Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions
Abstract
1. Introduction
2. Results
2.1. Effect of Caffeine on the Sleep–Wake Cycle and Locomotor Activity
2.2. Effect of Caffeine on Vigilance State Episodes
2.3. Effect of Caffeine on EEG Slow-Wave and Theta Activity
2.4. Effect of Caffeine on the EEG Power Spectrum of Waking and REM Sleep
2.5. Effect of Caffeine on the Neural Activity in the Lateral Hypothalamus
3. Discussion
3.1. Acute Effect of Caffeine on Vigilance States
3.2. Theta Activity in Different Sleep Stages
3.3. Activity in of the Lateral Hypothalamus
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Drug Treatment
4.4. EEG Data Acquisition
4.5. Data Analysis
4.6. Histology
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwierin, B.; Borbely, A.A.; Tobler, I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur. J. Pharmacol. 1996, 300, 163–171. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Landolt, H.P.; Dijk, D.J.; Gaus, S.E.; Borbely, A.A. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology 1995, 12, 229–238. [Google Scholar] [CrossRef]
- Daan, S.; Beersma, D.G.; Borbely, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Porkka-Heiskanen, T.; Strecker, R.E.; Thakkar, M.; Bjorkum, A.A.; Greene, R.W.; McCarley, R.W. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997, 276, 1265–1268. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef]
- Landolt, H.P. Sleep homeostasis: A role for adenosine in humans? Biochem. Pharmacol. 2008, 75, 2070–2079. [Google Scholar] [CrossRef]
- Bonati, M.; Latini, R.; Tognoni, G.; Young, J.F.; Garattini, S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab. Rev. 1984, 15, 1355–1383. [Google Scholar] [CrossRef]
- Arnaud, M.J. The pharmacology of caffeine. Prog. Drug Res. 1987, 31, 273–313. [Google Scholar] [CrossRef] [PubMed]
- Scheer, N.; McLaughlin, L.A.; Rode, A.; Macleod, A.K.; Henderson, C.J.; Wolf, C.R. Deletion of 30 murine cytochrome p450 genes results in viable mice with compromised drug metabolism. Drug Metab. Dispos. 2014, 42, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Estari, R.K.; Dong, J.; Chan, W.K.; Park, M.S.; Zhou, Z. Time effect of rutaecarpine on caffeine pharmacokinetics in rats. Biochem. Biophys. Rep. 2021, 28, 101121. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P.P. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 2008, 74, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W.; Jacobson, K.A.; Ukena, D. Adenosine receptors: Development of selective agonists and antagonists. Prog. Clin. Biol. Res. 1987, 230, 41–63. [Google Scholar] [PubMed]
- Roenneberg, T.; Daan, S.; Merrow, M. The art of entrainment. J. Biol. Rhythms. 2003, 18, 183–194. [Google Scholar] [CrossRef]
- Daan, S. The Colin, S. Pittendrigh Lecture. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J. Biol. Rhythms. 2000, 15, 195–207. [Google Scholar] [CrossRef]
- Meijer, J.H.; Rietveld, W.J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol. Rev. 1989, 69, 671–707. [Google Scholar] [CrossRef]
- Elliott, K.J.; Todd Weber, E.; Rea, M.A. Adenosine A1 receptors regulate the response of the hamster circadian clock to light. Eur. J. Pharmacol. 2001, 414, 45–53. [Google Scholar] [CrossRef]
- Sigworth, L.A.; Rea, M.A. Adenosine A1 receptors regulate the response of the mouse circadian clock to light. Brain Res. 2003, 960, 246–251. [Google Scholar] [CrossRef]
- van Diepen, H.C.; Lucassen, E.A.; Yasenkov, R.; Groenen, I.; Ijzerman, A.P.; Meijer, J.H.; Deboer, T. Caffeine increases light responsiveness of the mouse circadian pacemaker. Eur. J. Neurosci. 2014, 40, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Kobori, M.; Suzuki, T.; Ishida, N. Caffeine lengthens circadian rhythms in mice. Biochem. Biophys. Res. Commun. 2011, 410, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Antle, M.C.; Steen, N.M.; Mistlberger, R.E. Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport 2001, 12, 2901–2905. [Google Scholar] [CrossRef]
- Ruby, C.L.; Verbanes, N.M.; Palmer, K.N.; Zisk, C.F.; Bunion, D.J.; Marinos, L.N. Caffeine Delays Light-entrained Activity and Potentiates Circadian Photic Phase-resetting in Mice. J. Biol. Rhythms. 2018, 33, 523–534. [Google Scholar] [CrossRef]
- Jha, P.K.; Bouaouda, H.; Gourmelen, S.; Dumont, S.; Fuchs, F.; Goumon, Y.; Bourgin, P.; Kalsbeek, A.; Challet, E. Sleep Deprivation and Caffeine Treatment Potentiate Photic Resetting of the Master Circadian Clock in a Diurnal Rodent. J. Neurosci. 2017, 37, 4343–4358. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.J.; Semba, K. Immunohistochemical localization of caffeine-induced c-Fos protein expression in the rat brain. J. Comp. Neurol. 1998, 401, 89–108. [Google Scholar] [CrossRef]
- Murphy, J.A.; Deurveilher, S.; Semba, K. Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience 2003, 121, 269–275. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Luppi, P.H.; Clement, O.; Sapin, E.; Gervasoni, D.; Peyron, C.; Leger, L.; Salvert, D.; Fort, P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med. Rev. 2011, 15, 153–163. [Google Scholar] [CrossRef]
- McCormick, D.A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989, 12, 215–221. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal systems. Front. Biosci. 2003, 8, s438–s451. [Google Scholar] [CrossRef] [PubMed]
- Gaus, S.E.; Strecker, R.E.; Tate, B.A.; Parker, R.A.; Saper, C.B. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience 2002, 115, 285–294. [Google Scholar] [CrossRef]
- Sherin, J.E.; Elmquist, J.K.; Torrealba, F.; Saper, C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 1998, 18, 4705–4721. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, R.; Alam, N.; Steininger, T.L.; McGinty, D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998, 803, 178–188. [Google Scholar] [CrossRef]
- Deboer, T.; Vansteensel, M.J.; Detari, L.; Meijer, J.H. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat. Neurosci. 2003, 6, 1086–1090. [Google Scholar] [CrossRef]
- Deboer, T.; Ruijgrok, G.; Meijer, J.H. Short light-dark cycles affect sleep in mice. Eur. J. Neurosci. 2007, 26, 3518–3523. [Google Scholar] [CrossRef]
- Nunez, A.; Buno, W. The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front. Cell. Neurosci. 2021, 15, 649262. [Google Scholar] [CrossRef]
- Pelissier, A.L.; Gantenbein, M.; Bruguerolle, B. Caffeine-induced modifications of heart rate, temperature, and motor activity circadian rhythms in rats. Physiol. Behav. 1999, 67, 81–88. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P., Jr. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef]
- Rainnie, D.G.; Grunze, H.C.; McCarley, R.W.; Greene, R.W. Adenosine inhibition of mesopontine cholinergic neurons: Implications for EEG arousal. Science 1994, 263, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Gerashchenko, D.Y.; Mochizuki, T.; McCarthy, M.T.; Estabrooke, I.V.; Sears, C.A.; Saper, C.B.; Urade, Y.; Hayaishi, O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 2001, 107, 653–663. [Google Scholar] [CrossRef]
- Morairty, S.; Rainnie, D.; McCarley, R.; Greene, R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: A new mechanism for sleep promotion. Neuroscience 2004, 123, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Narishige, S.; Kuwahara, M.; Shinozaki, A.; Okada, S.; Ikeda, Y.; Kamagata, M.; Tahara, Y.; Shibata, S. Effects of caffeine on circadian phase, amplitude and period evaluated in cells in vitro and peripheral organs in vivo in PER2::LUCIFERASE mice. Br. J. Pharmacol. 2014, 171, 5858–5869. [Google Scholar] [CrossRef]
- Yanik, G.; Glaum, S.; Radulovacki, M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987, 403, 177–180. [Google Scholar] [CrossRef]
- Garrett, B.E.; Holtzman, S.G. D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol. Biochem. Behav. 1994, 47, 89–94. [Google Scholar] [CrossRef]
- Marin, M.T.; Zancheta, R.; Paro, A.H.; Possi, A.P.; Cruz, F.C.; Planeta, C.S. Comparison of caffeine-induced locomotor activity between adolescent and adult rats. Eur. J. Pharmacol. 2011, 660, 363–367. [Google Scholar] [CrossRef]
- El Yacoubi, M.; Ledent, C.; Menard, J.F.; Parmentier, M.; Costentin, J.; Vaugeois, J.M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br. J. Pharmacol. 2000, 129, 1465–1473. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Zimmerman, J.C.; Ronda, J.M.; Moore-Ede, M.C.; Weitzman, E.D. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep 1980, 2, 329–346. [Google Scholar]
- Bland, B.H. The physiology and pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 1986, 26, 1–54. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Tobler, I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005, 1050, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, U.; Kasicki, S. The frequency of rat’s hippocampal theta rhythm is related to the speed of locomotion. Brain Res. 1998, 796, 327–331. [Google Scholar] [CrossRef]
- Daly, J.W.; Shi, D.; Nikodijevic, O.; Jacobson, K.A. The role of adenosine receptors in the central action of caffeine. Pharmacopsychoecologia 1994, 7, 201–213. [Google Scholar] [PubMed]
- Bergel, A.; Deffieux, T.; Demene, C.; Tanter, M.; Cohen, I. Local hippocampal fast gamma rhythms precede brain-wide hyperemic patterns during spontaneous rodent REM sleep. Nat. Commun. 2018, 9, 5364. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Kovacs, E.M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005, 13, 1195–1204. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A.; Weinstein, Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Deboer, T.; Overeem, S.; Visser, N.A.; Duindam, H.; Frolich, M.; Lammers, G.J.; Meijer, J.H. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 2004, 129, 727–732. [Google Scholar] [CrossRef]
- Zhang, S.; Zeitzer, J.M.; Yoshida, Y.; Wisor, J.P.; Nishino, S.; Edgar, D.M.; Mignot, E. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep 2004, 27, 619–627. [Google Scholar] [CrossRef]
- Gautron, L.; Elmquist, J.K.; Williams, K.W. Neural control of energy balance: Translating circuits to therapies. Cell 2015, 161, 133–145. [Google Scholar] [CrossRef]
- Deurveilher, S.; Lo, H.; Murphy, J.A.; Burns, J.; Semba, K. Differential c-Fos immunoreactivity in arousal-promoting cell groups following systemic administration of caffeine in rats. J. Comp. Neurol. 2006, 498, 667–689. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Dalia, C.; Tafuri, D.; Monda, V.; Palmieri, F.; Dato, A.; Russo, A.; De Blasio, S.; Messina, A.; De Luca, V.; et al. Orexin-A controls sympathetic activity and eating behavior. Front. Psychol. 2014, 5, 997. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Kodama, T.; Siegel, J.M. Caffeine promotes glutamate and histamine release in the posterior hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R704–R710. [Google Scholar] [CrossRef] [PubMed]
- Elahdadi Salmani, M.; Sarfi, M.; Goudarzi, I. Hippocampal orexin receptors: Localization and function. Vitam. Horm. 2022, 118, 393–421. [Google Scholar] [CrossRef]
- Huang, Z.L.; Qu, W.M.; Eguchi, N.; Chen, J.F.; Schwarzschild, M.A.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005, 8, 858–859. [Google Scholar] [CrossRef]
- Franken, P.; Dijk, D.J.; Tobler, I.; Borbely, A.A. Sleep deprivation in rats: Effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol. 1991, 261, R198–R208. [Google Scholar] [CrossRef]
- Panagiotou, M.; Deboer, T. Chronic high-caloric diet accentuates age-induced sleep alterations in mice. Behav. Brain Res. 2019, 362, 131–139. [Google Scholar] [CrossRef]

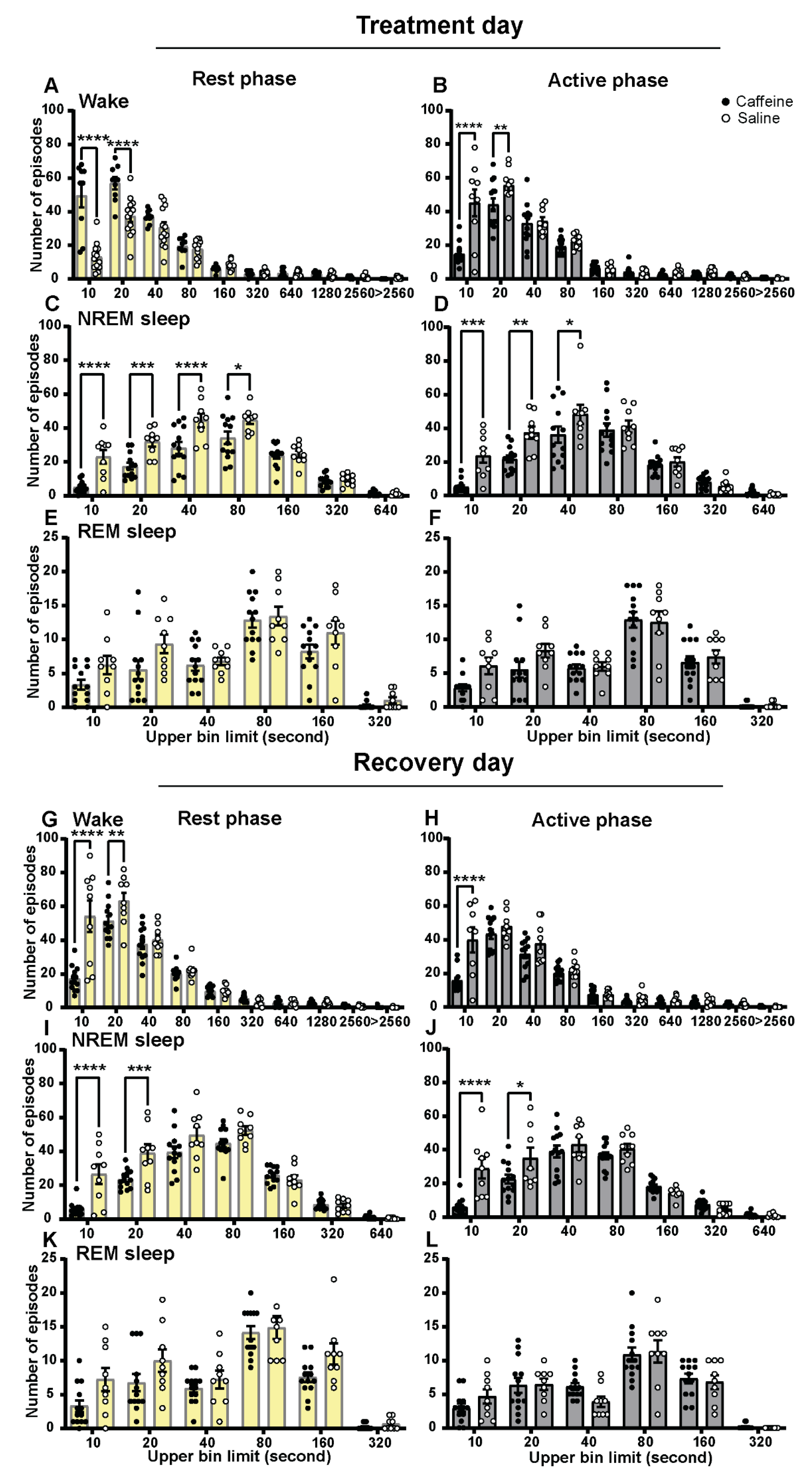
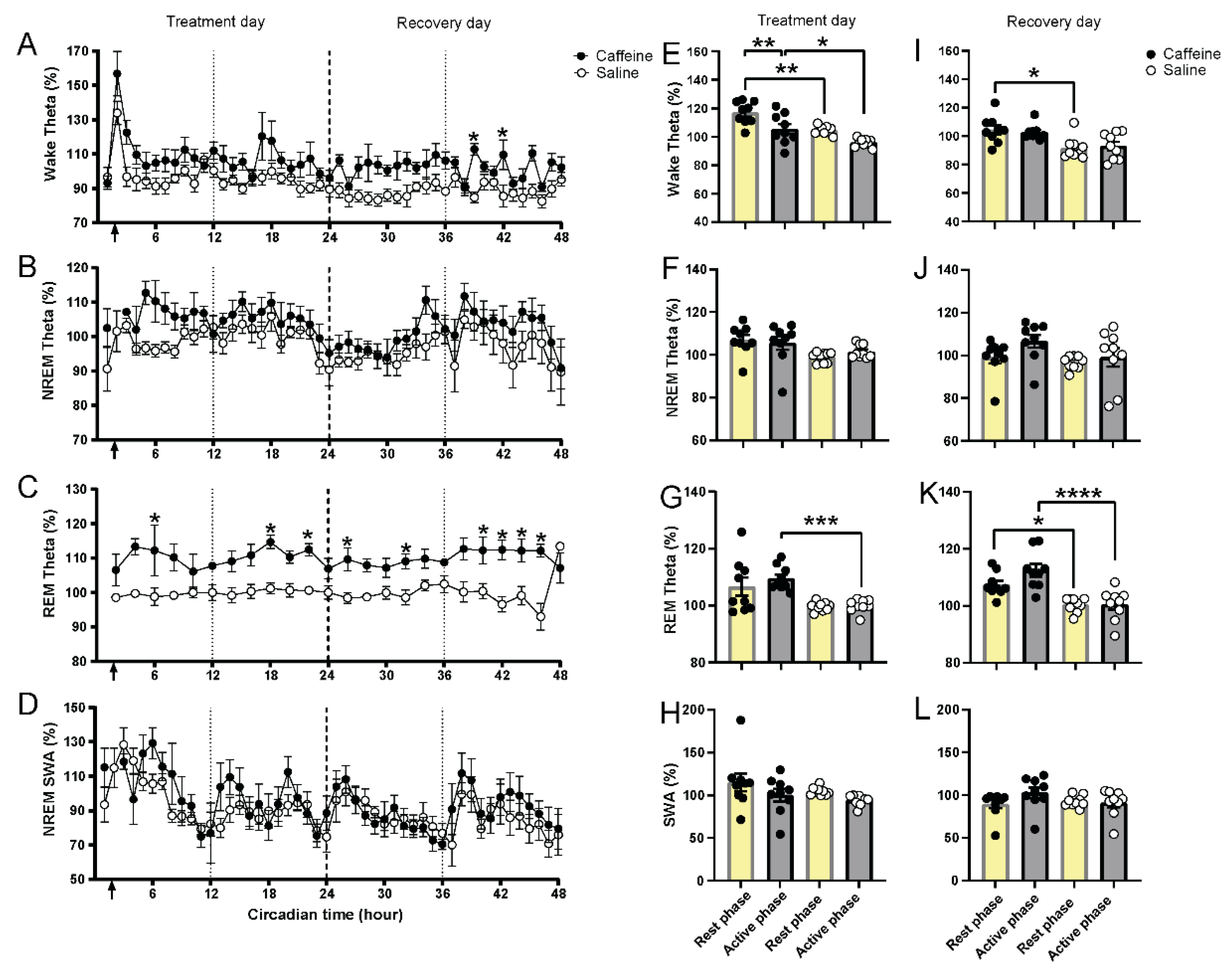
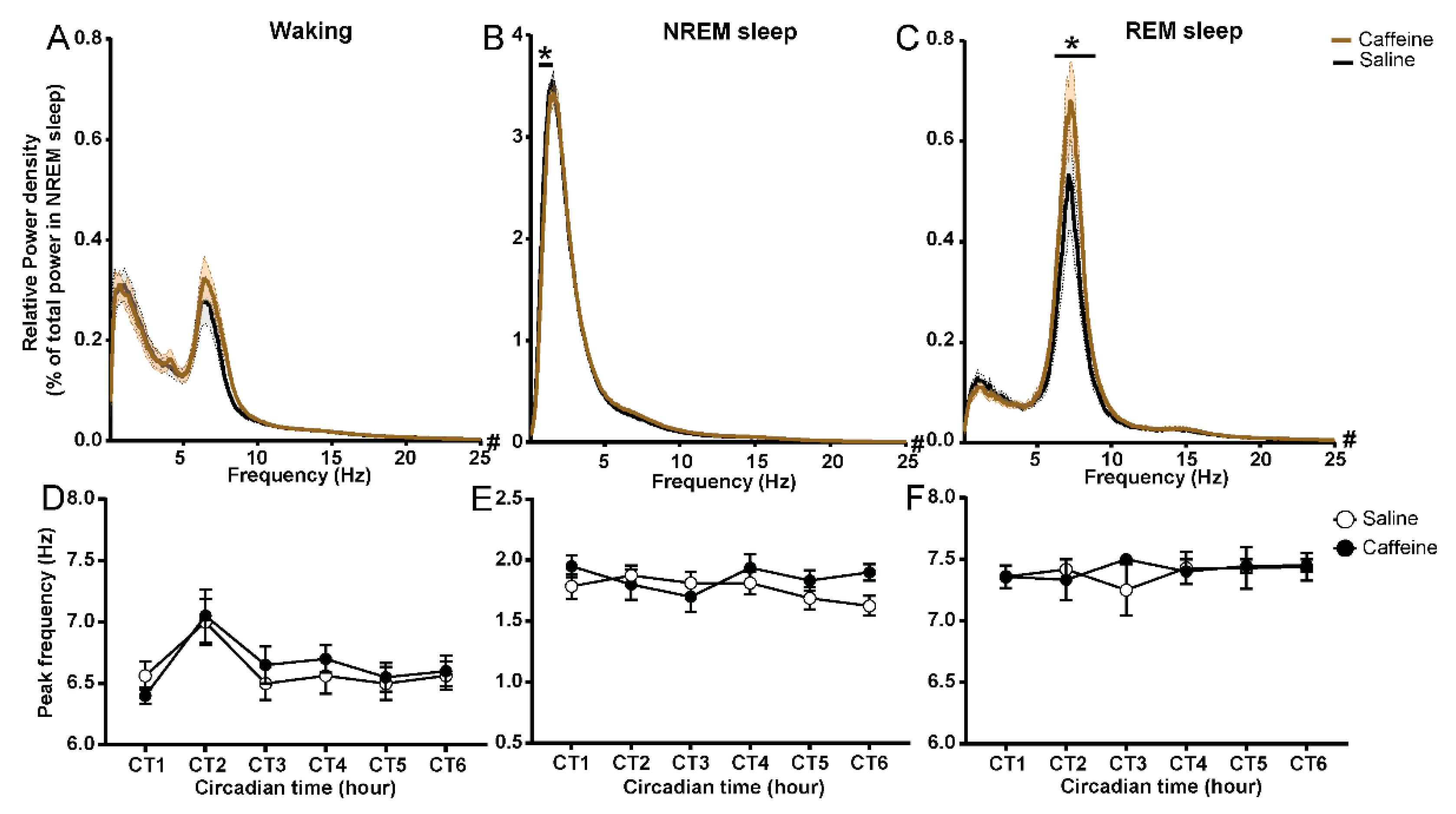
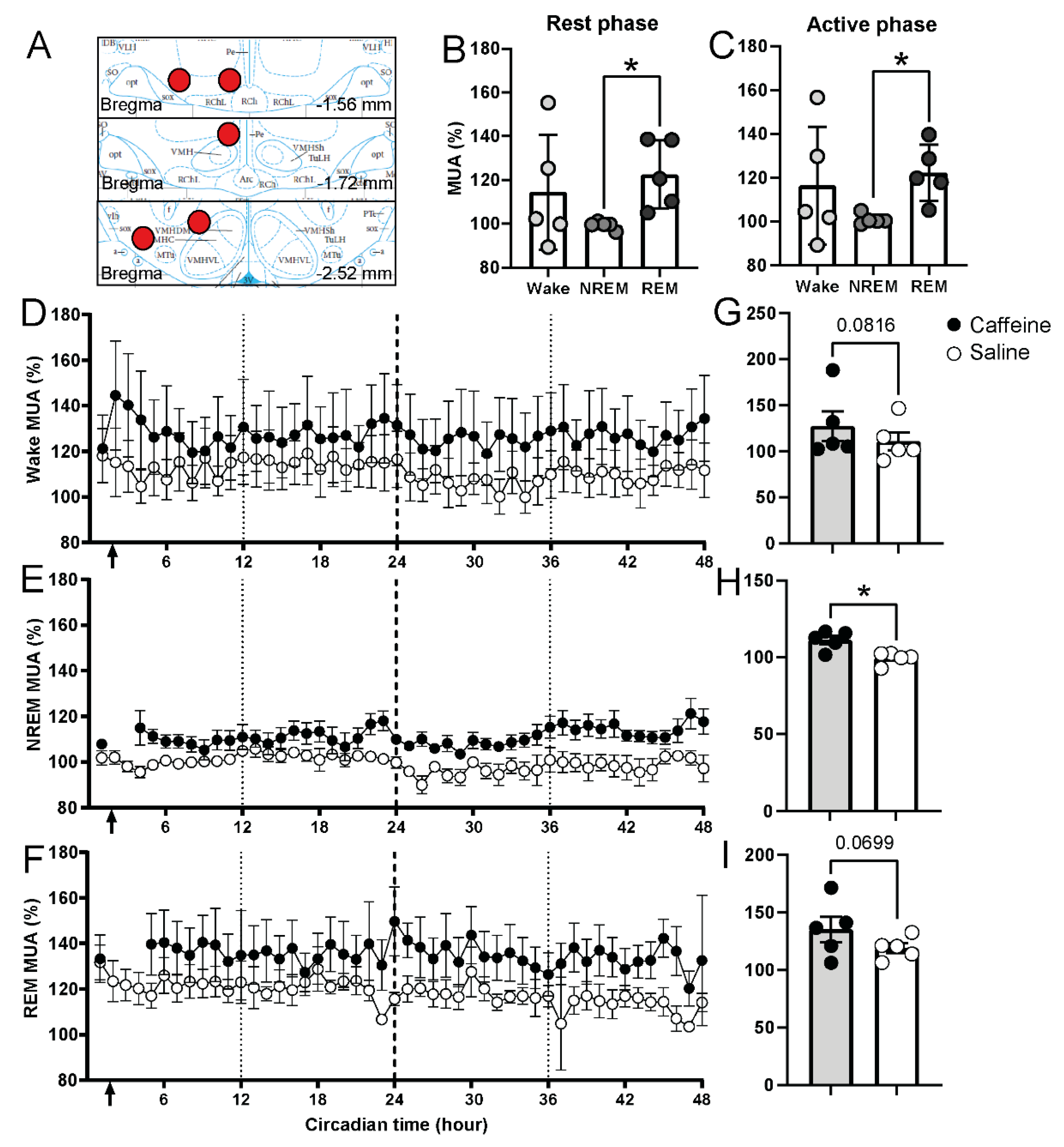
| Vigilance State and PIR | Time of Day | Treatment | Interaction |
|---|---|---|---|
| Treatment day | |||
| Waking | F (23, 480) = 3.159 | F (1, 480) = 4.427 | F (23, 480) = 2.565 |
| p < 0.0001 | p = 0.0359 | p = 0.0001 | |
| NREM sleep | F (23, 480) = 2.718 | F (1, 480) = 3.564 | F (23, 480) = 2.727 |
| P = 0.0596 | p = 0.0596 | p < 0.0001 | |
| REM sleep | F (23, 480) = 3.645 | F (1, 480) = 4.995 | F (23, 480) = 1.474 |
| p < 0.0001 | p = 0.0259 | p = 0.0733 | |
| REM/Total sleep | F (23, 451) = 2.050 | F (1, 451) = 4.157 | F (23, 451) = 0.8427 |
| p = 0.0031 | p = 0.0420 | ns 1 | |
| PIR | F (23, 480) = 9.019 | F (1, 480) = 6.516 | F (23, 480) = 4.506 |
| p < 0.0001 | p = 0.0110 | p < 0.0001 | |
| Recovery day | |||
| Waking | F (23, 456) = 2.291 | F (1, 456) = 0.2185 | F (23, 456) = 0.6089 |
| p = 0.0007 | ns | ns | |
| NREM sleep | F (23, 456) = 2.361 | F (1, 456) = 0.08843 | F (23, 456) = 0.5142 |
| p = 0.0004 | ns | ns | |
| REM sleep | F (23, 456) = 2.964 | F (1, 456) = 4.994 | F (23, 456) = 1.052 |
| p < 0.0001 | p = 0.0259 | ns | |
| REM/Total sleep | F (23, 442) = 1.979 | F (1, 442) = 0.6342 | F (23, 442) = 1.025 |
| p = 0.0048 | ns | ns | |
| PIR | F (23, 480) = 4.663 | F (1, 480) = 0.5301 | F (23, 480) = 0.6357 |
| p < 0.0001 | ns | ns |
| States | Time of Day | Treatment | Interaction |
|---|---|---|---|
| Treatment day | |||
| Theta in waking | F (23, 384) = 5.844 | F (1, 384) = 45.62 | F (23, 384) = 0.8229 |
| p < 0.0001 | p < 0.0001 | ns 1 | |
| Theta in NREM sleep | F (23, 345) = 1.676 | F (1, 345) = 25.89 | F (23, 345) = 0.5991 |
| p = 0.0278 | p < 0.0001 | ns | |
| Theta in REM sleep | F (11, 153) = 0.5196 | F (1, 153) = 60.51 | F (11, 153) = 0.3431 |
| ns | p < 0.0001 | ns | |
| SWA in NREM sleep | F (1, 339) = 5.955 | F (22, 339) = 3.652 | F (22, 339) = 0.8643 |
| p < 0.0001 | p = 0.0152 | ns | |
| Recovery day | |||
| Theta in waking | F (23, 384) = 1.174 | F (1, 384) = 80.43 | F (23, 384) = 0.9871 |
| ns | p < 0.0001 | ns | |
| Theta in NREM sleep | F (23, 362) = 2.214 | F (1, 362) = 15.37 | F (23, 362) = 0.3396 |
| p = 0.0012 | p = 0.0001 | ns | |
| Theta in REM sleep | F (11, 144) = 0.5267 | F (1, 144) = 59.84 | F (11, 144) = 1.162 |
| ns | p < 0.0001 | ns | |
| SWA in NREM sleep | F (23, 362) = 2.491 | F (1, 362) = 1.901 | F (23, 362) = 0.5437 |
| p = 0.0002 | ns | ns |
| States | Time of Day | Treatment | Interaction |
|---|---|---|---|
| Treatment day | |||
| Waking | F (23, 192) = 0.1205 | F (1, 192) = 10.85 | F (23, 192) = 0.1161 |
| ns 1 | p = 0.0012 | ns | |
| NREM sleep | F (21, 173) = 0.6057 | F (1, 173) = 74.28 | F (21, 173) = 0.5898 |
| ns | p < 0.0001 | ns | |
| REM sleep | F (20, 146) = 0.1174 | F (1, 146) = 19.31 | F (20, 146) = 0.2023 |
| ns | p < 0.0001 | ns | |
| Recovery day | |||
| Waking | F (23, 192) = 0.1227 | F (1, 192) = 21.47 | F (23, 192) = 0.06239 |
| ns | p < 0.0001 | ns | |
| NREM sleep | F (23, 188) = 1.282 | F (1, 188) = 144.3 | F (23, 188) = 0.3960 |
| ns | p < 0.0001 | ns | |
| REM sleep | F (23, 158) = 0.3248 | F (1, 158) = 46.23 | F (23, 158) = 0.1461 |
| ns | p < 0.0001 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deboer, T. Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions. Clocks & Sleep 2022, 4, 260-276. https://doi.org/10.3390/clockssleep4020023
Wang Y, Deboer T. Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions. Clocks & Sleep. 2022; 4(2):260-276. https://doi.org/10.3390/clockssleep4020023
Chicago/Turabian StyleWang, Yumeng, and Tom Deboer. 2022. "Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions" Clocks & Sleep 4, no. 2: 260-276. https://doi.org/10.3390/clockssleep4020023
APA StyleWang, Y., & Deboer, T. (2022). Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions. Clocks & Sleep, 4(2), 260-276. https://doi.org/10.3390/clockssleep4020023







