Measuring Food Anticipation in Mice
Abstract
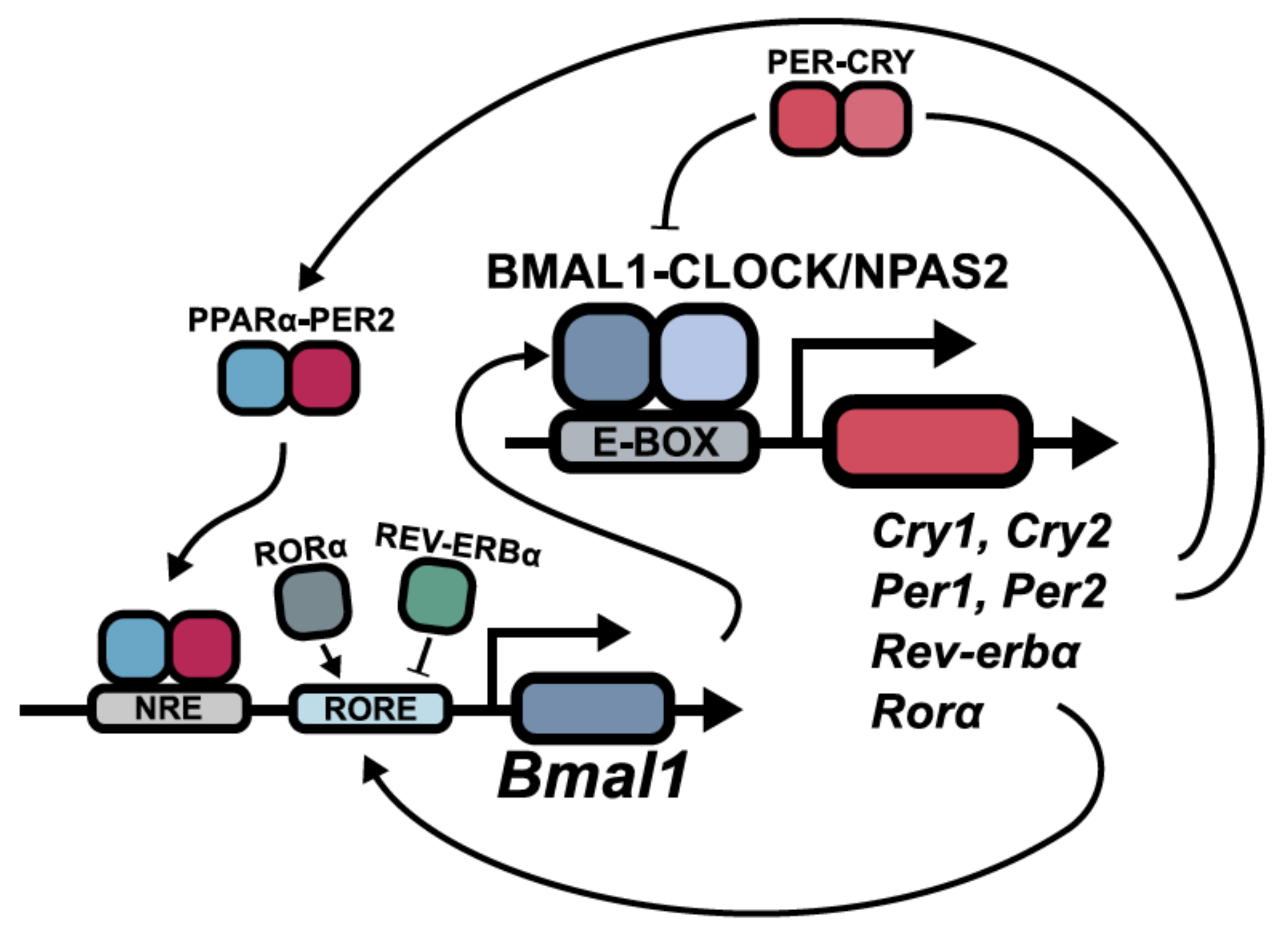
:1. Introduction
2. Protocols
2.1. Restricted Feeding Protocol
- Place min. 3-month-old experimental and control animals into individual cages and give them ad libitum access to standard chow (free access to unlimited amount of food).
- On the 7th, 14th and 21st day clean cages and measure mouse body mass and mass of food eaten.
- On the 21st day remove food just before lights off (ZT 12).
- Use the gathered data to calculate caloric requirements of individual mice. If there are slight differences in the weekly amount of food eaten during adaptation, the values of the final week should be used to determine the regular daily intake.
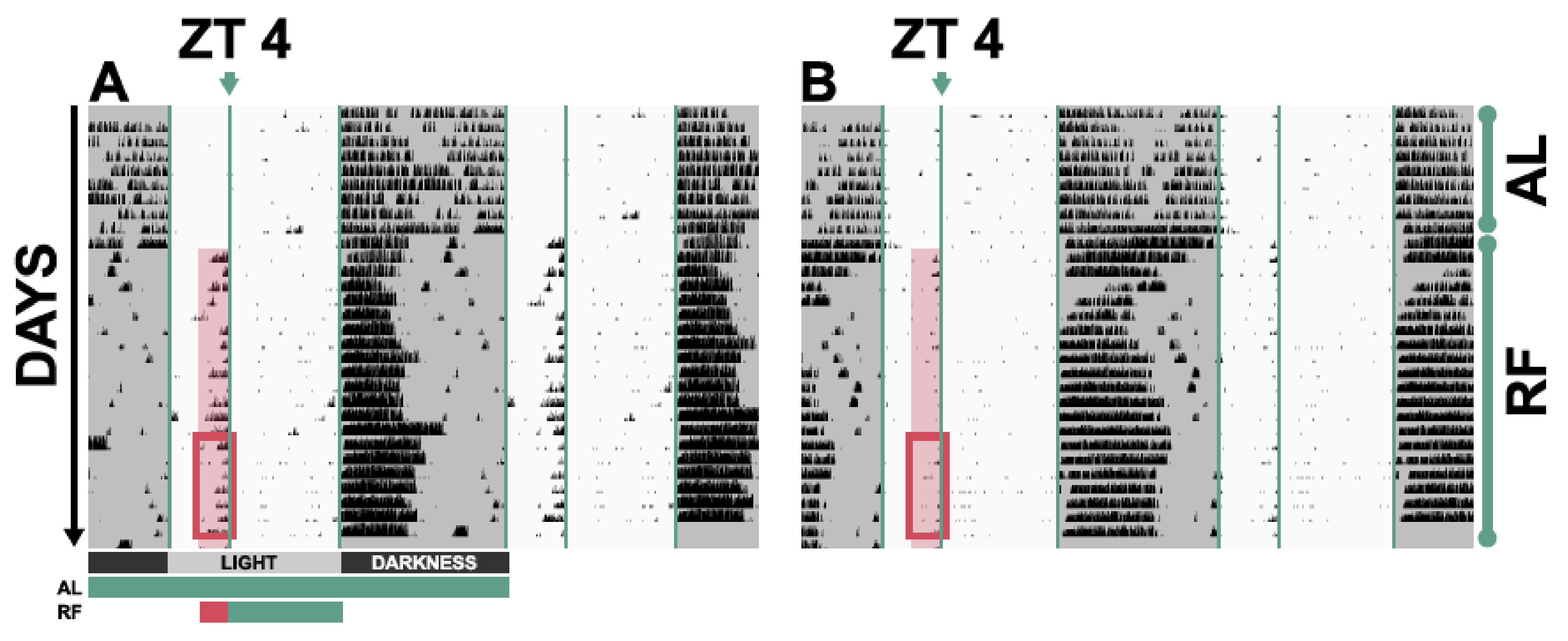
- For the following 3 weeks, starting on the 22nd day, challenge mice by limiting food access from ZT 4 to ZT 12 and by providing 80% of their regular daily intake in the first week and 70% of their regular intake in the following two weeks. Prepare portions of food in advance, so that time with animals is minimised. The body mass of animals should never drop below 80% of the mass they had in the third week of ad libitum feeding. In such an event, remove the mice from the experiment and house them with ad libitum access to food to recover.
- Analyse and compare activities and/or internal body temperature profiles of animals during the last week of ad libitum conditions: the activity levels and temperature profiles should be comparable.
- Analyse and compare activities and patterns of internal body temperature during ZT 2–4 of the last week of restricted feeding conditions.
2.2. Monitoring Activity Using Wheel-Running Cages
- Set up a system for wheel rotation monitoring.
- Validate wheel revolution count.
- Place the mice into wheel-running cages. Note: Special attention should be given to the amount of bedding and nesting material. Too much bedding and nesting will result in partial or total wheel blockage, which may produce false measurements.
- Perform restricted feeding experiment. Record wheel-running activity during the adaptation under ad libitum feeding as well as during the restricted feeding.
- Change cages once a week when weighing mice and food, just before lights off, so that disturbance of mice and their natural rhythms is minimal. If additional inspection of animals is absolutely necessary, do it just before lights off or during the beginning of the activity period of mice using night vision goggles.
- Export and analyse the data.
2.3. Surgical Implantation of Telemetric Transponders
- Prepare necessary medication using good laboratory practice to avoid post-surgical complications and potential abnormal behaviour due to infection and/or inflammation. Prepare all medication in a biosafety cabinet under aseptic conditions. We recommend using 0.9% NaCl infundibile for dilution of the medication rather than in-lab prepared sterile 0.9% NaCl which is not apyrogenic. We also suggest preparing the medication into apyrogenic vials that need to have the rubber stopper disinfected with alcohol. Prior to recovery of fluid from a vial, the syringe should contain a volume of air that will match and replace the volume of liquid required. Likewise, for injection into an empty vial, the plunger of the syringe should be released before the needle and syringe are removed from the vial, to allow removal of displaced air and adjustment of pressure. The liquid-air exchange can be done in multiple small steps by manipulating the plunger.
- 1.1.
- Anaesthetic: 2.4 mg ketamine hydrochloride and 0.01 mg medetomidine hydrochloride per 30 g mouse (80 mg/kg, 0.3 mg/kg). Usually, ketamine hydrochloride is available at 100 mg/mL and medetomidine hydrochloride is available at 1 mg/mL concentration. The appropriate anaesthetic can be prepared by taking 0.120 mL ketamine and diluting it in 0.9% NaCl to a total volume of 1 mL and by taking 0.100 mL medetomidine and diluting it in 0.9% NaCl to a total volume of 1 mL; mix the ketamine and medetomidine diluted solutions in a 2:1 ratio to get the anaesthetic that can be injected as 0.300 mL per 30 g mouse (and adjusted to mouse body mass) intraperitoneally [24].
- 1.2.
- Painkiller: 0.15–0.30 mg carprofen per 30 g mouse (5–10 mg/kg). Usually, carprofen is available at 50 mg/mL concentration. The appropriate painkiller can be prepared by taking 0.050 mL carprofen and diluting it in 0.9% NaCl to a total volume of 5 mL. This solution can be injected as 0.300–0.600 mL per 30 g mouse (and adjusted to mouse body mass) subcutaneously. Injecting the bolus is also a way to provide fluid.
- 1.3.
- Anti-sedation: 0.03 mg atipamezole hydrochloride per 30 g mouse. Usually, atipamezole hydrochloride is available at 5 mg/mL concentration. The appropriate anti-sedation can be prepared by taking 0.040 mL atipamezole hydrochloride and diluting it in 0.9% NaCl to 0.800 mL. This solution can be injected as 0.120 mL per 30 g mouse (and adjusted to mouse body mass) intraperitoneally.
- Clean and sterilize surgical tools and implant. Surgical tools can be sterilized by heat or with a liquid sterilizing agent. The implants can usually be sterilized using liquid sterilizing agents, such as benzalkonium chloride. Consult the manufacturer’s manual. Before use, wash implants with 0.9% NaCl infundibile.
- Weigh the mouse and calculate required doses of medication.
- If you are using an implant that can be fixed into place with stitching material, attach the stitching material to the transponder.
- Immobilize the mouse by grasping the skin fold at the rear of its neck and holding its tail. Manoeuvre it into a head-down position, so that the intestines of the mouse move towards the upper part of the abdominal cavity. This will allow intraperitoneal injection of medication with the intestines being out of the way of the needle.
- Intraperitoneally inject mouse with anaesthetic according to institutional rules and animal experimentation legislation. Place the mouse in its cage and wait for it to become unresponsive. Then roughly clean the mouse of bedding and place it onto an aseptic and heated (42 °C) working surface. Disinfect the belly, for example, with povidone-iodine. Check depth of anaesthesia by pinching skin between toes. Avoid pinching the toe itself as this can cause injury and pain after surgery.
- Apply hydrogel to eyes to prevent drying out.
- Make a vertical incision of a length of 2 cm that stops around 1 cm below the rib cage. Skin can be shaved before the surgery.
- Put the chip into the abdominal cavity, not too close to the skin to avoid false measurements and fasten it in place according to manufacturer’s instructions. Pull the stitching material out through the wound, so it can be fastened into place when doing the suture.
- Close the wound; we use a simple interrupted suture with 3–5 stitches.
- Apply non-steroid anti-inflammatory medication subcutaneously. Apply anti-sedation intraperitoneally (see 5).
- Put the mouse into a cage on a heating mat, place food inside of the cage for easy access. Food can be pre-soaked in water. Nesting material soaked in water (or a piece of apple) can be placed close to the food so the mouse can chew on it. The recovery cage should not have bedding as this could go into the airway of the mouse.
- Monitor mouse at least every 30 min until fully awake and mobile. If necessary, re-apply hydrogel to eyes. Place the mouse into its home cage. Allow at least 3–5 days of recovery before recording.
- After the implantation of transponders and recovery, proceed to the experimental protocol. After surgery, perform ad libitum feeding for 3 weeks in order to monitor potentially abnormal behaviour (activity patterns, temperature fluctuations) and to establish average daily temperature rhythms and regular food intake. Determine the regular food intake when the implant is in place, because the abdominal implant’s physical volume may affect the amount of food eaten.
- Perform the restricted feeding experiment and record internal body temperature and activity with the telemetrics system. Analyse the data.
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rew. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavan, R.; Feillet, C.; Costa, S.S.; Delorme, J.E.; Okabe, T.; Ripperger, J.A.; Albrecht, U. Liver-derived ketone bodies are necessary for food anticipation. Nat. Commun. 2016, 7, 10580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schibler, U.; Ripperger, J.; Brown, S.A. Peripheral circadian oscillators in mammals: Time and food. J. Biol. Rhythms 2003, 18, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistlberger, R.E. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994, 18, 171–195. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B. It’s all in the timing: Many clocks, many outputs. J. Biol. Rhythms 2004, 19, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E. Food-anticipatory circadian rhythms: Concepts and methods. Eur. J. Neurosci. 2009, 30, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Downes, M.; Ruth, T.Y.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear Receptor Expression Links the Circadian Clock to Metabolism. Cell 2006, 25, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.A. Mind your rhythms: An important role for circadian genes in neuroprotection. J. Clin. Investig. 2013, 123, 4994–4996. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Molecular Mechanisms in Mood Regulation Involving the Circadian Clock. Front Neurol. 2017, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian Timing in Cancer Treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J. Cancer Clocks Out for Lunch: Disruption of Circadian Rhythm and Metabolic Oscillation in Cancer. Front. Cell. Dev. Biol. 2016, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lee, C.C. The circadian clock: Pacemaker and tumour suppressor. Nat. Rev. Cancer. 2003, 3, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Mendoza, J.; Dardente, H.; Pévet, P. Neurogenetics of food anticipation. Eur. J. Neurosci. 2009, 30, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Albrecht, U.; Challet, E. Behavioural food anticipation in clock genes deficient mice: Confirming old phenotypes, describing new phenotypes. Genes Brain Behav. 2010, 9, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.-A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the Circadian Clock in the Liver by Feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Langmesser, S.; Tallone, T.; Bordon, A.; Rusconi, S.; Albrecht, U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol. 2008, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, J.; Albrecht, U. Clock-Controlled Genes; Springer Nature: New York, NY, USA, 2009. [Google Scholar]
- Jud, C.; Schmutz, I.; Hampp, G.; Oster, H.; Albrecht, U. A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol. Proced. Online 1997, 7, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arras, M.; Autenried, P.; Rettich, A.; Spaeni, D.; Rülicke, T. Optimization of intraperitoneal injection anesthesia in mice: Drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 2001, 51, 443–456. [Google Scholar] [PubMed]
- Feillet, C.A.; Ripperger, J.A.; Magnone, M.C.; Dulloo, A.; Albrecht, U.; Challet, E. Lack of Food Anticipation in Per2 Mutant Mice. Curr. Biol. 2006, 16, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Vital View Data Acquisition System, Instruction Manual, Software Version 5.1, Starr Life Sciences: Oakmont, PA, USA.
- Tan, K.; Knight, Z.A.; Friedman, J.M. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab. 2014, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.D.; Lamont, E.W.; Rodrigues, T.; Abizaid, A. Isolating neural correlates of the pacemaker for food anticipation. PLoS ONE 2012, 7, e36117. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Yamazaki, S. The Mysterious Food-Entrainable Oscillator: Insights from Mutant and Engineered Mouse Models. J. Biol. Rhythms 2018, 33, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J. Search for the feeding-entrainable circadian oscillator: A complex proposition. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R1524–R1526. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, T.; Ripperger, J.A.; Albrecht, U. Measuring Food Anticipation in Mice. Clocks & Sleep 2019, 1, 65-74. https://doi.org/10.3390/clockssleep1010007
Martini T, Ripperger JA, Albrecht U. Measuring Food Anticipation in Mice. Clocks & Sleep. 2019; 1(1):65-74. https://doi.org/10.3390/clockssleep1010007
Chicago/Turabian StyleMartini, Tomaz, Jürgen A. Ripperger, and Urs Albrecht. 2019. "Measuring Food Anticipation in Mice" Clocks & Sleep 1, no. 1: 65-74. https://doi.org/10.3390/clockssleep1010007
APA StyleMartini, T., Ripperger, J. A., & Albrecht, U. (2019). Measuring Food Anticipation in Mice. Clocks & Sleep, 1(1), 65-74. https://doi.org/10.3390/clockssleep1010007





