Objective Food Intake in Night and Day Shift Workers: A Laboratory Study
Abstract
:1. Introduction
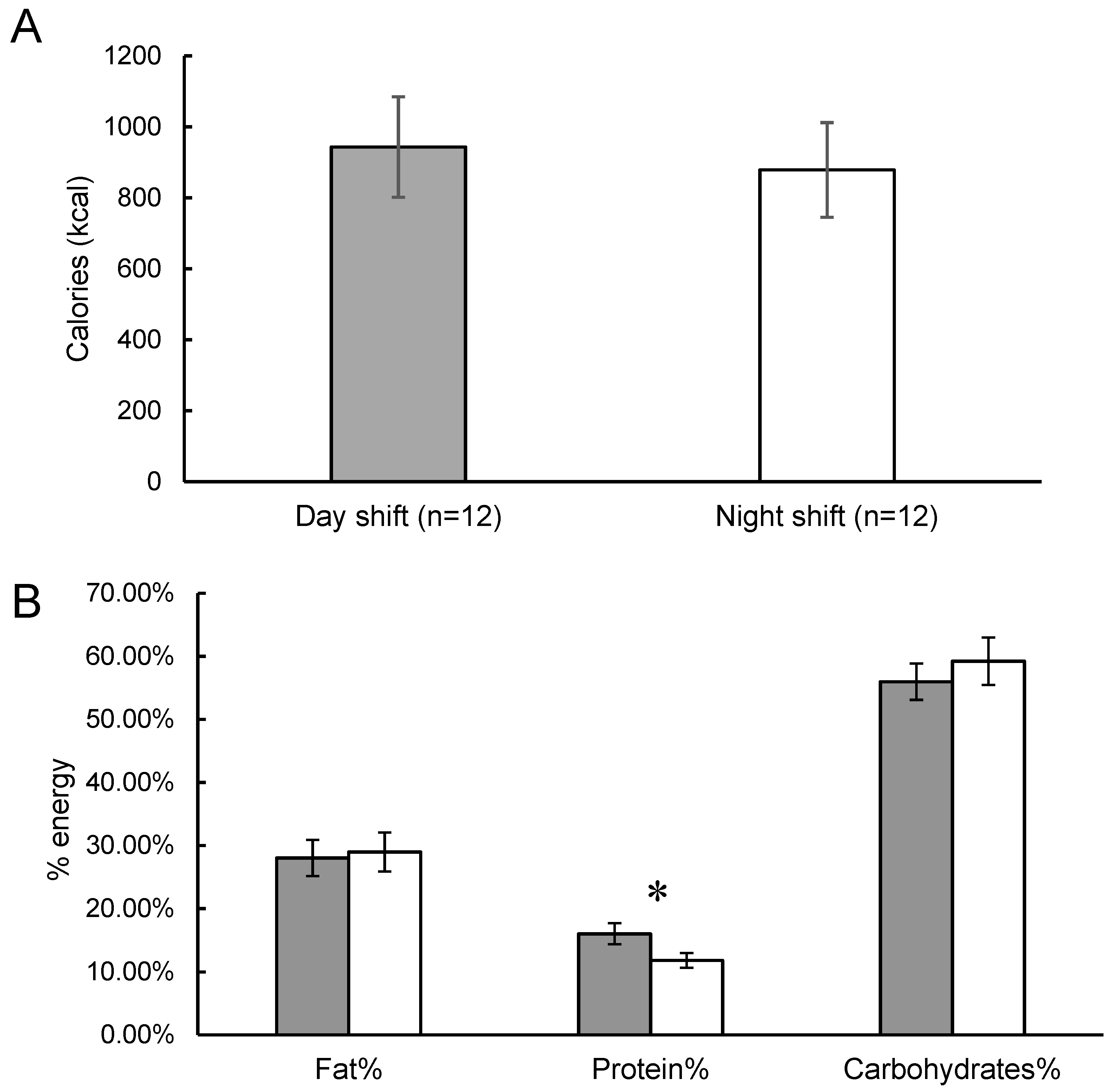
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Atlanta, GA, USA, 2017.
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Alterman, T.; Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Calvert, G.M. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Grandner, M.A.; St-Onge, M.-P. The role of sleep in the control of food intake. Am. J. Lifestyle Med. 2014, 8, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Wright, K.P. Sleep loss and fatigue in shift work and shift work disorder. Sleep Med. Clin. 2009, 4, 257–271. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, O.M.; Cain, S.W.; O’connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra43. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.P.; Bonnell, E.K.; Huggins, C.E. Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiol. Int. 2016, 33, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B. Energy Balance Measurement Working Group. Energy balance measurement: When something is not better than nothing. Int. J. Obes. (Lond.) 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.W.; Filtness, A.J.; Phillips, C.L.; Anderson, C. Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work Environ. Health 2015, 41, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—Effects on habits, metabolism, and performance. Scand. J. Work Environ. Health 2010, 36, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Padilha, H.G.; Crispim, C.A.; Zimberg, I.Z.; Folkard, S.; Tufik, S.; de Mello, M.T. Metabolic responses on the early shift. Chronobiol. Int. 2010, 27, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Kawano, Y.; Maeda, I.; Yoshizaki, T.; Sunami, A.; Yokoyama, Y.; Matsumoto, H.; Hida, A.; Komatsu, T.; Togo, F. Association of body mass index with lifestyle and rotating shift work in J apanese female nurses. Obesity 2014, 22, 2489–2493. [Google Scholar] [PubMed]
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010, 11, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlheim, L.S.; Allison, D.B.; Heshka, S.; Heymsfield, S.B. Do unsuccessful dieters intentionally underreport food intake? Int. J. Eat. Disord. 1998, 24, 259–266. [Google Scholar] [CrossRef]
- De Castro, J.M. Eating behavior: Lessons from the real world of humans. Nutrition 2000, 16, 800–813. [Google Scholar] [CrossRef]
- Venti, C.A.; Votruba, S.B.; Franks, P.W.; Krakoff, J.; Salbe, A.D. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am. J. Clin. Nutr. 2010, 91, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Finlayson, G.; Dalton, M.; Caudwell, P.; Blundell, J.E. Metabolic Phenotyping Guidelines: Studying eating behaviour in humans. J. Endocrinol. 2014, 222, G1–G12. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, M.M.; Rolls, B.J. Favouring more rigour when investigating human eating behaviour is like supporting motherhood and apple pie: A response to Robinson, Bevelander, Field, and Jones (2018). Appetite 2018, 130, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Lassen, A.; Mahnke, C.; Schultes, B.; Schiöth, H.B.; Born, J.; Lange, T. Acute sleep deprivation reduces energy expenditure in healthy men. Am. J. Clin. Nutr. 2011, 93, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brondel, L.; Romer, M.A.; Nougues, P.M.; Touyarou, P.; Davenne, D. Acute partial sleep deprivation increases food intake in healthy men. Am. J. Clin. Nutr. 2010, 91, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Kasza, K.; Schoeller, D.A.; Penev, P.D. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 2009, 89, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Hallschmid, M.; Jauch-Chara, K.; Wilms, B.; Benedict, C.; Lehnert, H.; Born, J.; Schultes, B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am. J. Clin. Nutr. 2009, 90, 1476–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Onge, M.P.; Roberts, A.L.; Chen, J.; Kelleman, M.; O’Keeffe, M.; RoyChoudhury, A.; Jones, P.J. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr. 2011, 94, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargent, C.; Zhou, X.; Matthews, R.W.; Darwent, D.; Roach, G.D. Daily rhythms of hunger and satiety in healthy men during one week of sleep restriction and circadian misalignment. Int. J. Environ. Res. Public Health 2016, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. Obesity: The protein leverage hypothesis. Obes. Rev. 2005, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Harnack, L.; Pereira, M.A. Assessment of the accuracy of nutrient calculations of five popular nutrition tracking applications. Public Health Nutr. 2018, 21, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic sleep/wake identification from wrist activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef] [PubMed]
| Food Item | Serving Size (g) | Energy (kcal) | Fat (%) | Protein (%) | Carbohydrate (%) |
|---|---|---|---|---|---|
| High fat | |||||
| Soft cheese | 156 | 483 | 73 | 27 | 0 |
| Peanut butter | 188 | 1190 | 71 | 13 | 16 |
| Oreo cookies | 130 | 668 | 38 | 2 | 60 |
| Chocolate kisses | 47 | 268 | 29 | 28 | 43 |
| High protein | |||||
| Greek yogurt | 320 | 298 | 18 | 31 | 51 |
| Protein bar | 140 | 583 | 29 | 28 | 43 |
| High carbohydrate | |||||
| Strawberry jam | 44 | 116 | 0 | 0 | 100 |
| Grape jam | 41 | 139 | 0 | 0 | 100 |
| White bread | 118 | 325 | 9 | 11 | 80 |
| Fruits | |||||
| Apples | 391 | 203 | 0 | 0 | 100 |
| Bananas | 362 | 301 | 3 | 4 | 93 |
| Raisins | 62 | 204 | 0 | 4 | 96 |
| Beverages | |||||
| Apple juice | 436 | 221 | 0 | 0 | 100 |
| Orange juice | 441 | 252 | 0 | 2 | 98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lauren, S.; Chang, B.P.; Shechter, A. Objective Food Intake in Night and Day Shift Workers: A Laboratory Study. Clocks & Sleep 2019, 1, 42-49. https://doi.org/10.3390/clockssleep1010005
Chen Y, Lauren S, Chang BP, Shechter A. Objective Food Intake in Night and Day Shift Workers: A Laboratory Study. Clocks & Sleep. 2019; 1(1):42-49. https://doi.org/10.3390/clockssleep1010005
Chicago/Turabian StyleChen, Yichi, Shaza Lauren, Bernard P. Chang, and Ari Shechter. 2019. "Objective Food Intake in Night and Day Shift Workers: A Laboratory Study" Clocks & Sleep 1, no. 1: 42-49. https://doi.org/10.3390/clockssleep1010005
APA StyleChen, Y., Lauren, S., Chang, B. P., & Shechter, A. (2019). Objective Food Intake in Night and Day Shift Workers: A Laboratory Study. Clocks & Sleep, 1(1), 42-49. https://doi.org/10.3390/clockssleep1010005






