Hormone Targets for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia: A Narrative Review
Abstract
:1. Introduction
- (1)
- Does the treatment of melatonin improve insomnia in women with schizophrenia at the end of the reproductive lifespan?
- (2)
- Does the use of estradiol, progesterone, and other sex hormones make a difference when treating insomnia?
- (3)
- Does raloxifene improve insomnia in postmenopausal women with schizophrenia?
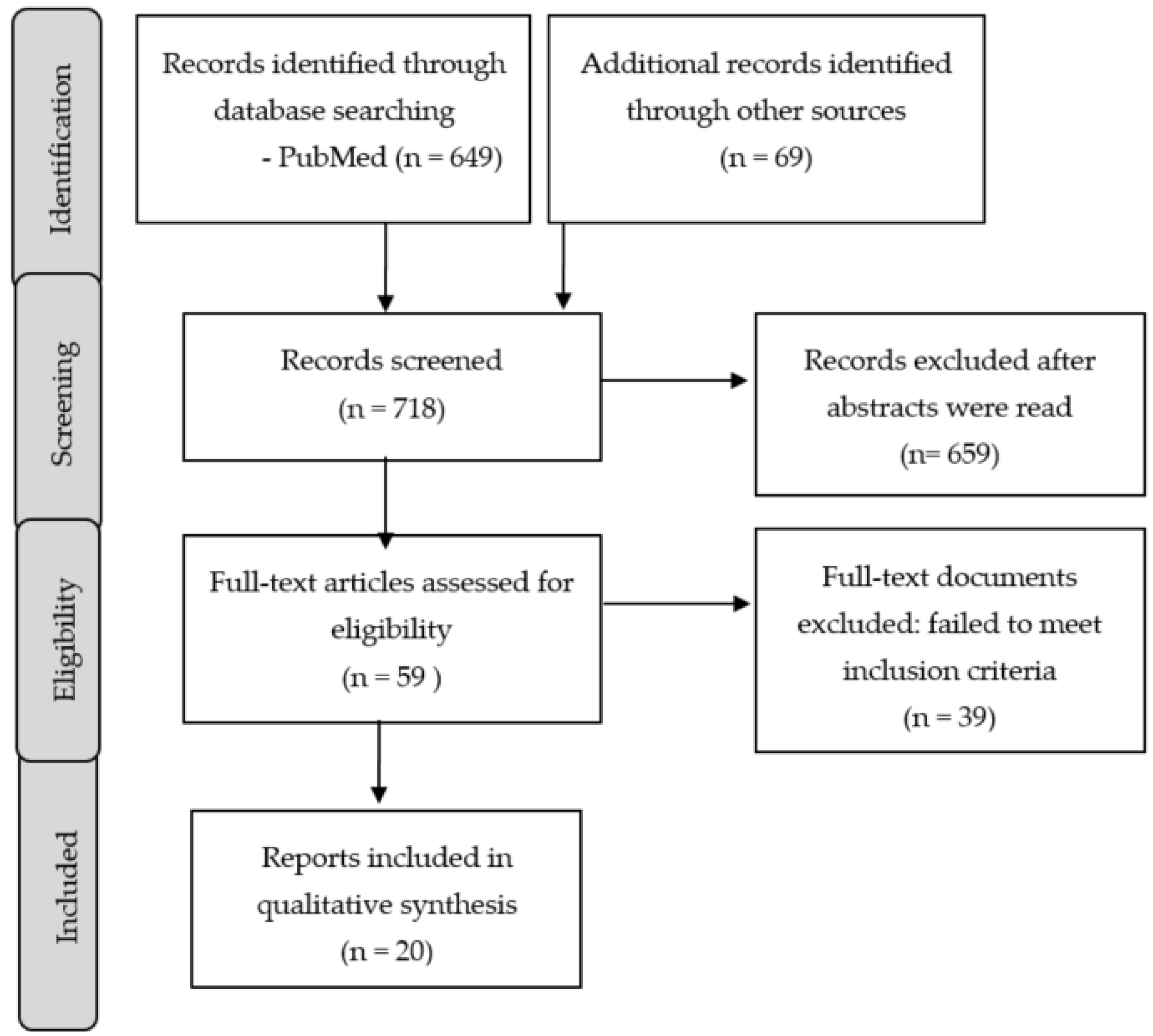
2. Methods
3. Results
3.1. Melatonin for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia
3.2. Sex Hormones for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia
3.2.1. Estradiol Use to Treat Sleep Disorders
3.2.2. Progesterone Use to Treat Sleep Disorders
3.2.3. Testosterone and Dehydroepiandrosterone (as a Precursor of Testosterone) to Treat Sleep Disorders
3.2.4. Raloxifene Use to Treat Sleep Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Reeve, S.; Sheaves, B.; Freeman, D. Sleep Disorders in Early Psychosis: Incidence, Severity, and Association with Clinical Symptoms. Schizophr. Bull. 2019, 45, 287–295. [Google Scholar] [CrossRef]
- Waite, F.; Myers, E.; Harvey, A.G.; Espie, C.A.; Startup, H.; Sheaves, B.; Freeman, D. Treating Sleep Problems in Patients with Schizophrenia. Behav. Cogn. Psychother. 2016, 44, 273–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.X.; Lam, S.P.; Zhang, J.; Yu, M.W.; Chan, J.W.; Chan, C.S.; Espie, C.A.; Freeman, D.; Mason, O.; Wing, Y.K. Sleep Disturbances and Suicide Risk in an 8-Year Longitudinal Study of Schizophrenia-Spectrum Disorders. Sleep 2016, 39, 1275–1282. [Google Scholar] [CrossRef]
- Miller, B.J.; Parker, C.B.; Rapaport, M.H.; Buckley, P.F.; McCall, W.V. Insomnia and suicidal ideation in nonaffective psychosis. Sleep 2019, 42, zsy215. [Google Scholar] [CrossRef]
- Stummer, L.; Markovic, M.; Maroney, M.E. Pharmacologic Treatment Options for Insomnia in Patients with Schizophrenia. Medicines 2018, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Monti, J.M.; BaHammam, A.S.; Pandi-Perumal, S.R.; Bromundt, V.; Spence, D.W.; Cardinali, D.P.; Brown, G.M. Sleep and circadian rhythm dysregulation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 209–216. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.K.; Kim, S.K.; Cho, A.R.; Kim, J.W.; Yim, S.V.; Chung, J.H. Association of polymorphism in the promoter of the melatonin receptor 1A gene with schizophrenia and with insomnia symptoms in schizophrenia patients. J. Mol. Neurosci. 2011, 45, 304–308. [Google Scholar] [CrossRef]
- Afonso, P.; Figueira, M.L.; Paiva, T. Sleep-promoting action of the endogenous melatonin in schizophrenia compared to healthy controls. Int. J. Psychiatry Clin. Pract. 2011, 15, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bersani, G.; Mameli, M.; Garavini, A.; Pancheri, P.; Nordio, M. Reduction of night/day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Neuro Endocrinol. Lett. 2003, 24, 181–184. [Google Scholar] [PubMed]
- Shirayama, Y.; Takahashi, M.; Suzuki, M.; Tsuruoka, Y.; Sato, K. Effects of Add-on Ramelteon on Cognitive Impairment in Patients with Schizophrenia: An Open-label Pilot Trial. Clin. Psychopharmacol. Neurosci. 2014, 12, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, N.; Gurvich, C.; Hudaib, A.R.; Gavrilidis, E.; Kulkarni, J. Dissecting the syndrome of schizophrenia: Associations between symptomatology and hormone levels in women with schizophrenia. Psychiatry Res. 2019, 280, 112510. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Gurvich, C.; Hudaib, A.R.; Gavrilidis, E.; de Castella, R.A.; Thomas, E.H.; Kulkarni, J. Serum estradiol as a blood-based biomarker predicting hormonal treatment outcomes in women with schizophrenia. Psychoneuroendocrinology 2021, 126, 105165. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Y.H.; Huang, S.Q.; Chen, H.; Li, Z.F.; Li, X.X.; Li, X.S.; Cheng, Y. Serum Progesterone and Testosterone Levels in Schizophrenia Patients at Different Stages of Treatment. J. Mol. Neurosci. 2021, 71, 1168–1173. [Google Scholar] [CrossRef]
- Gogos, A.; Ney, L.J.; Seymour, N.; Van Rheenen, T.E.; Felmingham, K.L. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? Br. J. Pharmacol. 2019, 176, 4119–4135. [Google Scholar] [CrossRef]
- Buoli, M.; Caldiroli, A.; Serati, M.; Grassi, S.; Altamura, A.C. Sex Steroids and Major Psychoses: Which Role for DHEA-S and Progesterone. Neuropsychobiology 2016, 73, 178–183. [Google Scholar] [CrossRef]
- Ray, P.; Mandal, N.; Sinha, V.K. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch. Womens Ment. Health 2020, 23, 113–122. [Google Scholar] [CrossRef]
- Van den Buuse, M.; Mingon, R.L.; Gogos, A. Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci. Lett. 2015, 607, 72–76. [Google Scholar] [CrossRef]
- Frye, C.A.; Sora, I. Progesterone reduces hyperactivity of female and male dopamine transporter knockout mice. Behav. Brain Res. 2010, 209, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogos, A.; van den Buuse, M. Comparing the effects of 17β-oestradiol and the selective oestrogen receptor modulators, raloxifene and tamoxifen, on prepulse inhibition in female rats. Schizophr. Res. 2015, 168, 634–639. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Bernardo, M.; Penadés, R.; Arias, B.; Ruiz Cortés, V.; Seeman, M.V.; Catalán, R. Do FSH/LH ratio and gonadal hormone levels predict clinical improvement in postmenopausal schizophrenia women? Arch. Womens Ment. Health 2017, 20, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Schaedel, Z.; Holloway, D.; Bruce, D.; Rymer, J. Management of sleep disorders in the menopausal transition. Post. Reprod. Health 2021, 27, 209–214. [Google Scholar] [CrossRef]
- Caretto, M.; Giannini, A.; Simoncini, T. An integrated approach to diagnosing and managing sleep disorders in menopausal women. Maturitas 2019, 128, 1–3. [Google Scholar] [CrossRef]
- Proserpio, P.; Marra, S.; Campana, C.; Agostoni, E.C.; Palagini, L.; Nobili, L.; Nappi, R.E. Insomnia and menopause: A narrative review on mechanisms and treatments. Climacteric 2020, 23, 539–549. [Google Scholar] [CrossRef]
- Duan, C.; Jenkins, Z.M.; Castle, D. Therapeutic use of melatonin in schizophrenia: A systematic review. World J. Psychiatry 2021, 11, 463–476. [Google Scholar] [CrossRef]
- Borba, C.P.; Fan, X.; Copeland, P.M.; Paiva, A.; Freudenreich, O.; Henderson, D.C. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J. Clin. Psychopharmacol. 2011, 31, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Sahbaz, C.; Özer, O.F.; Kurtulmus, A.; Kırpınar, I.; Sahin, F.; Guloksuz, S. Evidence for an association of serum melatonin concentrations with recognition and circadian preferences in patients with schizophrenia. Metab. Brain. Dis. 2019, 34, 865–868. [Google Scholar] [CrossRef]
- Maiti, R.; Mishra, B.R.; Jena, M.; Mishra, A.; Nath, S. Effect of Haloperidol and Risperidone on Serum Melatonin and GAP-43 in Patients with Schizophrenia: A Prospective Cohort Study. Clin. Psychopharmacol. Neurosci. 2021, 19, 125–134. [Google Scholar] [CrossRef]
- Palagini, L.; Manni, R.; Aguglia, E.; Amore, M.; Brugnoli, R.; Bioulac, S.; Bourgin, P.; Micoulaud Franchi, J.A.; Girardi, P.; Grassi, L.; et al. International Expert Opinions and Recommendations on the Use of Melatonin in the Treatment of Insomnia and Circadian Sleep Disturbances in Adult Neuropsychiatric Disorders. Front. Psychiatry 2021, 12, 688890. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, P.A.; Micoulaud Franchi, J.A.; Lopez, R.; Schroder, C.M.; Membres du Consensus Mélatonine SFRMS. The use of melatonin in adult psychiatric disorders: Expert recommendations by the French institute of medical research on sleep (SFRMS). Encephale 2019, 45, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Maiti, R.; Mishra, B.R.; Jena, M.; Nath, S.; Sahu, P. Effect of add-on ramelteon therapy on sleep and circadian rhythm disruption in patients with schizophrenia: A randomized controlled trial. Eur. Neuropsychopharmacol. 2020, 31, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, P.N.; Andrade, C.; Bhakta, S.G.; Singh, N.M. Melatonin in schizophrenic outpatients with insomnia: A double-blind, placebo-controlled study. J. Clin. Psychiatry 2007, 68, 237–241. [Google Scholar] [CrossRef]
- Shamir, E.; Laudon, M.; Barak, Y.; Anis, Y.; Rotenberg, V.; Elizur, A.; Zisapel, N. Melatonin improves sleep quality of patients with chronic schizophrenia. J. Clin. Psychiatry 2000, 61, 373–377. [Google Scholar] [CrossRef]
- Baandrup, L.; Lindschou, J.; Winkel, P.; Gluud, C.; Glenthoj, B.Y. Prolonged-release melatonin versus placebo for benzodiazepine discontinuation in patients with schizophrenia or bipolar disorder: A randomised, placebo-controlled, blinded trial. World J. Biol. Psychiatry 2016, 17, 514–524. [Google Scholar] [CrossRef]
- Lord, C.; Sekerovic, Z.; Carrier, J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol. Biol. 2014, 62, 302–310. [Google Scholar] [CrossRef]
- Bezerra, A.G.; Andersen, M.L.; Pires, G.N.; Tufik, S.; Hachul, H. Effects of hormonal contraceptives on sleep—A possible treatment for insomnia in premenopausal women. Sleep Sci. 2018, 11, 129–136. [Google Scholar] [CrossRef]
- Kalleinen, N.; Aittokallio, J.; Lampio, L.; Kaisti, M.; Polo-Kantola, P.; Polo, O.; Heinonen, O.J.; Saaresranta, T. Sleep during menopausal transition: A 10-year follow-up. Sleep 2021, 44, zsaa283. [Google Scholar] [CrossRef]
- Morssinkhof, M.W.L.; van Wylick, D.W.; Priester-Vink, S.; van der Werf, Y.D.; den Heijer, M.; van den Heuvel, O.A.; Broekman, B.F.P. Associations between sex hormones, sleep problems and depression: A systematic review. Neurosci. Biobehav. Rev. 2020, 118, 669–680. [Google Scholar] [CrossRef]
- Cai, H.; Zhou, X.; Dougherty, G.G.; Reddy, R.D.; Haas, G.L.; Montrose, D.M.; Keshavan, M.; Yao, J.K. Pregnenolone-progesterone-allopregnanolone pathway as a potential therapeutic target in first-episode antipsychotic-naïve patients with schizophrenia. Psychoneuroendocrinology 2018, 90, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Glue, P. Progesterone loading as a strategy for treating postpartum depression. Hum. Psychopharmacol. 2020, 35, e2731. [Google Scholar] [CrossRef] [PubMed]
- Sander, B.; Muftah, A.; Sykes Tottenham, L.; Grummisch, J.A.; Gordon, J.L. Testosterone and depressive symptoms during the late menopause transition. Biol. Sex Differ. 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Kianimehr, G.; Fatehi, F.; Hashempoor, S.; Khodaei-Ardakani, M.R.; Rezaei, F.; Nazari, A.; Kashani, L.; Akhondzadeh, S. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: A randomized double-blind and placebo controlled trial. Daru 2014, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Albertazzi, P.; Missiroli, N.; Pedrini, D.; Salgarello, M. Effects of raloxifene on mood, sleep, libido and cognitive function in postmenopausal healthy women: A pilot study. Maturitas 2004, 48, 59–63. [Google Scholar] [CrossRef]
- Biri, A.; Korucuoglu, U.; Ilhan, M.N.; Ciftci, B.; Bozkurt, N.; Guner, H. Evaluation of the sexual function and quality of life in raloxifene treated postmenopausal women. Arch. Gynecol. Obstet. 2009, 279, 505–509. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zheng, W.; Li, X.H.; Cai, D.B.; Yang, X.H.; Ungvari, G.S.; Ng, C.H.; Wang, X.P.; Kulkarni, J.; Grigg, J.; et al. Adjunctive raloxifene for postmenopausal women with schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophr. Res. 2018, 197, 288–293. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Wang, Y.; Li, X. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: A meta-analysis of randomized controlled trials. Arch. Womens Ment. Health 2018, 21, 31–41. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Vishnoi, S.; Kaushik, M.; Parveen, K.; Tabassum, H.; Akram, M.; Parvez, S. Reversal of Schizophrenia-like Symptoms and Cholinergic Alterations by Melatonin. Arch. Med. Res. 2019, 50, 295–303. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Aina, O.A.; Onaolapo, O.J. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed. Pharmacother. 2017, 92, 373–383. [Google Scholar] [CrossRef]
- Afonso, A.C.; Pacheco, F.D.; Canever, L.; Wessler, P.G.; Mastella, G.A.; Godoi, A.K.; Hubbe, I.; Bischoff, L.M.; Bialecki, A.V.S.; Zugno, A.I. Schizophrenia-like behavior is not altered by melatonin supplementation in rodents. An. Acad. Bras. Cienc. 2020, 92, e20190981. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.S.; El-Deek, H.E. Melatonin modulates inflammatory mediators and improves olanzapine-induced hepatic steatosis in rat model of schizophrenia. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 64–75. [Google Scholar] [PubMed]
- Igwe, S.C.; Brigo, F. Does Melatonin and Melatonin Agonists Improve the Metabolic Side Effects of Atypical Antipsychotics?: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2018, 16, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.; Coroa, M.; Madeira, N. Treatment Options for Insomnia in Schizophrenia: A Systematic Review. Pharmacopsychiatry 2019, 52, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Arad, M.; Weiner, I. Contrasting effects of increased and decreased dopamine transmission on latent inhibition in ovariectomized rats and their modulation by 17beta-estradiol: An animal model of menopausal psychosis? Neuropsychopharmacology 2010, 35, 1570–1582. [Google Scholar] [CrossRef] [Green Version]
- González-Rodríguez, A.; Seeman, M.V. Pharmacotherapy for schizophrenia in postmenopausal women. Expert. Opin. Pharmacother. 2018, 19, 809–821. [Google Scholar] [CrossRef]
- Labad, J.; Cobo, J.; Núñez, C.; Creus, M.; García-Parés, G.; Cuadras, D.; Franco, J.; Miquel, E.; Reyes, J.C.; Marcó-García, S.; et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: A 24-week double-blind, randomized, parallel, placebo-controlled trial. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 729–737. [Google Scholar]
- Kaskie, R.E.; Graziano, B.; Ferrarelli, F. Schizophrenia and sleep disorders: Links, risks, and management challenges. Nat. Sci. Sleep 2017, 9, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Baker, F.C.; de Zambotti, M.; Colrain, I.M.; Bei, B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Kornstein, S.G.; Clayton, A.H.; Bao, W.; Guico-Pabia, C.J. A pooled analysis of the efficacy of desvenlafaxine for the treatment of major depressive disorder in perimenopausal and postmenopausal women. J. Womens Health 2015, 24, 281–290. [Google Scholar] [CrossRef]
| Author and Year of Publication | Study Design | Sample N | Comparison Groups | Assessment | Results |
|---|---|---|---|---|---|
| Mishra A et al., 2020 [33] | Randomized rater-blinded | 120 (49 F; 71 M) | Ramelteon plus risperidone or haloperidol, and control group receiving haloperidol or risperidone. | Baseline serum melatonin; serum AANAT; urinary melatonin; Pittsburgh Sleep Quality Index (PSQI). | Increase in night-time melatonin levels, AANAT serum, and urinary melatonin, and decrease in PSQI scores in ramelteon group. |
| Suresh Kumar et al., 2007 [34] | Randomised, double-blind | 40 (13 F; 27 M) | Melatonin (3–12 mg) and placebo. | 15-item structured questionnaire about sleep functioning. | Melatonin improved the sleep functioning better than placebo. |
| Shamir E et al., 2000 [35] | Randomised, double-blind, cross-over | 19 (7 F; 12 M) | Controlled-release melatonin (2 mg) and placebo. | Activity- and rest-derived sleep/awake episodes. | Melatonin improved sleep efficiency and increased sleep duration. |
| Animal Studies | |
|---|---|
| Estrogens are neuroprotective and show a positive influence on behavioral symptoms in animal models of schizophrenia (e.g., prepulse inhibition in rats). | Estrogens may influence sleep regulation. |
| Human Studies | |
| Estrogens may vary according to the phases of the menstrual cycle. High estrogen levels are associated with improvements in psychotic symptoms. | Estrogens and progesterone differ according to the phases of the menstrual cycle in premenopausal women and are found to influence sleep. |
| Sleep architecture worsens during the menopausal transition. Insomnia is frequent at menopause. | Loss of estrogens is associated with sleep disturbances at menopause. In schizophrenic women, this association may be stronger. |
| Hormone | Hypothesis | Findings |
|---|---|---|
| Estradiol | Estradiol is capable of preventing dopamine D1/D2-receptor-mediated disruptions of sensorimotor gating in animal models. | Is estradiol a potential target for the treatment of insomnia? No results for postmenopausal populations. |
| Progesterone | Brexanolone, a synthetic allopregnanolone, prevents depression-like behaviors in animal models. | The psychotropic properties of progesterone have not been evaluated in postmenopausal schizophrenic patients. |
| Testosterone | High testosterone–estradiol ratio at menopause. Testosterone implicated in physiopathology of schizophrenia. | The use of testosterone to treat insomnia has not been evaluated. |
| Dehydroepiandrosterone (DHEA) | Precursors of androgens in women may be effective for the treatment of insomnia at menopause. | Potentially effective for the treatment of psychotic or cognitive symptoms; no results for insomnia. |
| Raloxifene (SERMs) | Positive effect on sleep in healthy women. | Potential effectiveness in postmenopausal schizophrenia. Future studies should consider insomnia as a primary outcome. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, A.; Haba-Rubio, J.; Usall, J.; Natividad, M.; Soria, V.; Labad, J.; Monreal, J.A. Hormone Targets for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia: A Narrative Review. Clocks & Sleep 2022, 4, 52-65. https://doi.org/10.3390/clockssleep4010007
González-Rodríguez A, Haba-Rubio J, Usall J, Natividad M, Soria V, Labad J, Monreal JA. Hormone Targets for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia: A Narrative Review. Clocks & Sleep. 2022; 4(1):52-65. https://doi.org/10.3390/clockssleep4010007
Chicago/Turabian StyleGonzález-Rodríguez, Alexandre, José Haba-Rubio, Judith Usall, Mentxu Natividad, Virginia Soria, Javier Labad, and José A. Monreal. 2022. "Hormone Targets for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia: A Narrative Review" Clocks & Sleep 4, no. 1: 52-65. https://doi.org/10.3390/clockssleep4010007
APA StyleGonzález-Rodríguez, A., Haba-Rubio, J., Usall, J., Natividad, M., Soria, V., Labad, J., & Monreal, J. A. (2022). Hormone Targets for the Treatment of Sleep Disorders in Postmenopausal Women with Schizophrenia: A Narrative Review. Clocks & Sleep, 4(1), 52-65. https://doi.org/10.3390/clockssleep4010007









