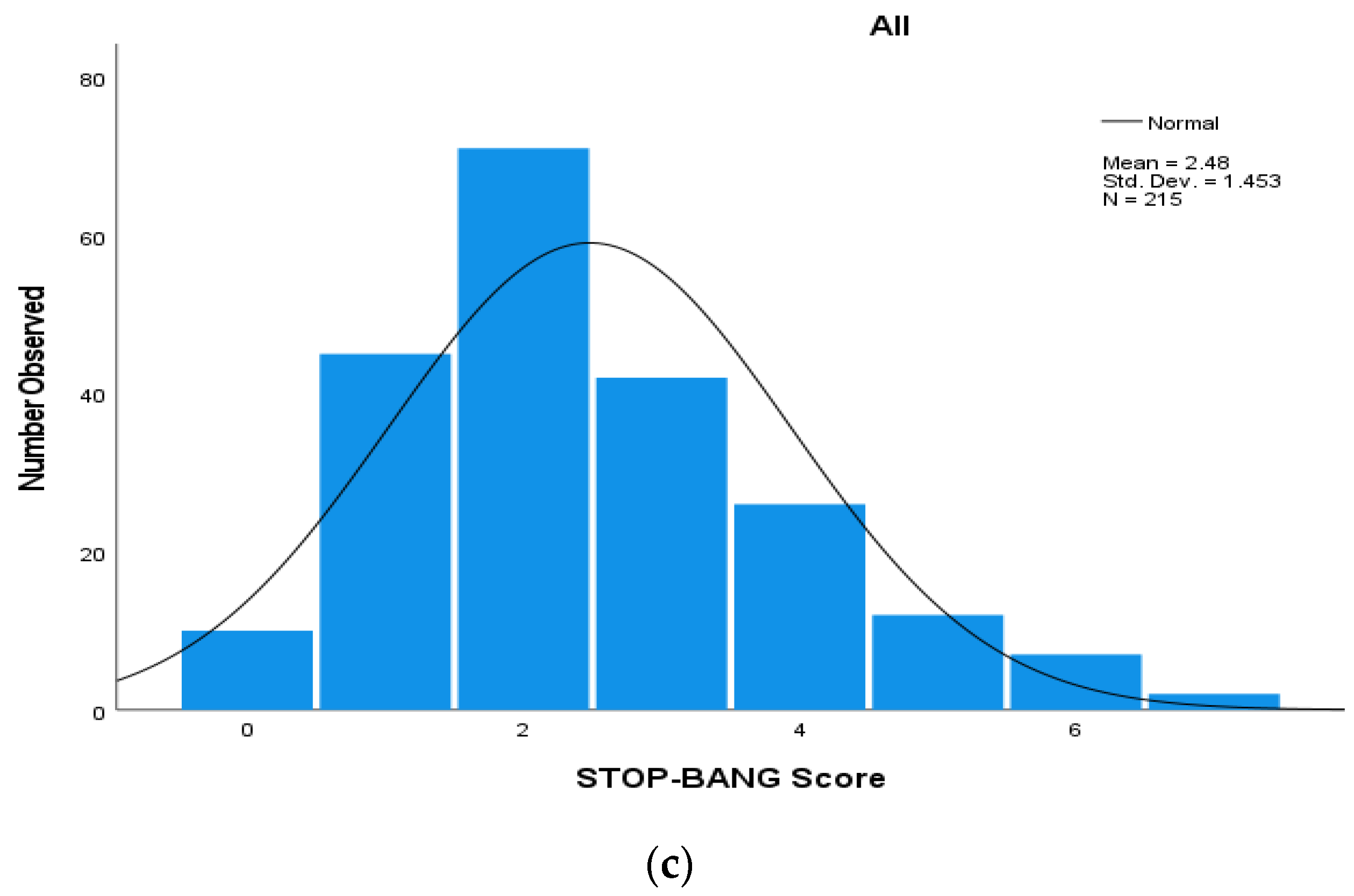
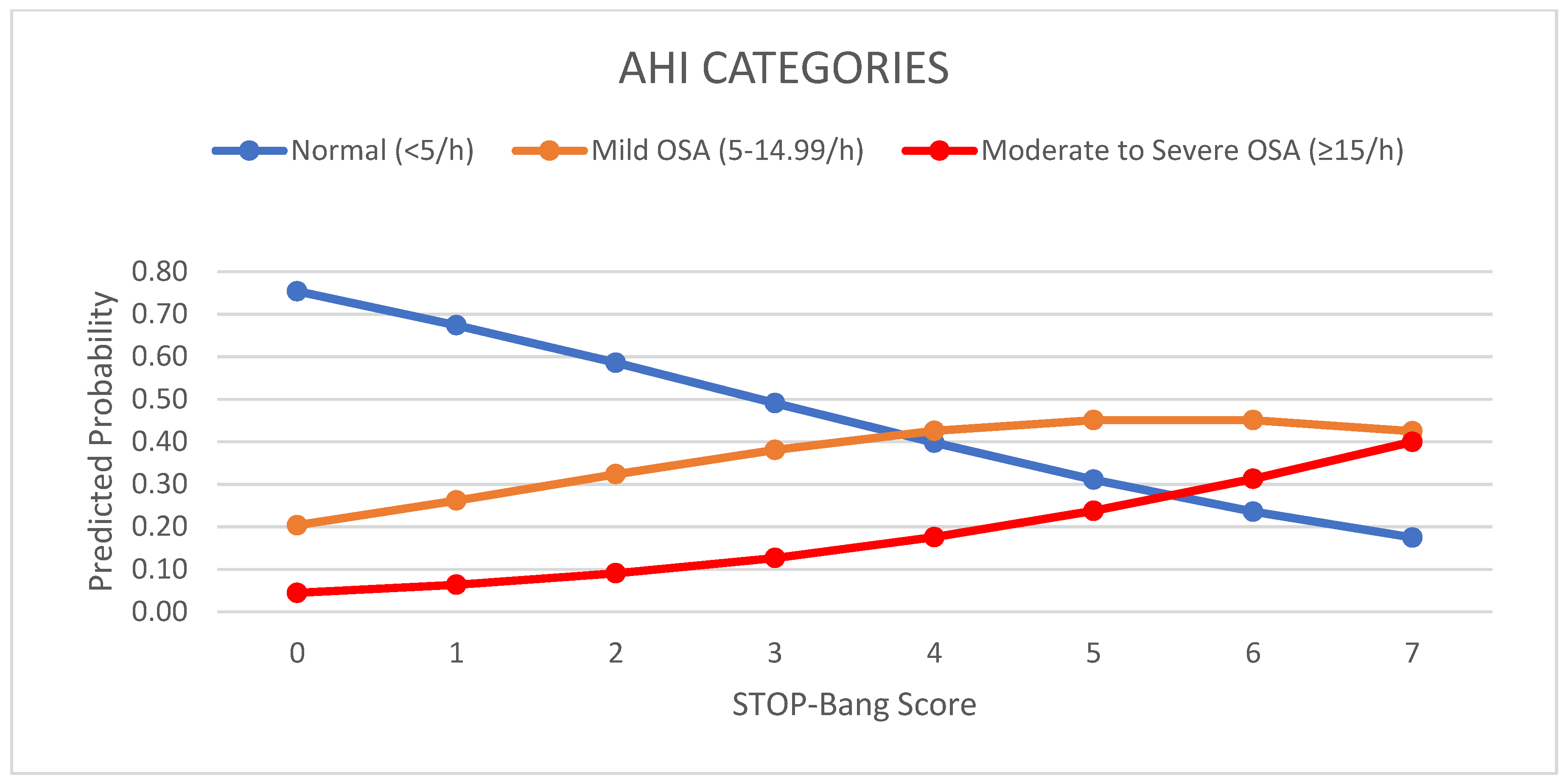
| Parameter | All | Males | Females | p Value 4 |
|---|---|---|---|---|
| N | 215 | 88 | 127 | |
| Loud snoring 1 (%) | 28.8 | 27.3 | 29.9 | 0.673 |
| Tired/Sleepy 1 (%) | 67.4 | 54.5 | 76.4 | 0.001 |
| Observed apnea 1 (%) | 20.5 | 19.3 | 21.3 | 0.729 |
| Blood pressure 1 (%) | 19.5 | 12.5 | 24.4 | 0.030 |
| BMI 2 (kg/m2) | 30.9 ± 8.1 (17.3–64.1) | 28.9 ± 7.3 (17.4–63.3) | 32.2 ± 8.4 (17.3–64.1) | 0.004 |
| Age 2 (years) | 37.7 ± 14.9 (18–76) | 35.5 ± 14.4 (18–76) | 39.3 ± 15.1 (18–73) | 0.068 |
| Neck size 2 (cm) | 37.1 ± 5.3 (21.0–61.0) | 38.2 ± 5.5 (30.0–61.0) | 36.4 ± 5.1 (21.0–52.0) | 0.016 |
| Gender (%) | 40.9 | 59.1 | ||
| STOP-Bang Score 2 | 2.5 ± 1.5 (0–7) | 2.8 ± 1.4 (1–7) | 2.3 ± 1.5 (0–7) | 0.010 |
| AHI 3 | 8.1 ± 11.6 (0–87.6) | 9.4 ± 11.8 (0–61.4) | 7.2 ± 11.5 (0–87.6) | 0.168 |
| STOP-Bang Score 1 | Apnea/Hypopnea Index | ||
|---|---|---|---|
| Normal (<5/h) | Mild OSA (5–14.99/h) | Moderate-to-Severe OSA (≥15/h) | |
| 0 | 5 | 4 | 1 |
| 1 | 33 | 8 | 4 |
| 2 | 42 | 27 | 2 |
| 3 | 18 | 18 | 6 |
| 4 | 11 | 12 | 3 |
| 5 | 5 | 2 | 5 |
| 6 | 3 | 2 | 2 |
| 7 | 0 | 0 | 2 |
| 8 | 0 | 0 | 0 |
| Total | 117 (54.4%) | 73 (34.0%) | 25 (11.6%) |
| Predictive Parameters | All OSA AHI ≥ 5/h | Moderate-to Severe OSA AHI ≥ 15/h |
|---|---|---|
| N | 98 | 25 |
| Prevalence | 45.6% | 11.6% |
| STOP-Bang score ≥ 3 | ||
| Sensitivity | 53.1% | 72.0% |
| Specificity | 68.4% | 62.6% |
| PPV | 58.4% | 20.2% |
| NPV | 63.5% | 94.4% |
| AUC (SE) | 0.61 (SE = 0.039) | 0.67 (SE = 0.056) |
| 95% CI | 0.53–0.69 | 0.56–0.78 |
| STOP-Bang score ≥ 4 | ||
| Sensitivity | 28.6% | 48.0% |
| Specificity | 83.8% | 81.6% |
| PPV | 59.6% | 25.5% |
| NPV | 58.3% | 92.3% |
| AUC (SE) | 0.56 (SE = 0.04) | 0.65 (SE = 0.064) |
| 95% CI | 0.48–0.64 | 0.52–0.77 |
| STOP-Bang score ≥ 5 | ||
| Sensitivity | 13.3% | 36.0% |
| Specificity | 93.2% | 93.7% |
| PPV | 61.9% | 42.9% |
| NPV | 56.2% | 91.8% |
| AUC (SE) | 0.53 (SE = 0.04) | 0.65 (SE = 0.067) |
| 95% CI | 0.45–0.61 | 0.52–0.78 |
| Loud Snoring | ||
| Sensitivity | 37.8% | 60.0% |
| Specificity | 78.6% | 75.3% |
| PPV | 59.7% | 24.2% |
| NPV | 60.1% | 93.5% |
| AUC (SE) | 0.58 (SE = 0.039) | 0.68 (SE = 0.060) |
| 95% CI | 0.50–0.66 | 0.56–0.80 |
| Loud snoring and Obese | ||
| Sensitivity | 26.7% | 39.3% |
| Specificity | 88.3% | 84.4% |
| PPV | 65.1% | 25.6% |
| NPV | 59.5% | 91.1% |
| AUC (SE) | 0.58 (SE = 0.038) | 0.64 (SE = 0.064) |
| 95% CI | 0.51–0.65 | 0.51–0.77 |
| Predictive Parameters | All OSA AHI ≥ 5/h | Moderate-to-Severe OSA AHI ≥ 15/h | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| N | 45 | 53 | 13 | 12 |
| Prevalence | 51.1% | 41.7% | 14.8% | 9.4% |
| STOP-Bang score ≥ 3 | ||||
| Sensitivity | 60.0% | 47.2% | 92.3% | 50.0% |
| Specificity | 67.4% | 68.9% | 61.3% | 63.5% |
| PPV | 65.9% | 52.1% | 29.3% | 12.5% |
| NPV | 61.75% | 64.6% | 97.9% | 92.4% |
| AUC (SE) | 0.64 (SE = 0.06) | 0.58 (SE = 0.052) | 0.77 (SE = 0.06) | 0.57 (SE = 0.09) |
| 95% CI | 0.52–0.76 | 0.48–0.68 | 0.65–0.88 | 0.39–0.75 |
| STOP-Bang score ≥ 4 | ||||
| Sensitivity | 35.6% | 22.6% | 69.2% | 25.0% |
| Specificity | 76.7% | 87.8% | 77.3% | 84.3% |
| PPV | 61.5% | 57.1% | 34.6% | 14.3% |
| NPV | 53.2% | 61.3% | 93.5% | 91.5% |
| AUC (SE) | 0.56 (SE = 0.06) | 0.55 (SE = 0.05) | 0.73 (SE = 0.08) | 0.55 (SE = 0.09) |
| 95% CI | 0.44–0.68 | 0.45–0.65 | 0.57–0.89 | 0.37–0.73 |
| STOP-Bang score ≥ 5 | ||||
| Sensitivity | 15.6% | 11.3% | 46.2% | 25.0% |
| Specificity | 93.0% | 93.2% | 94.7% | 93.0% |
| PPV | 70.0% | 54.5% | 60.0% | 27.3% |
| NPV | 51.3% | 59.5% | 91.0% | 92.2% |
| AUC (SE) | 0.54 (SE = 0.06) | 0.52 (SE = 0.05) | 0.70 (SE = 0.09) | 0.59 (SE = 0.09) |
| 95% CI | 0.42–0.66 | 0.42–0.62 | 0.52–0.88 | 0.41–0.77 |
| Loud Snoring | ||||
| Sensitivity | 40.0% | 35.8% | 76.9% | 41.7% |
| Specificity | 86.0% | 74.3% | 81.3% | 71.3% |
| PPV | 75.0% | 50.0% | 41.7% | 13.2% |
| NPV | 57.8% | 61.8% | 95.3% | 91.1% |
| AUC (SE) | 0.63 (SE = 0.06) | 0.55 (SE = 0.05) | 0.79 (SE = 0.07) | 0.57 (SE = 0.09) |
| 95% CI | 0.51–0.75 | 0.45–0.65 | 0.65–0.93 | 0.39–0.75 |
| Loud snoring and Obese | ||||
| Sensitivity | 24.4% | 30.2% | 46.2% | 33.3% |
| Specificity | 95.3% | 79.7% | 90.7% | 76.5% |
| PPV | 84.6% | 51.6% | 46.2% | 12.9% |
| NPV | 54.7% | 61.5% | 90.7% | 91.7% |
| AUC (SE) | 0.60 (SE = 0.06) | 0.55 (SE = 0.05) | 0.68 (SE = 0.09) | 0.55 (SE = 0.09) |
| 95% CI | 0.48–0.72 | 0.45–0.65 | 0.50–0.86 | 0.37–0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosman, J.A.; Karunanayake, C.P.; Fenton, M.; Ramsden, V.R.; Seeseequasis, J.; Mike, D.; Seesequasis, W.; Neubuhr, M.; Skomro, R.; Kirychuk, S.; et al. STOP-Bang Score and Prediction of Severity of Obstructive Sleep Apnea in a First Nation Community in Saskatchewan, Canada. Clocks & Sleep 2022, 4, 535-548. https://doi.org/10.3390/clockssleep4040042
Dosman JA, Karunanayake CP, Fenton M, Ramsden VR, Seeseequasis J, Mike D, Seesequasis W, Neubuhr M, Skomro R, Kirychuk S, et al. STOP-Bang Score and Prediction of Severity of Obstructive Sleep Apnea in a First Nation Community in Saskatchewan, Canada. Clocks & Sleep. 2022; 4(4):535-548. https://doi.org/10.3390/clockssleep4040042
Chicago/Turabian StyleDosman, James A., Chandima P. Karunanayake, Mark Fenton, Vivian R. Ramsden, Jeremy Seeseequasis, Delano Mike, Warren Seesequasis, Marie Neubuhr, Robert Skomro, Shelley Kirychuk, and et al. 2022. "STOP-Bang Score and Prediction of Severity of Obstructive Sleep Apnea in a First Nation Community in Saskatchewan, Canada" Clocks & Sleep 4, no. 4: 535-548. https://doi.org/10.3390/clockssleep4040042
APA StyleDosman, J. A., Karunanayake, C. P., Fenton, M., Ramsden, V. R., Seeseequasis, J., Mike, D., Seesequasis, W., Neubuhr, M., Skomro, R., Kirychuk, S., Rennie, D. C., McMullin, K., Russell, B. P., Koehncke, N., Abonyi, S., King, M., & Pahwa, P. (2022). STOP-Bang Score and Prediction of Severity of Obstructive Sleep Apnea in a First Nation Community in Saskatchewan, Canada. Clocks & Sleep, 4(4), 535-548. https://doi.org/10.3390/clockssleep4040042





