Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Experimental Procedures
2.3. Data Collection and Analyses
2.3.1. Bifurcation Symmetry Index (BSI)
2.3.2. Entrainment Quotient in T15/T30
2.3.3. DimDim Data
2.3.4. Statistical Tests
3. Results
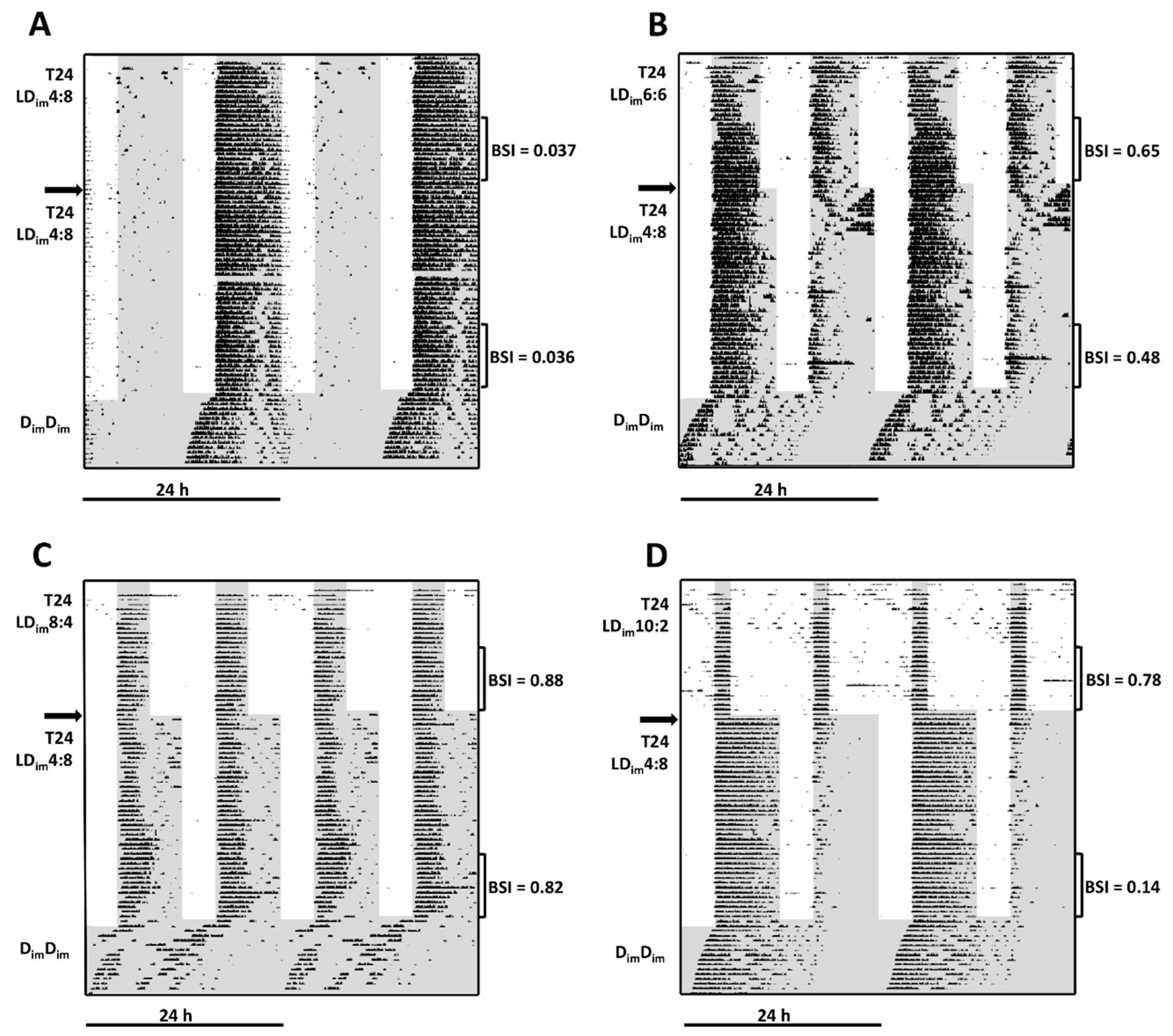
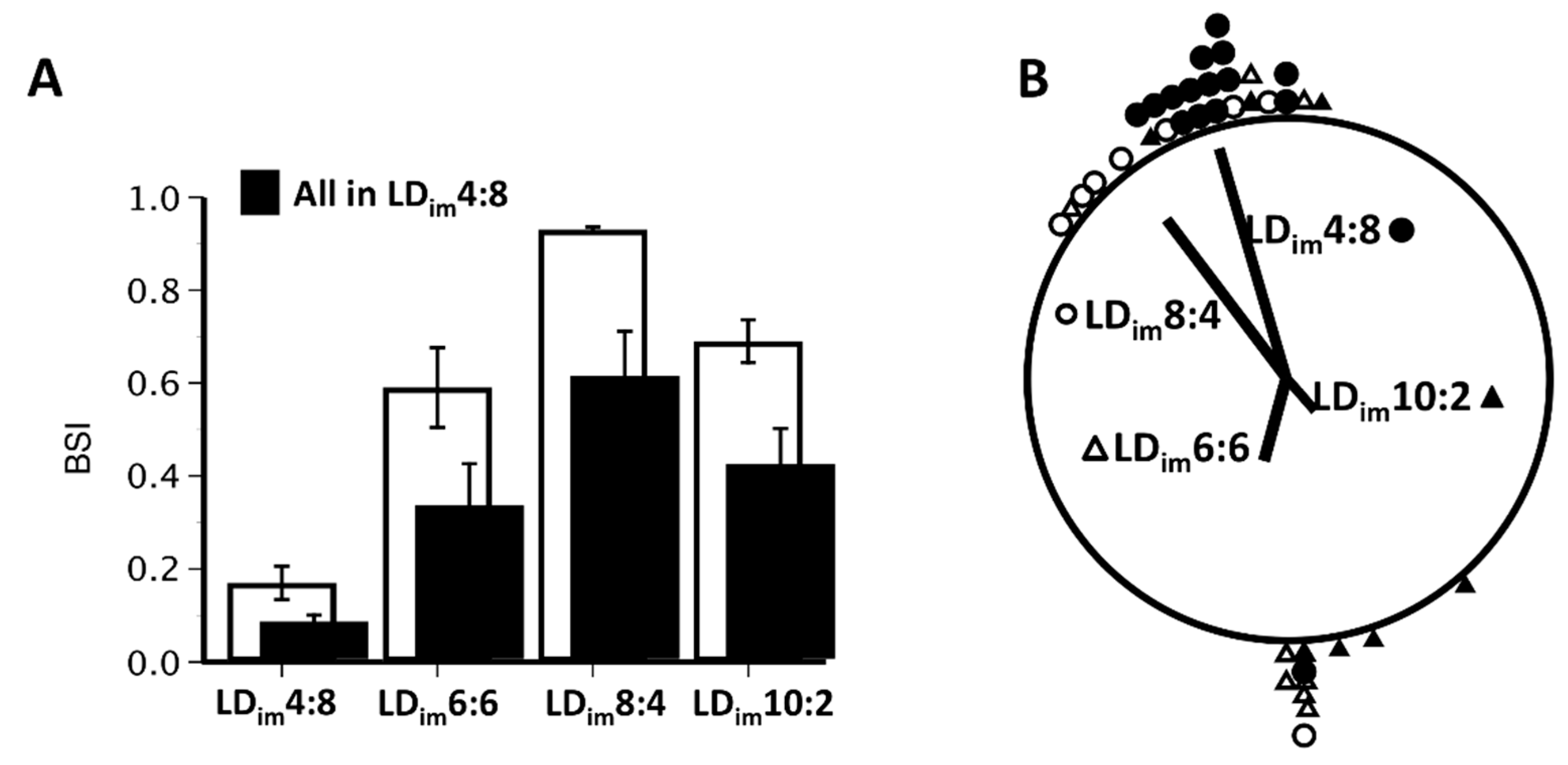
3.1. Experiment 1
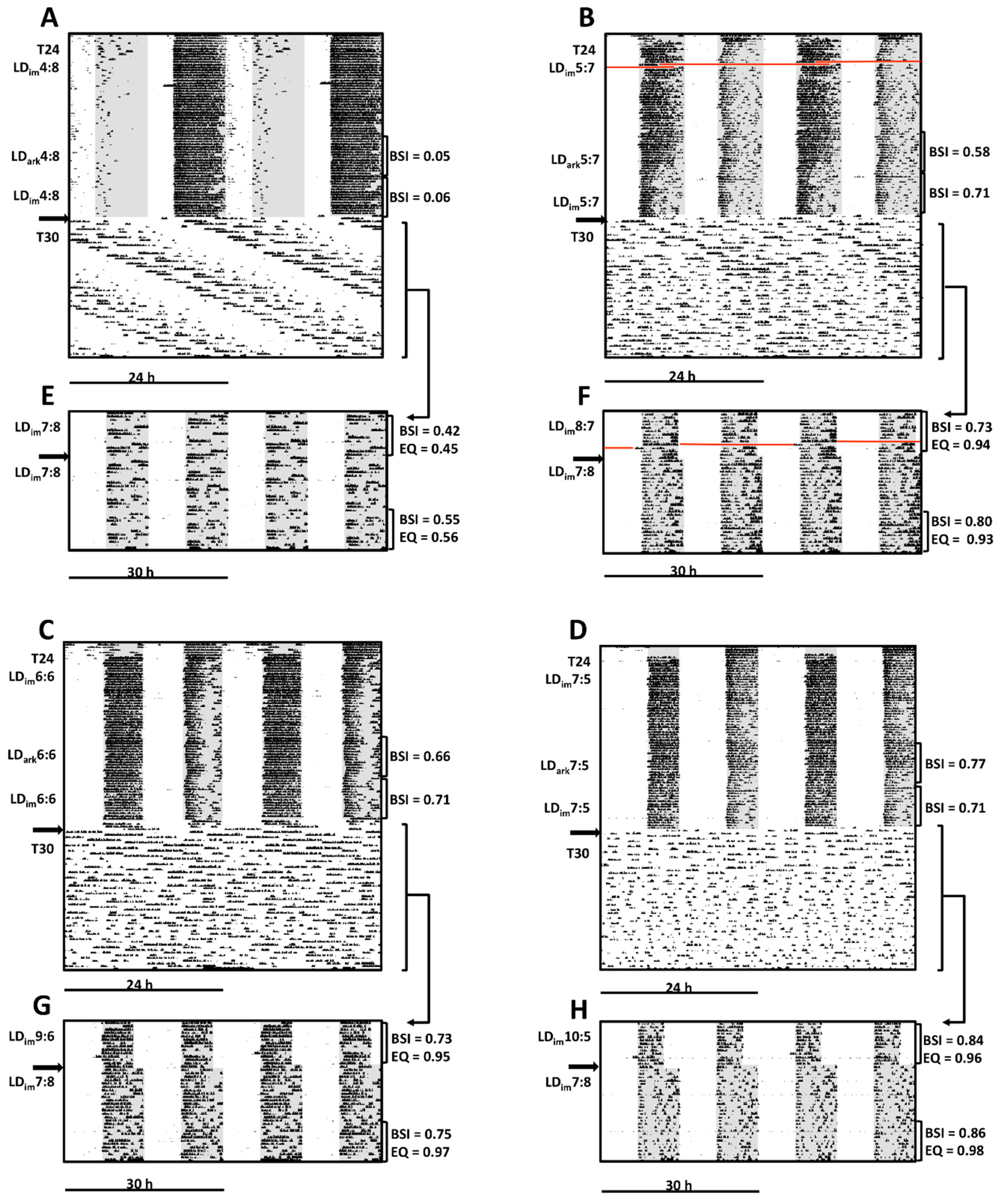
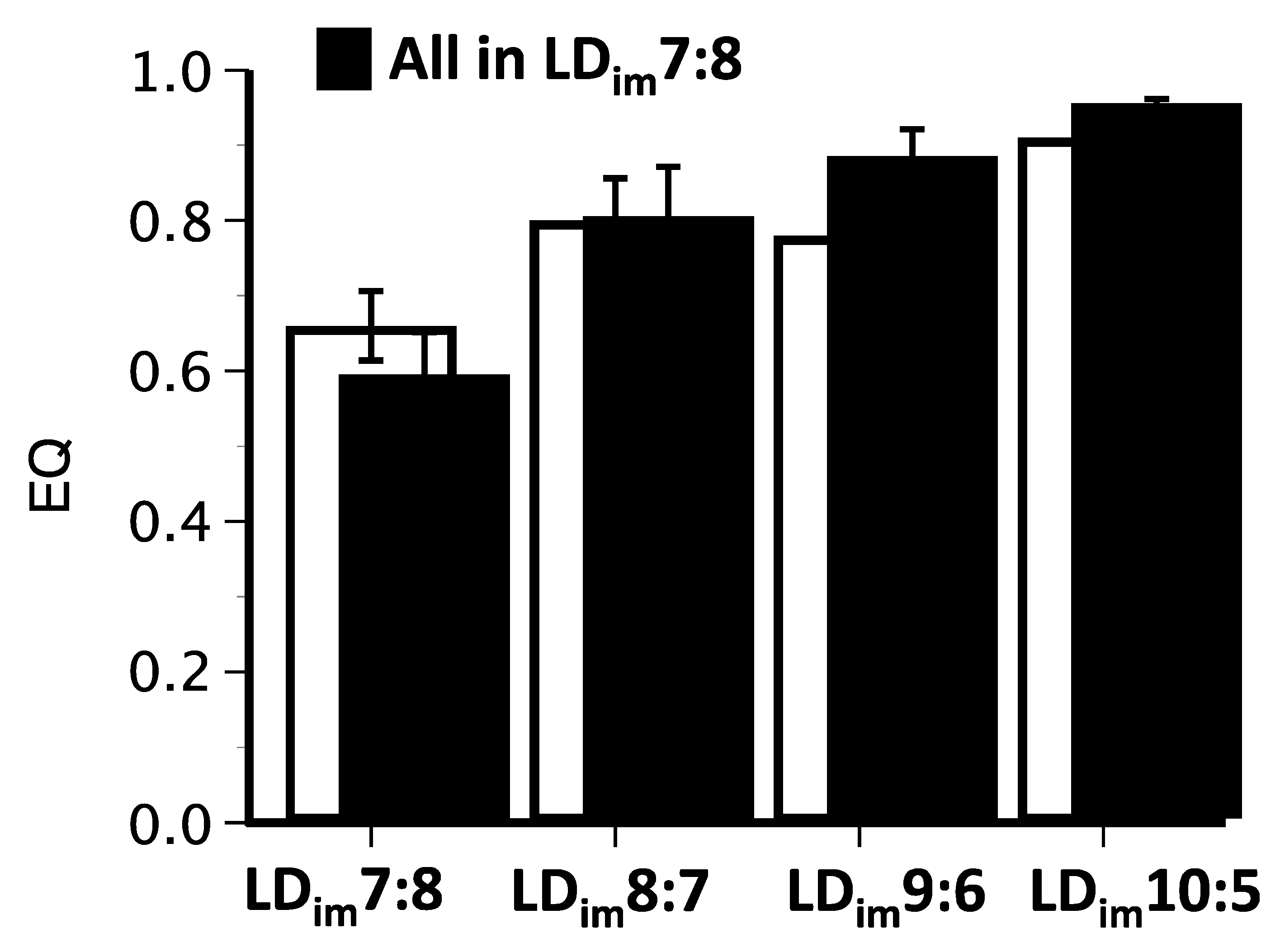
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LDLD | light:dark:light:dark |
| SCN | suprachiasmatic nuclei |
| T12 | 12 h cycle length |
| BSI | Bifurcation symmetry index |
| EQ | Entrainment quotient |
| LDim | Light:dim |
| DimDim | Constant dim light |
| PRC | Phase response curve |
| FRP | Free-running period |
References
- Moore, R.Y. The Suprachiasmatic Nucleus and the Circadian Timing System. Prog. Mol. Biol. Transl. Sci. 2013, 119, 1–28. [Google Scholar] [PubMed]
- Takahashi, J.S. Transcriptisonal architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties SCN: Suprachiasmatic nucleus. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Merrow, M.; Roenneberg, T. Photoperiodism in Neurospora crassa. J. Biol. Rhythm. 2004, 19, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sumova, A.; Travnickova, Z.; Peters, R.; Schwartz, W.J.; Illnerova, H. The rat suprachiasmatic nucleus is a clock for all seasons. Proc. Natl. Acad. Sci. USA 1995, 92, 7754–7758. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, P.; Vanin, S.; Yoshii, T.; Rieger, D.; Hermann, C.; Dusik, V.; Kyriacou, C.P.; Helfrich-Förster, C.; Costa, R. Drosophila clock neurons under natural conditions. J. Biol. Rhythm. 2013, 28, 3–14. [Google Scholar] [CrossRef]
- Myung, J.; Pauls, S.D. Encoding seasonal information in a two-oscillator model of the multi-oscillator circadian clock. Eur. J. Neurosci. 2018, 48, 2718–2727. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The Stability and Lability of Spontaneous Frequency. J. Comp. Physiol. A Sens. Neural Behav. Physiol. A 1976, 106, 223–252. [Google Scholar] [CrossRef]
- Azzi, A.; Dallmann, R.; Casserly, A.; Rehrauer, H.; Patrignani, A.; Maier, B.; Kramer, A.; Brown, S.A. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat. Neurosci. 2014, 17, 377–382. [Google Scholar] [CrossRef]
- VanderLeest, H.T.; Houben, T.; Michel, S.; Deboer, T.; Albus, H.; Vansteensel, M.J.; Block, G.D.; Meijer, J.H. Seasonal Encoding by the Circadian Pacemaker of the SCN. Curr. Biol. 2007, 17, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Sumová, A.; Bendová, Z.; Sládek, M.; Iková, Z.K.Č.; Illnerová, H. Seasonal molecular timekeeping within the rat circadian clock. Physiol. Res. 2004, 53, S167–S176. [Google Scholar]
- Gorman, M.R.; Zucker, I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus. Am. J. Physiol. 1997, 272, R887–R895. [Google Scholar] [CrossRef]
- Ruby, N.F.; Saran, A.; Kang, T.; Franken, P.; Heller, H.C. Siberian hamsters free run or become arrhythmic after a phase delay of the photocycle. Am. J. Physiol. 1996, 271, R881–R890. [Google Scholar] [CrossRef]
- Puchalski, W.; Lynch, G.R. Circadian characteristics of Djungarian hamsters: Effects of photoperiodic pretreatment and artificial selection. Am. J. Physiol. Integr. Comp. Physiol. 1991, 261, R670–R676. [Google Scholar] [CrossRef]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R539–R546. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S.; Elliott, J.; Takamura, T. The Circadian Component in Photoperiodic Induction. Ciba Found. Symp. 1984, 104, 26–41. [Google Scholar]
- Glickman, G.; Webb, I.C.; Elliott, J.A.; Baltazar, R.M.; Reale, M.E.; Lehman, M.N.; Gorman, M.R. Photic Sensitivity for Circadian Response to Light Varies with Photoperiod. J. Biol. Rhythm. 2012, 27, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Glickman, G.L.; Harrison, E.M.; Elliott, J.A.; Gorman, M.R. Increased photic sensitivity for phase resetting but not melatonin suppression in Siberian hamsters under short photoperiods. Horm. Behav. 2014, 65, 301–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorman, M.R.; Elliott, J.A. Entrainment of 2 Subjective Nights by Daily Light:Dark:Light:Dark Cycles in 3 Rodent Species. J. Biol. Rhythm. 2003, 18, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Walbeek, T.J.; Sun, J.; Johnson, J.; Poonawala, Q.; Gorman, M.R. Extraordinary behavioral entrainment following circadian rhythm bifurcation in mice. Sci. Rep. 2016, 6, 38479. [Google Scholar] [CrossRef] [PubMed]
- Walbeek, T.J.; Gorman, M.R. Simple Lighting Manipulations Facilitate Behavioral Entrainment of Mice to 18-h Days. J. Biol. Rhythm. 2017, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Gorman, M.R.; Elliott, J.A. Exceptional entrainment of circadian activity rhythms with manipulations of rhythm waveform in male Syrian hamsters. Yale J. Biol. Med. 2019, 92, 187–199. [Google Scholar] [PubMed]
- Harrison, E.M.; Gorman, M.R. Changing the Waveform of Circadian Rhythms: Considerations for Shift-Work. Front. Neurol. 2012, 3, 72. [Google Scholar] [CrossRef]
- Gorman, M.R.; Elliott, J.A.; Evans, J.A. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol. Int. 2003, 20, 233–248. [Google Scholar] [CrossRef]
- Gorman, M.R.; Elliott, J.A. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 2004, 190, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Walbeek, T.J.; Joye, D.A.M.; Mishra, I.; Gorman, M.R. Physiological, behavioral and environmental factors influence bifurcated circadian entrainment in mice, under review. under review.
- Gorman, M.R.; Steele, N.A. Phase angle difference alters coupling relations of functionally distinct circadian oscillators revealed by rhythm splitting. J. Biol. Rhythm. 2006, 21, 195–205. [Google Scholar] [CrossRef]
- Gorman, M.R.; Lee, T.M. Daily novel wheel running reorganizes and splits hamster circadian activity rhythms. J. Biol. Rhythm. 2001, 16, 541–551. [Google Scholar] [CrossRef]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Circadian entrainment and phase resetting differ markedly under dimly illuminated versus completely dark nights. Behav. Brain Res. 2005, 162, 116–126. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Janik, D. Behavioral Decoupling of Circadian Rhythms. J. Biol. Rhythm. 1993, 8, 57–65. [Google Scholar] [CrossRef]
- Raiewski, E.E.; Elliott, J.A.; Evans, J.A.; Glickman, G.L.; Gorman, M.R. Twice daily melatonin peaks in Siberian but not Syrian hamsters under 24 h Light:Dark:Light:Dark cycles. Chronobiol. Int. 2012, 29, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Silver, R.; Gorman, M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J. Biol. Rhythm. 2010, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Naito, E.; Nakao, N.; Tei, H.; Yoshimura, T.; Ebihara, S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-h light and 5-h dark schedule. J. Biol. Rhythm. 2007, 22, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Gorman, M.R. Rapid Adjustment of Circadian Clocks to Simulated Travel to Time Zones across the Globe. J. Biol. Rhythm. 2015, 30, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Harrison, E.M.; Sun, J.; May, D.; Ng, A.; Welsh, D.K.; Gorman, M.R. Circadian rhythm bifurcation induces flexible phase resetting by reducing circadian amplitude. Eur. J. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gorman, M.R. Exotic photoperiods induce and entrain split circadian activity rhythms in hamsters. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2001, 187, 793–800. [Google Scholar] [CrossRef]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Dim nighttime illumination interacts with parametric effects of bright light to increase the stability of circadian rhythm bifurcation in hamsters. Chronobiol. Int. 2011, 28, R156–R157. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr. Biol. 2009, 19, R156–R157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Individual differences in circadian waveform of Siberian hamsters under multiple lighting conditions. J. Biol. Rhythm. 2012, 27, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Abraham, U.; Granada, A.E.; Westermark, P.O.; Heine, M.; Kramer, A.; Herzel, H. Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 2010, 6, 438. [Google Scholar] [CrossRef]
- Schmal, C.; Herzog, E.D.; Herzel, H. Measuring Relative Coupling Strength in Circadian Systems. J. Biol. Rhythm. 2018, 33, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Erzberger, A.; Hampp, G.; Granada, A.E.; Albrecht, U.; Herzel, H. Genetic redundancy strengthens the circadian clock leading to a narrow entrainment range. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Suzuki, T.; Mizoro, Y.; Kori, H.; Okada, K.; Chen, Y.; Fustin, J.-M.; Yamazaki, F.; Mizuguchi, N.; Zhang, J.; et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 2013, 342, 85–90. [Google Scholar] [CrossRef] [PubMed]
| Experiment 1 | # Cycles @ T | Photoperiod Conditions | |||
| Precondition | 10 @ 24 h | LDark14:10 (n = 40) | |||
| Phase 1—Bifurcation Induction | 56 @ 12 h | LDim4:8 (n = 16) | LDim6:6 (n = 8) | LDim8:4 (n = 8) | LDim10:2 (n = 8) |
| Phase 2—Bifurcation After-effects | 84 @ 12 h | LDim4:8 | LDim4:8 | LDim4:8 | LDim4:8 |
| Phase 3—Constant Conditions | 14 @ 24 h | DimDim | DimDim | DimDim | DimDim |
| Experiment 2 | # Cycles @ T | Photoperiod Conditions | |||
| Precondition | 10 @ 24 h | LDark14:10 (n = 44) | |||
| Phase 1a/1b—Bifurcation Induction | 56 @ 12 h | LDim4:8 (n = 10) | LDim5:7 (n = 12) | LDim6:6 (n = 12) | LDim7:5 (n = 10) |
| Phase 1c—Dark nights | 28 @ 12 h | LDark4:8 | LDark5:7 | LDark6:6 | LDark7:5 |
| Phase 1d—Dim nights | 28 @ 12 h | LDim4:8 | LDim5:7 | LDim6:6 | LDim7:5 |
| Phase 2—T15 Entrainment | 26 @ 15 h | LDim7:8 | LDim8:7 | LDim9:6 | LDim10:5 |
| Phase 3—T15 After-effects | 56 @ 15 h | LDim7:8 | LDim7:8 | LDim7:8 | LDim7:8 |
| Group | Kruskal–Wallis Test | ||||
|---|---|---|---|---|---|
| Phase 1 (T24 Photoperiods) | LDim4:8 | LDim6:6 | LDim8:4 | LDim10:2 | |
| BSI | 0.17 ± 0.04 A | 0.59 ± 0.09 B | 0.93 ± 0.01 C | 0.69 ± 0.05 B | H(3) = 28.9; p < 0.001 |
| Periodogram-12 h | 349 ± 28 A | 691 ± 58 B | 869 ± 51 C | 461 ± 28 D | H(3) = 28.7; p < 0.001 |
| Periodogram-24 h | 633 ± 50 A | 208 ± 68 B | 3 ± 1 C | 13 ± 3 B | H(3) = 30.3; p < 0.001 |
| Phase 2 (T24 After-effects) | LDim4:8 | LDim4:8:8 | LDim4:8 | LDim4:8 | |
| BSI | 0.08 ± 0.02 A | 0.33 ± 0.09 B | 0.61 ± 0.10 B | 0.42 ± 0.08 B | H(3) = 18.7; p < 0.001 |
| Periodogram-12 h | 235 ± 19 | 318 ± 66 | 418 ± 70 | 264 ± 58 | H(3) = 4.3; ns |
| Periodogram-24 h | 646 ± 53 A | 372 ± 87 B | 169 ± 101 B | 224 ± 83 B | H(3) = 18.0; p < 0.001 |
| Phase 3 (Constant dim) | DimDim | DimDim | DimDim | DimDim | |
| Onset FRP (h) | 23.68 ± 0.03 A | 23.71 ± 0.05 A | 23.45 ± 0.09 B | 23.81 ± 0.03 A | H(3) = 11.7; p < 0.01 |
| Periodogram FRP (h) | 23.70 ± 0.04 | 23.70 ± 0.08 | 23.58 ± 0.08 | 23.73 ± 0.04 | H(3) = 2.7; ns |
| Peak periodogram power | 553 ± 68 A | 341 ± 56 AB | 369 ± 98 AB | 229 ± 69 B | H(3) = 8.9; p < 0.05 |
| n | 16 | 8 | 8 | 7 | |
| Group | |||||
|---|---|---|---|---|---|
| Phase 1c (T24-Photoperiods) * | LDark4:8 | LDark5:7 | LDark6:6 | LDark7:5 | Kruskal–Wallis Test |
| BSI | 0.25 ± 0.07 A | 0.47 ± 0.07 AB | 0.55 ± 0.05 BC | 0.71 ± 0.05 C | H(3) = 17.1; p < 0.001 |
| Periodogram-12 h | 336 ± 42 A | 594 ± 60 B | 661 ± 66 B | 667 ± 81 B | H(3) = 12.7; p < 0.01 |
| Periodogram-24 h | 451 ± 90 A | 298 ± 70 AB | 173 ± 34 B | 82 ± 27 C | H(3) = 15.2; p < 0.01 |
| Phase 1d (T24-Photoperiods) | LDim4:8 | LDim5:7 | LDim6:6 | LDim7:5 | |
| BSI | 0.22 ± 0.04 A | 0.55 ± 0.09 AB | 0.61 ± 0.06 B | 0.70 ± 0.03 B | H(3) = 16.7; p < 0.001 |
| Periodogram-12 h | 271 ± 38 | 382 ± 94 | 410 ± 65 | 412 ± 82 | H(3) = 1.9; ns |
| Periodogram-24 h | 364 ± 76 A | 105 ± 35 B | 107 ± 30 B | 61 ± 14 B | H(3) = 14.5; p < 0.01 |
| Phase 2 (T30-Photoperiods) | LDim7:8 | LDim8:7 | LDim9:6 | LDim10:5 | |
| BSI | 0.41 ± 0.05 A | 0.57 ± 0.07 AB | 0.66 ± 0.05 BC | 0.75 ± 0.02 C | H(3) = 17.4; p < 0.001 |
| Periodogram-12 h | 225 ± 34 | 390 ± 99 | 401 ± 75 | 411 ± 72 | H(3) = 3.7; ns |
| Periodogram-24 h | 4.6 ± 2.2 | 3.5 ± 1.0 | 6.8 ± 2.3 | 1.8 ± 0.5 | H(3) = 3.9; ns |
| Periodogram 23-26 h | 175 ± 43 A | 81 ± 34 B | 48 ± 20 B | 21 ± 6 B | H(3) = 16.0; p < 0.01 |
| EQ | 0.59 ± 0.06 A | 0.80 ± 0.07 B | 0.88 ± 0.04 BC | 0.95 ± 0.01 C | H(3) = 18.0; p < 0.001 |
| % activity in light | 2.7 ± 0.4 | 3.6 ± 0.7 | 4.9 ± 1.2 | 3.0 ± 0.5 | H(3) = 1.6; ns |
| Phase 3 (T30-After-effects) | LDim7:8 | LDim7:8 | LDim7:8 | LDim7:8 | |
| BSI | 0.55 ± 0.05 | 0.63 ± 0.05 | 0.63 ± 0.05 | 0.72 ± 0.03 | H(3) = 5.8; ns |
| Periodogram-15 h | 119 ± 14 | 205 ± 46 | 145 ± 21 | 109 ± 22 | H(3) = 3.1; ns |
| Periodogram-30 h | 1.6 ± 0.6 | 4.0 ± 2.2 | 1.7 ± 0.8 | 1.6 ± 0.6 | H(3) = 1.0; ns |
| Periodogram 23-26 h | 78 ± 29 A | 43 ± 19 AB | 66 ± 29 AB | 8 ± 2 B | H(3) = 14.1; p < 0.01 |
| EQ | 0.66 ± 0.05 A | 0.80 ± 0.06 AB | 0.78 ± 0.06 AB | 0.91 ± 0.03 B | H(3) = 11.2; p < 0.05 |
| % activity in light | 2.9 ± 0.6 | 3.3 ± 1.0 | 2.8 ± 0.6 | 2.2 ± 0.3 | H(3) = 1.5; ns |
| n | 10 | 11 ** | 12 | 10 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Joye, D.A.M.; Farkas, A.H.; Gorman, M.R. Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice. Clocks & Sleep 2019, 1, 290-305. https://doi.org/10.3390/clockssleep1030025
Sun J, Joye DAM, Farkas AH, Gorman MR. Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice. Clocks & Sleep. 2019; 1(3):290-305. https://doi.org/10.3390/clockssleep1030025
Chicago/Turabian StyleSun, Jonathan, Deborah A. M. Joye, Andrew H. Farkas, and Michael R. Gorman. 2019. "Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice" Clocks & Sleep 1, no. 3: 290-305. https://doi.org/10.3390/clockssleep1030025
APA StyleSun, J., Joye, D. A. M., Farkas, A. H., & Gorman, M. R. (2019). Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice. Clocks & Sleep, 1(3), 290-305. https://doi.org/10.3390/clockssleep1030025





